Abstract
Probiotic Lactobacillus species are known to exert health benefits in hosts when administered in adequate quantities. A systematic safety assessment of the strains must be performed before the Lactobacillus strains can be designated as probiotics for human consumption. In this study, we selected Lactobacillus fermentum IDCC 3901, L. gasseri IDCC 3101, L. helveticus IDCC 3801, and L. salivarius IDCC 3551 as representative Lactobacilli probiotic strains and investigated their probiotic properties and potential risks through phenotypic and genomic characterization. Various assays including antimicrobial resistance, biogenic amine production, L-/D-lactate production, acute oral toxicity, and antipathogenic effect were performed to evaluate the safety of the four Lactobacillus strains. Genomic analysis using whole genome sequencing was performed to investigate virulence and antibiotic resistance genes in the genomes of the selected probiotic strains. The phenotypes of the strains such as enzymatic activity and carbohydrate utilization were also investigated. As a result, antibiotic resistances of the four Lactobacillus species were detected; however, neither antibiotic resistance-related genes nor virulence genes were found by genomic analysis. Moreover, the four Lactobacillus species did not exhibit hemolytic activity or β-glucuronidase activity. The biogenic amine production and oral acute toxicity were not shown in the four Lactobacillus species, whereas they produced D-lactate with minor ratio. The four Lactobacillus species exhibited antipathogenic effect to five pathogenic microorganisms. This study provides a way to assess the potential risks of four different Lactobacillus species and validates the safety of all four strains as probiotics for human consumption.
1. Introduction
Probiotics are live microorganisms that confer health benefits to the host when administered in adequate amounts [1]. Lactic acid bacteria (LAB), including Lactobacilli, are well-known probiotics. Lactobacilli are Gram-positive, rod-shaped bacteria. Lactobacilli are traditionally used for fermentation of dairy, vegetables, and beverages [2]. In dairy industry, L. helveticus, L. delbruekii subsp. bulgaricus, L. paracasei, and L. rhamnosus are important in cheese fermentation [3]. L. delbruekii subsp. bulgaricus is also a traditional yogurt bacterium [4]. L. plantarum and L. brevis are usually isolated from fermented vegetables [5]. Moreover, wine fermentation includes some Lactobacilli such as L. plantarum, L. brevis, L. paracasei [6]. Lactobacilli are important members of the human gut microbiota, and some strains are known for their varied health benefits [7]. Lactobacillus species are extensively studied for their intestinal regulation by immunomodulatory mechanism [8]. Several representative strains of Lactobacilli with probiotic potential have been consumed as dietary supplements including L. rhamnosus, L. helveticus, L. fermentum, L. gasseri, L. bulgaricus, L. acidophilus, L. casei, and L. reuteri [9].
Various biological activities of probiotic nature have been reported in Lactobacilli species, and the demand for such probiotic lactobacilli species for intake in promoting human health has been present for decades. Therefore, oral intake of probiotic Lactobacilli should be proven safe for humans by a systematic safety assessment. Genome analysis can be used for performing safety evaluation because it can provide genetic information about probiotic strains, such as the presence of virulence genes and antibiotic resistance genes in their genome.
Previously, we showed that four probiotics strains, L. fermentum IDCC 3901, L. gasseri IDCC 3101, L. helveticus IDCC 3801, and L. salivarius IDCC 3551 have 76-87% acid tolerance, 96–97% bile tolerance, 42–53% adhesion to Caco-2 cells, and 70–72% competitive exclusion against pathogenic bacteria [10]. Furthermore, we applied quadruple coating to these strains to improve these probiotics properties in the manufacturing process [10,11]. Fermented milk by L. helveticus IDCC 3801 alleviated memory deficit by reducing beta-amyloid in a rat model [12]. In this study, four Lactobacillus strains, L. fermentum IDCC 3901, L. gasseri IDCC 3101, L. helveticus IDCC 3801, and L. salivarius IDCC 3551, were selected and evaluated. This study provides a way to assess the potential risks of four Lactobacillus species using various phenotypic and genomic analyses, including whole genome sequence analysis, minimum inhibitory concentration (MIC) test, β-hemolytic activity assay, enzymatic activity assay, carbohydrate utilization test, biogenic amine production analysis, L-/D-lactate production analysis, and oral acute toxicity tests. Finally, the antipathogenic activities of the strains were investigated in terms of preventing or relieving chronic inflammation in the gastrointestinal tract. Thus, the systematic analysis performed in this study validates the use of these four specific Lactobacillus strains as safe probiotic strains.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
L. fermentum IDCC 3901, L. gasseri IDCC 3101, L. helveticus IDCC 3801, and L. salivarius IDCC 3551 were stored at −80 °C by Ildong Bioscience (Pyeongtaek-si, Gyeonggi-do, Korea). All strains were grown in De Man, Rogosa, and Sharpe (MRS) medium (BD Difco, Franklin Lakes, NJ, USA) at 37 °C. All four Lactobacillus strains were used for probiotic manufacturing at Ildong Bioscience (Table 1).

