The Effect of the Gallbladder Environment during Chronic Infection on Salmonella Persister Cell Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Minimum Inhibitory Concentration
2.3. Planktonic Persister Assays
2.4. Biofilm Persister Assays
2.5. Statistical Analysis
3. Results
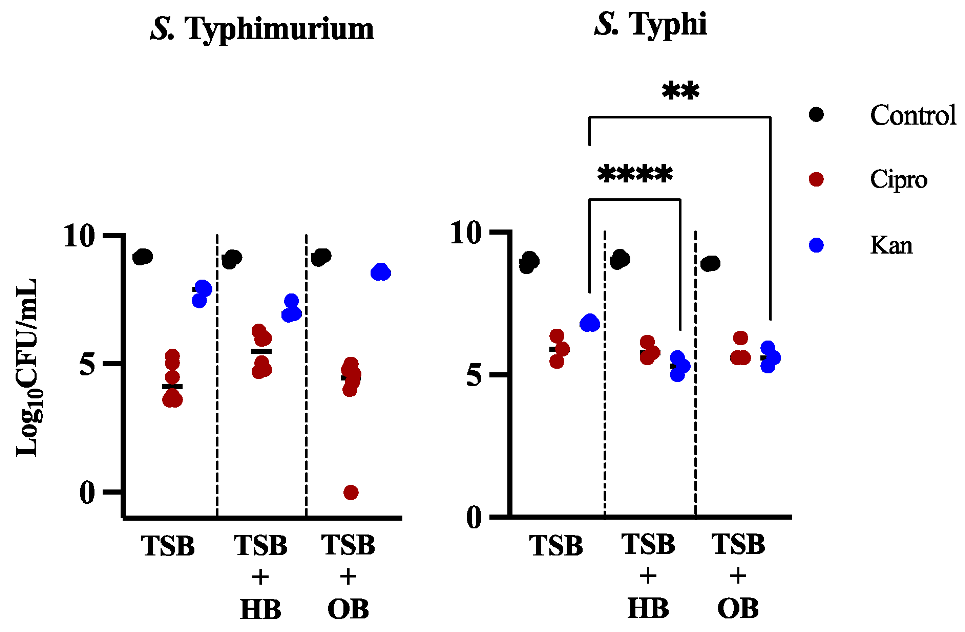
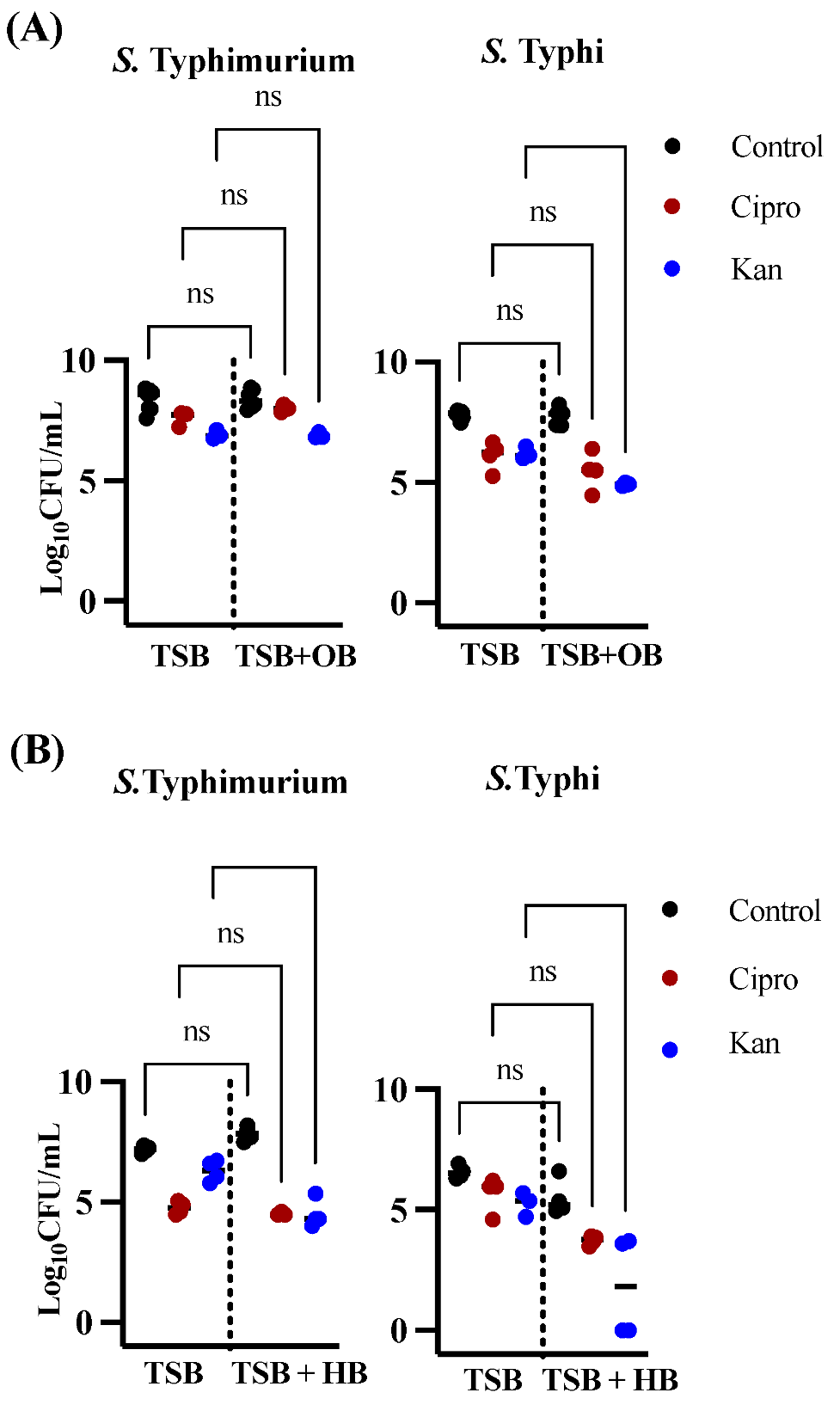
3.1. Effects of Bile in Persister Formation of Various Salmonella Populations