Table 1.
Probiotic strains used in this study.
For the antipathogenic experiment, the five pathogenic microorganisms including Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, Streptococcus pneumonia ATCC 49619, Bacillus cereus ATCC 14579, and Salmonella Typhimurium ATCC 13311 were purchased from the American Type Culture Collection (ATCC). The culture conditions of each pathogenic microorganism are listed in Table 2.

Table 2.
Pathogenic bacteria used in this study.
2.2. Genomic Analysis
Genomic DNA of the Lactobacillus strains L. fermentum IDCC 3901, L. gasseri IDCC 3101, and L. helveticus IDCC 3801 was extracted using a Maxwell 16 LEV Blood DNA Kit and a Maxwell 16 Buccal Swab LEV DNA Purification Kit (Promega Co., Madison, WI, USA) according to the manufacturer’s instructions. Genomic DNA was extracted from the L. salivarius IDCC 3551 using the Wizard Genomic DNA Purification Kit (Promega Co., USA) according to the manufacturer’s instructions. Genome sequencing was performed by Macrogen Inc. (Korea) using a PacBio RS II instrument (Pacific Biosciences of California Inc., Menlo Park, CA, USA) on an Illumina platform (Illumina Inc., San Diego, CA, USA). The average nucleotide identity (ANI) value was calculated using an ANI calculator (Kostas Lab).
2.3. Identification of Antibiotic Resistance, Virulence Genes, and Mobile Elements
To identify putative antibiotic resistance genes, the assembled sequences were compared to the reference sequences in the ResFinder database (https://cge.cbs.dtu.dk/services/ResFinder/) using ResFinder 3.2 software (accessed on 1 July 2021) [13]. The search parameters for the analysis were sequence identity of >80% and coverage of >60%. To analyze virulence genes, VFDB (http://www.mgc.ac.cn/VFs/, accessed on 1 July 2021) was used with the BLASTn algorithm [14]. The thresholds for identification were as follows: identity > 70%, coverage > 70%, and E-value < 1E-5. In addition, mobile elements were searched using the BLASTP algorithm (for transposases and plasmids) and the PHASTER web-based program (for prophage regions) [15].
2.4. Determination of Minimum Inhibitory Concentrations (MICs)
The four Lactobacillus strains were assessed for susceptibility to ampicillin, vancomycin, gentamicin, kanamycin, streptomycin, erythromycin, clindamycin, tetracycline, and chloramphenicol (Sigma-Aldrich, St. Louis, MO, USA). Cultures of each strain and antibiotics were mixed in a 96-well microplate and anaerobically incubated at 37 °C for 18–20 h. The optical density was measured using a microplate reader (BioTek, Winooski, VT, USA).
2.5. β-Hemolytic and Enzymatic Activities
Each Lactobacillus species was grown overnight at 37 °C in MRS medium, and the culture was streaked on sheep blood agar plates (BD Difco). The plates were incubated overnight at 37 °C. A clear zone around the colony demonstrated hemolytic activity. For all samples, Staphylococcus aureus subsp. aureus ATCC 25923 was used as a positive control.