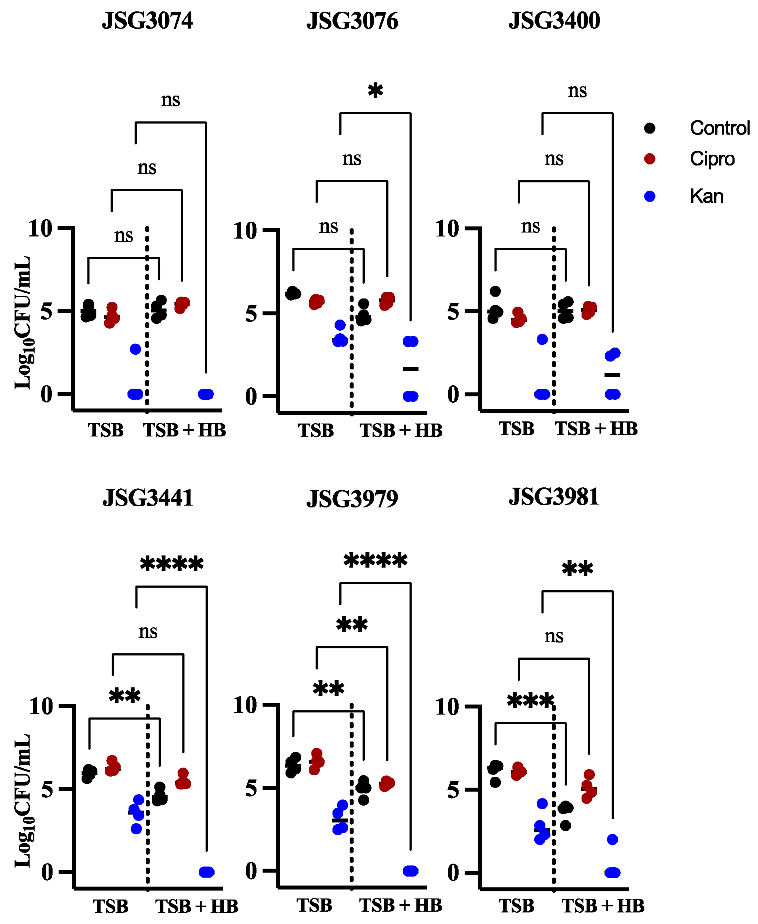
3.2. Human Bile Has a Varied Effect on the Amount of Persister Cells Found after Ciprofloxacin or Kanamycin Treatment in Biofilms of S. Typhi Clinical Isolates
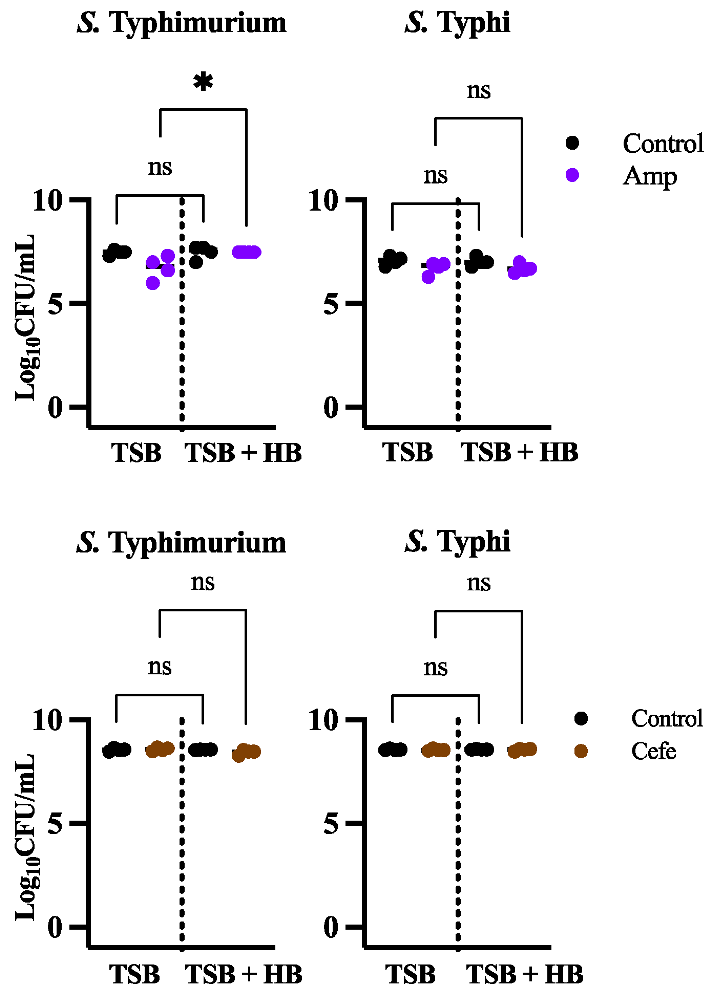
3.3. The Cellular Target of an Antibiotic Influences the Amount of Persisters
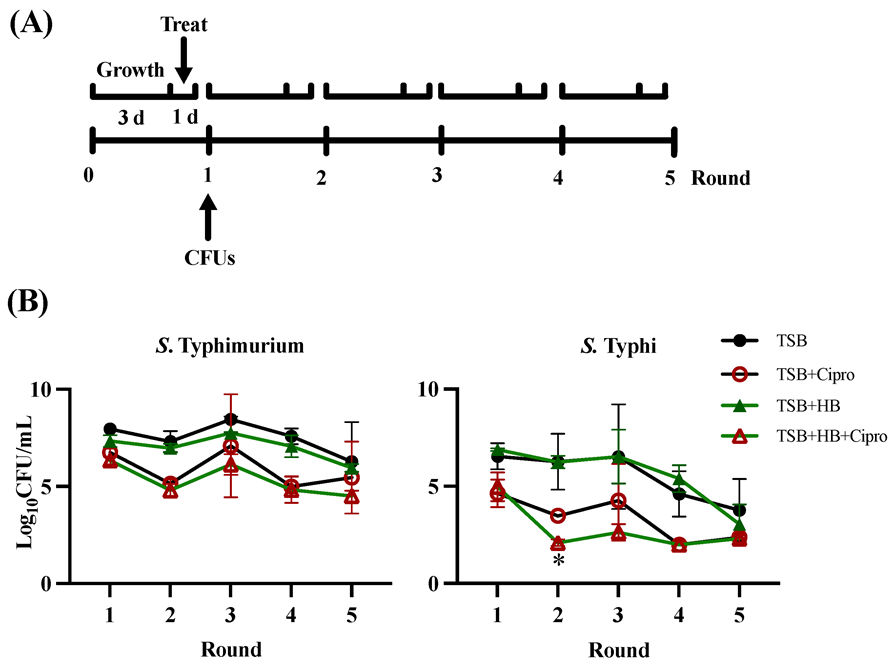
3.4. Multiple Rounds of Ciprofloxacin Treatment in GS Conditions affects S. Typhimurium and S. Typhi Differently
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chambers, H.; McPhee, S.; Papadakis, M.; Tierney, L. Current Medical Diagnosis and Treatment; McGraw-Hill Medical: New York, NY, USA, 2008; Available online: https://scholar.google.com/scholar_lookup?title=Current%20Medical%20Diagnosis%20and%20Treatment&author=H.F.%20Chambers&publication_year=2008 (accessed on 25 August 2021).