The enzyme activities of all four strains were determined using an API ZYM kit (BIOMÉRIUX, Marcy-l’Étoile, France) with 19 different substrates. Briefly, the four Lactobacillus strains were cultured overnight at 37 °C in MRS medium. The cultures of each strain were harvested by centrifugation at 6000 rpm for 8 min at 4 °C and each cell pellet was resuspended in a sterile saline solution to adjust the final cell concentration to 1 × 109 CFU/mL. The resuspended cells were inoculated into a well-type plate provided by the manufacturer, and the plate was incubated at 37 °C for 4 h. ZYM A and ZYM B solutions were then added to wells, and the color changes were observed after 5 min at room temperature.
2.6. Carbohydrate Utilization
The carbohydrate utilization ability was determined using an API 50 CHL/CHB Kit (BIOMÉRIUX, Marcy-l’Étoile, France) and 49 different carbohydrates. The cultures of four Lactobacillus strains were harvested by centrifugation at 6000 rpm for 8 min at 4 °C and each cell pellet (6 × 108 CFU/mL) was resuspended in API 50 CHL medium. The resuspended cells were inoculated into a well-type plate provided by manufacturer, and the plate was incubated at 37 °C for 48 h. The color changes were then observed.
2.7. Biogenic Amine (BA) Production
The supernatant from the overnight cultures of each Lactobacillus species was obtained by centrifugation at 6000 rpm and filtrated through a 0.22 µm pore size membrane. An aliquot of each supernatant (0.5 mL) was mixed with the same aliquot of 0.1 M HCl and filtered through a 0.45 µm membrane for extraction of BAs. For the derivatization, 1 mL of the extracted BAs was incubated at 70 °C for 10 min, which was followed by addition of 200 µL of saturated NaHCO3, 20 µL of 2 M NaOH, and 0.5 mL of dansyl chloride (10 mg/mL acetone). The derivatized BAs were mixed with 200 µL of L-proline (100 mg/mL H2O) and incubated in darkness at room temperature for 15 min. Acetonitrile (HPLC grade; Sigma-Aldrich, St. Louis, MO) was added to obtain a final volume of 5 mL. High-performance chromatography was performed to separate and quantify the BAs using an HPLC instrument (Agilent 1260, Agilent Technologies, CA, USA) equipped with a C18 column (YMC-Triart, 4.6 × 250 mm, YMC, Kyoto, Japan) and a UV detector (G7115A, Agilent Technologies, CA). Acetonitrile solution (acetonitrile: H2O = 67:33, v/v) was used as the mobile phase at a constant flow rate of 0.8 mL/min. Calibration of each BA, including tyramine, histamine, putrescine, 2-phenethylamine, cadaverine, and tryptamine, was used to quantify BAs (Sigma-Aldrich, St. Louis, MO, USA).
2.8. D-/L-Lactate Production
L- and D-lactate levels were measured using an assay kit (Megazyme, Bray, Ireland) according to the manufacturer’s protocol. The supernatant (0.1 mL) from the culture was mixed with 1.5 mL of H2O, 0.5 mL of buffer solution (pH 10.0), 0.1 mL of NAD+ solution, and 0.02 mL of glutamate-pyruvate transaminase (GPT) and incubated at room temperature for 3 min. Subsequently, 0.02 mL of lactate dehydrogenase (LDH; 2000 U/mL) was added to the reaction mixture. The absorbance of D-lactate was measured at 340 nm until the reaction stopped. The concentrations of L- and D-lactate were then calculated using equations from the manufacturer’s protocol.
2.9. Acute Oral Toxicity
Acute oral toxicity tests were performed at the Korea Testing and Research Institute (Hwasun-gun, Jeollanam-do, Korea). The rats used in this study were bred under the following environmental conditions: 21.1–22.3 °C, 40.5–58.0% relative humidity, 12 h-light/dark cycle, 150–300 Lux of illumination, 270 × 500 × 200 mm (W× D × H) cage size, and less than three rats per cage. The rats were allowed free access to food (Rodent Diet 20 5053; Labdiet, St. Louis, MO, USA) and water. Four groups of female rats were divided by age (9–10 weeks) and administered doses (mg/kg B.W.) of Lactobacilli powder solution in sterile distilled water. Clinical signs, changes in body weight, and necropsy findings were investigated during the test period of 14 days.