- Ristori, C.; Rodríguez, H.; Vicent, P.; Ferreccio, C.; García, J.; Lobos, H.; D’Ottone, K. Persistence of the Salmonella typhi-paratyphi carrier state after gallbladder removal. Bull. Pan Am. Health Organ. 1982, 16, 361–366. [Google Scholar] [PubMed]
- Crawford, R.W.; Rosales-Reyes, R.; Ramirez-Aguilar, M.d.l.L.; Chapa-Azuela, O.; Alpuche-Aranda, C.; Gunn, J.S. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc. Natl. Acad. Sci. USA 2010, 107, 4353–4358. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Escobedo, G.; Marshall, J.; Gunn, J.S. Chronic and acute infection of the gall bladder by Salmonella Typhi: Understanding the carrier state. Nat. Rev. Genet. 2011, 9, 9–14. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Genet. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Bigger, J.W. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 1944, 244, 497–500. [Google Scholar] [CrossRef]
- Wood, T.K.; Knabel, S.J.; Kwan, B.W. Bacterial Persister Cell Formation and Dormancy. Appl. Environ. Microbiol. 2013, 79, 7116–7121. [Google Scholar] [CrossRef]
- Wilmaerts, D.; Windels, E.; Verstraeten, N.; Michiels, J. General Mechanisms Leading to Persister Formation and Awakening. Trends Genet. 2019, 35, 401–411. [Google Scholar] [CrossRef]
- Helaine, S.; Kugelberg, E. Bacterial persisters: Formation, eradication, and experimental systems. Trends Microbiol. 2014, 22, 417–424. [Google Scholar] [CrossRef]
- Cohen, N.R.; Lobritz, M.A.; Collins, J.J. Microbial Persistence and the Road to Drug Resistance. Cell Host Microbe 2013, 13, 632–642. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells and the riddle of biofilm survival. Biochemistry 2005, 70, 267–274. [Google Scholar] [CrossRef]
- Moyed, H.S.; Bertrand, K.P. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J. Bacteriol. 1983, 155, 768–775. [Google Scholar] [CrossRef]
- Moyed, H.S.; Broderick, S.H. Molecular cloning and expression of hipA, a gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J. Bacteriol. 1986, 166, 399–403. [Google Scholar] [CrossRef]
- Conlon, B.P.; Rowe, S.E.; Gandt, A.B.; Nuxoll, A.S.; Donegan, N.P.; Zalis, E.A.; Clair, G.; Adkins, J.N.; Cheung, A.L.; Lewis, K. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 2016, 1, 16051. [Google Scholar] [CrossRef]
- Shan, Y.; Gandt, A.B.; Rowe, S.E.; Deisinger, J.P.; Conlon, B.P.; Lewis, K. ATP-Dependent Persister Formation in Escherichia coli. mBio 2017, 8, e02267-16. [Google Scholar] [CrossRef]
- Pontes, M.H.; Groisman, E.A. Slow growth determines nonheritable antibiotic resistance in Salmonella enterica. Sci. Signal. 2019, 12, eaax3938. [Google Scholar] [CrossRef]
- Pontes, M.H.; Groisman, E.A. A Physiological Basis for Nonheritable Antibiotic Resistance. mBio 2020, 11, e00817-2. [Google Scholar] [CrossRef]
- Begley, M.; Gahan, C.G.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef]
- Hernández, S.B.; Cota, I.; Ducret, A.; Aussel, L.; Casadesús, J. Adaptation and Preadaptation of Salmonella enterica to Bile. PLOS Genet. 2012, 8, e1002459. [Google Scholar] [CrossRef]
- González, J.F.; Alberts, H.; Lee, J.; Doolittle, L.; Gunn, J.S. Biofilm Formation Protects Salmonella from the Antibiotic Ciprofloxacin In Vitro and In Vivo in the Mouse Model of chronic Carriage. Sci. Rep. 2018, 8, 222. [Google Scholar] [CrossRef]
- Melvin, P.; Weinstein, M. Dilution AST for Aerobically Grown Bacteria—CLSI, 11th ed.; CLSI: Wayne, PA, USA, 2018; Available online: https://clsi.org/standards/products/microbiology/documents/m07/ (accessed on 10 August 2021).