2.10. Antipathogenic Effect
The four Lactobacillus strains were grown overnight at 37 °C in MRS medium. The cell-free supernatant was obtained using centrifugation at 6000 rpm for 8 min at 4 °C and filtered through a 0.22 μm pore size membrane. Five pathogenic microorganisms, including Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, Streptococcus pneumonia ATCC 49619, Bacillus cereus ATCC 14579, and Salmonella Typhimurium ATCC 13311, were adjusted to an initial cell density of 1.5 x 108 CFU/mL using the McFarland standard (BioMerieux, Marcy-I’Etoile, France). The cell-free supernatant of each strain was then mixed with each pathogen. The mixture was incubated in a 96-well microplate under the optimal culture conditions of each pathogen. The optical density was measured using a microplate reader at time 0 and 24 h. The antimicrobial effect of the four Lactobacillus strains was determined by complete inhibition of the cell growth of pathogens.
3. Results
3.1. Whole Genome Sequence Analysis
Whole genome sequencing was performed for all four of the selected Lactobacillus strains, L. fermentum IDCC 3901, L. gasseri IDCC 3101, L. helveticus IDCC 3801, and L. salivarius IDCC 3551. Based on these results, the four Lactobacillus strains were confirmed as Limosilactobacillus fermentum (previously classified as Lactobacillus fermentum), L. gasseri, L. helveticus, and L. salivarius, respectively. Information regarding the size of the genome, GC content, and CDSs is listed in Table 3. The genome sequences of the four strains were analyzed to identify antibiotic resistance and virulence genes. None of the four strains had genes related to general antibiotic resistance, such as aminoglycosides, beta-lactams, MLS-macrolide, lincosamide, phenicol, and tetracycline (Table 4). According to the BLASTn algorithm and VFDB, no virulence genes were found in the genomes of the four Lactobacillus strains. Thus, genome sequencing results indicated the safety of L. fermentum IDCC 3901, L. gasseri IDCC 3101, L. helveticus IDCC 3801, and L. salivarius IDCC 3551 for human consumption.

Table 3.
Genomic information of four Lactobacillus species.

Table 4.
Minimal inhibitory concentrations and antibiotic resistance genes of four Lactobacillus species.
3.2. Determination of MICs
MIC tests were performed for the four Lactobacillus species with ampicillin, vancomycin, gentamicin, kanamycin, streptomycin, erythromycin, clindamycin, tetracycline, and chloramphenicol. L. fermentum IDCC 3901, L. gasseri IDCC 3101, and L. salivarius IDCC 3551 were resistant to gentamicin, kanamycin, and streptomycin. However, L. helveticus IDCC 3801 was resistant only to gentamicin and kanamycin. Apart from these results, the four species were also susceptible to other antibiotics; according to genome sequence analysis, they exhibit intrinsic antibiotic resistance to certain antibiotics. Intrinsic antibiotic resistance against aminoglycosides, such as gentamicin, kanamycin, and streptomycin, has been found in many Lactobacillus species [16]. Despite the presence of antibiotic resistance as revealed through the MIC analysis, no gene related to resistance against these antibiotics was detected in the genome sequences of the four Lactobacillus strains.
3.3. β-Hemolytic and Enzyme Activity
Ideally, hemolytic activity must be absent in probiotic strains. Hemolytic activity is the ability to lyse red blood cells, resulting in the destruction of hemoglobin. Based on the β-hemolytic activity test, the four Lactobacillus species did not exhibit hemolytic activity. L. fermentum IDCC 3901, L. gasseri IDCC 3101, L. helveticus IDCC 3801, and L. salivarius IDCC 3551 did not show clear zones on sheep blood agar, whereas the positive control S. aureus subsp. aureus ATCC 25923 showed a clear zone (Figure 1).