- Gonzalez-Escobedo, G.; Gunn, J.S. Gallbladder Epithelium as a Niche for Chronic Salmonella Carriage. Infect. Immun. 2013, 81, 2920–2930. [Google Scholar] [CrossRef] [PubMed]
- Effa, E.E.; Lassi, Z.S.; Critchley, J.A.; Garner, P.; Sinclair, D.; Olliaro, P.L.; Bhutta, Z.A. Fluoroquinolones for treating typhoid and paratyphoid fever (enteric fever). Cochrane Database Syst. Rev. 2011, 2011, CD004530. [Google Scholar] [CrossRef] [PubMed]
- Thaver, D.; Zaidi, A.K.M.; Critchley, J.; Azmatullah, A.; Madni, S.A.; Bhutta, Z.A. A comparison of fluoroquinolones versus other antibiotics for treating enteric fever: Meta-analysis. BMJ 2009, 338, b1865. [Google Scholar] [CrossRef] [PubMed]
- Schiøler, H.; Christiansen, E.D.; Høybye, G.; Rasmussen, S.N.; Greibe, J. The Danish Salca-Group Biliary Calculi in Chronic Salmonella Carriers and Healthy Controls: A Controlled Study. Scand. J. Infect. Dis. 1983, 15, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Dinbar, A.; Altmann, G.; Tulcinsky, D. The treatment of chronic biliary Salmonella carriers. Am. J. Med. 1969, 47, 236–242. [Google Scholar] [CrossRef]
- Drescher, S.P.M.; Gallo, S.W.; Ferreira, P.M.A.; Ferreira, C.A.S.; De Oliveira, S.D. Salmonella enterica persister cells form unstable small colony variants after in vitro exposure to ciprofloxacin. Sci. Rep. 2019, 9, 7232. [Google Scholar] [CrossRef]
- Narimisa, N.; Amraei, F.; Kalani, B.S.; Jazi, F.M. Evaluation of gene expression and protein structural modeling involved in persister cell formation in Salmonella Typhimurium. Braz. J. Microbiol. 2020, 52, 207–217. [Google Scholar] [CrossRef]
- Spoering, A.L.; Lewis, K. Biofilms and Planktonic Cells of Pseudomonas aeruginosa Have Similar Resistance to Killing by Antimicrobials. J. Bacteriol. 2001, 183, 6746–6751. [Google Scholar] [CrossRef]
- Waters, E.M.; Rowe, S.E.; O’Gara, J.P.; Conlon, B.P. Convergence of Staphylococcus aureus Persister and Biofilm Research: Can Biofilms Be Defined as Communities of Adherent Persister Cells? PLOS Pathog. 2016, 12, e1006012. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Genet. 2019, 17, 441–448. [Google Scholar] [CrossRef]
- Verstraete, L.; Bergh, B.V.D.; Verstraeten, N.; Michiels, J. Ecology and evolution of antibiotic persistence. Trends Microbiol. 2021, 30, 466–479. [Google Scholar] [CrossRef]
- Trampari, E.; Holden, E.R.; Wickham, G.J.; Ravi, A.; Martins, L.D.O.; Savva, G.M.; Webber, M.A. Exposure of Salmonella biofilms to antibiotic concentrations rapidly selects resistance with collateral tradeoffs. NPJ Biofilms Microbiomes 2021, 7, 3. [Google Scholar] [CrossRef]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef]
- Ronneau, S.; Hill, P.W.; Helaine, S. Antibiotic persistence and tolerance: Not just one and the same. Curr. Opin. Microbiol. 2021, 64, 76–81. [Google Scholar] [CrossRef]
- Pu, Y.; Li, Y.; Jin, X.; Tian, T.; Ma, Q.; Zhao, Z.; Lin, S.-Y.; Chen, Z.; Li, B.; Yao, G.; et al. ATP-Dependent Dynamic Protein Aggregation Regulates Bacterial Dormancy Depth Critical for Antibiotic Tolerance. Mol. Cell 2019, 73, 143–156. [Google Scholar] [CrossRef]
- Helaine, S.; Cheverton, A.M.; Watson, K.G.; Faure, L.M.; Matthews, S.A.; Holden, D.W. Internalization of Salmonella by Macrophages Induces Formation of Nonreplicating Persisters. Science 2014, 343, 204–208. [Google Scholar] [CrossRef]