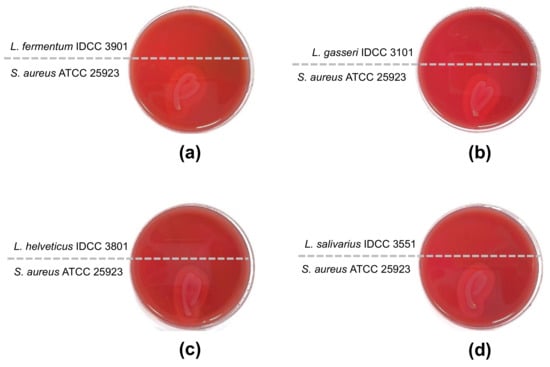
Figure 1.
Test for β-hemolytic activity. S. aureus subsp. aureus ATCC 25923 was used for a positive control and showed β-hemolytic activity. (a) L. fermentum IDCC 3901; (b) L. gasseri IDCC 3101; (c) L. helveticus IDCC 3801; (d) L. salivarius IDCC 3551.
The enzymatic activity assay included 19 enzymes involved in carbohydrate, lipid, and vitamin metabolism. The results of enzymatic activity are presented in Table 5. L. salivarius IDCC 3551 did not display esterase activity, whereas the other strains did. L. fermentum IDCC 3901 and L. salivarius IDCC 3551 were positive for valine arylamidase activity. L. helveticus IDCC 3801 and L. salivarius IDCC 3551 exhibited activity for cystine arylamidase. Only L. fermentum IDCC 3901 had naphthol-AS-BI-phosphohydrolase activity, whereas the other strains did not. L. helveticus IDCC 3801 did not exhibit ɑ-galactosidase activity, whereas the other strains did. Only L. fermentum IDCC 3901 showed ɑ-glucosidase activity, whereas the other strains did not. L. gasseri IDCC 3101 and L. helveticus IDCC 3801 exhibited activity for β-glucosidase. Only L. gasseri IDCC 3101 exhibited N-acetyl-β-glucosaminidase activity. β-Glucuronidase may be related to colon cancer because it produces carcinogenic compounds [17]. However, the four lactobacilli did not present β-glucuronidase activity, so they are free of the safety concerns of β-glucuronidase.

Table 5.
Enzymatic activities of four Lactobacillus species.
3.4. Carbohydrate Utilization
Lactic acid bacteria have different carbohydrate metabolisms. Thus, investigation of carbohydrate utilization in different probiotic strains can provide important phenotypic characterization. The API 50 CH test results for the four Lactobacillus strains are listed in Table 6. L. fermentum IDCC 3901 metabolized L-arabinose, ribose, gluconate, and 2-keto-gluconate, whereas the other species did not. L. gasseri IDCC 3101 metabolized d-turanose and d-tagatose. L. salivarius IDCC 3551 metabolizes mannitol, sorbitol, and l-arabitol. All four Lactobacillus strains could metabolize galactose, d-glucose, d-fructose, esculine, maltose, lactose, and sucrose.

Table 6.
API 50 CH test result of the four Lactobacillus strains tested in this study.
3.5. Production of Biogenic Amine and Lactate
The four selected Lactobacillus strains were also evaluated for biogenic amine production because biogenic amines are potential health risks [18]. According to HPLC analysis, none of the four Lactobacillus species produced biogenic amines, including tyramine, histamine, putrescine, 2-phenethylamine, cadaverine, and tryptamine (Table S1).
Some lactic acid bacteria produce lactate with two isomers, L-lactate and D-lactate, depending on environmental conditions [19]. D-lactate cannot be metabolized in the human body; therefore, D-lactate can accumulate, resulting in acidosis [20]. However, recent studies have demonstrated that the accumulation of D-lactate occurs only in cases of impaired D-lactate metabolism [13]. The four Lactobacillus strains selected in this study produced both L- and D-lactate isomers at varying ratios (Table S2). However, the predominant production of L-lactate compared with that of D-lactate was noticed in all four Lactobacillus strains, indicating that they were less problematic for consumption.