- Stapels, D.A.C.; Hill, P.W.S.; Westermann, A.J.; Fisher, R.A.; Thurston, T.L.; Saliba, A.-E.; Blommestein, I.; Vogel, J.; Helaine, S. Salmonella persisters undermine host immune defenses during antibiotic treatment. Science 2018, 362, 1156–1160. [Google Scholar] [CrossRef]
- Hill, P.W.; Moldoveanu, A.L.; Sargen, M.; Ronneau, S.; Glegola-Madejska, I.; Beetham, C.; Fisher, R.A.; Helaine, S. The vulnerable versatility of Salmonella antibiotic persisters during infection. Cell Host Microbe 2021, 29, 1757–1773. [Google Scholar] [CrossRef]
- Bartell, J.A.; Cameron, D.R.; Mojsoska, B.; Haagensen, J.A.J.; Pressler, T.; Sommer, L.M.; Lewis, K.; Molin, S.; Johansen, H.K. Bacterial persisters in long-term infection: Emergence and fitness in a complex host environment. PLOS Pathog. 2020, 16, e1009112. [Google Scholar] [CrossRef]
- LaFleur, M.D.; Qi, Q.; Lewis, K. Patients with Long-Term Oral Carriage Harbor High-Persister Mutants of Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 39–44. [Google Scholar] [CrossRef]
- Mulcahy, L.R.; Burns, J.L.; Lory, S.; Lewis, K. Emergence of Pseudomonas aeruginosa Strains Producing High Levels of Persister Cells in Patients with Cystic Fibrosis. J. Bacteriol. 2010, 192, 6191–6199. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, B.; Michiels, J.E.; Wenseleers, T.; Windels, E.M.; Boer, P.V.; Kestemont, D.; De Meester, L.; Verstrepen, K.J.; Verstraeten, N.; Fauvart, M.; et al. Frequency of antibiotic application drives rapid evolutionary adaptation of Escherichia coli persistence. Nat. Microbiol. 2016, 1, 16020. [Google Scholar] [CrossRef] [PubMed]
- Said, H.M. (Ed.) Front Matter. In Physiology of the Gastrointestinal Tract, 6th ed.; Academic Press: Cambridge, MA, USA, 2018; pp. i–ii. [Google Scholar]
| % In Planktonic | % In Biofilm | ||||
|---|---|---|---|---|---|
| Organism | Media | Ciprofloxacin | Kanamycin | Ciprofloxacin | Kanamycin |
| S. Typhimurium | Control | 3.07 × 10−4 | 4.61 | 4.89 | 13.73 |
| HB | 0.01 | 1.18 | 0.04 | 0.08 | |
| OB | 6.67 × 10−4 | 25.78 | 21.55 | 6.70 | |
| S. Typhi | Control | 0.12 | 0.69 | 12.16 | 4.12 |
| HB | 0.07 | 0.02 | 0.52 | 0.20 | |
| OB | 0.12 | 0.06 | 1.67 | 0.08 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, J.F.; Hitt, R.; Laipply, B.; Gunn, J.S. The Effect of the Gallbladder Environment during Chronic Infection on Salmonella Persister Cell Formation. Microorganisms 2022, 10, 2276. https://doi.org/10.3390/microorganisms10112276
González JF, Hitt R, Laipply B, Gunn JS. The Effect of the Gallbladder Environment during Chronic Infection on Salmonella Persister Cell Formation. Microorganisms. 2022; 10(11):2276. https://doi.org/10.3390/microorganisms10112276
Chicago/Turabian StyleGonzález, Juan F., Regan Hitt, Baileigh Laipply, and John S. Gunn. 2022. "The Effect of the Gallbladder Environment during Chronic Infection on Salmonella Persister Cell Formation" Microorganisms 10, no. 11: 2276. https://doi.org/10.3390/microorganisms10112276
APA StyleGonzález, J. F., Hitt, R., Laipply, B., & Gunn, J. S. (2022). The Effect of the Gallbladder Environment during Chronic Infection on Salmonella Persister Cell Formation. Microorganisms, 10(11), 2276. https://doi.org/10.3390/microorganisms10112276