3.6. Acute Oral Toxicity
Acute oral toxicity was investigated using a single-dose acute oral toxicity test. No deaths occurred, and no clinical signs were observed in the rats during the study. There were no significant body weight changes in rats administered Lactobacillus species (Table 7). Furthermore, no abnormal necropsy findings were observed. These results indicate that the four Lactobacillus species did not negatively affect the health of the rats.

Table 7.
Body weight changes of rats administered the four Lactobacillus species.
3.7. Antipathogenic Effects of the Four Lactobacillus Strains
Antimicrobial activity is an important criterion for the selection of microorganisms to be used as probiotics [21]. Here, the four Lactobacillus strains completely inhibited bacterial growth of intestinal and pneumonia pathogens, including Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, Streptococcus pneumonia ATCC 49619, Bacillus cereus ATCC 14579, and Salmonella Typhimurium ATCC 13311 (Figure 2). Interestingly, the pH of the cell-free supernatant of L. fermentum IDCC 3901, L. gasseri IDCC 3101, L. helveticus IDCC 3801, and L. salivarius IDCC 3551 were 4.85 ± 0.95, 3.82 ± 0.04, 3.67 ± 0.03, and 3.90 ± 0.10, respectively. Considering that pKa value of acetic acid is 4.75, acetic acids produced by these strains would be antipathogenic substances [22]. Lactic acids produced by L. fermentum IDCC 3901, L. gasseri IDCC 3101, L. helveticus IDCC 3801, and L. salivarius IDCC 3551 were measured at 831.36 mg/L ± 26.42, 1520.70 mg/L ± 6.53, 79.67 mg/L ± 20.16, and 1585.03 mg/L ± 800.69, respectively. In addition, the Lactobacillus genus is known to produce bacteriocin, small antibacterial peptide [23]. Pathogens such as S. aureus, E. faecalis, B. cereus, and S. Typhimurium are known to cause gastrointestinal disease by destruction of the epithelial structures. Among them, Salmonella species are known as main food poisoning bacteria, causing mucosal ulceration [24]. Furthermore, S. pneumonia is a bacterium that affects the respiratory system, causing pneumonia, bronchitis, and otitis media [25]. Thus, these four strains would be suggested as health functional food in terms of preventing or relieving chronic inflammation in the gastrointestinal tract.
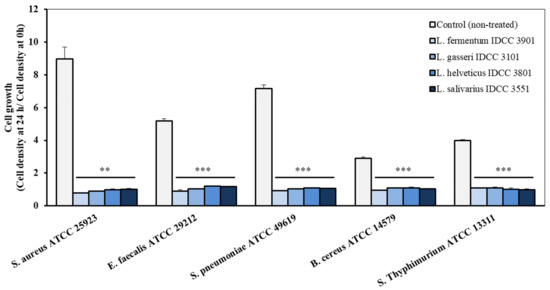
Figure 2.
Antimicrobial effect of the cell-free supernatant of the four Lactobacillus strains on pathogenic microorganisms. (**, p < 0.01; ***, p < 0.001 vs. control group).
4. Discussion
According to the result of the genomic analysis in our study, genes related to antibiotic resistance or virulence were not found in the four Lactobacillus species. However, the strains were revealed to be resistant to some antibiotics by the MIC tests. The antibiotic resistance of some Lactobacillus species was studied from other several studies [26,27], and that of our four Lactobacillus species was comparable to these results. Some gram-positive bacteria have hemolytic activity for iron uptake and host defense [28]. β-Hemolytic activity, a complete lysis of hemoglobin, was not detected from any of the four Lactobacillus species. Bacterial β-glucuronidase might be related to colorectal cancer [29]; therefore, the β-glucuronidase activity should be tested for probiotic strains for human consumption. Our results showed that none of the four Lactobacillus strains had β-glucuronidase activity. Biogenic amines are tentatively harmful elements and have a recommended level in foods according to the Food and Drug Administration (FDA) [30]. Potential toxic compounds, iogenic amines are produced by some bacteria, which is why the biogenic amine production test was conducted for probiotic strains. In our study, none of the four Lactobacillus species produced biogenic amines. D-lactate has been considered to be a factor of acidosis in the human body because of its accumulation. However, D-lactate acidosis may not be a concern to healthy humans [13]. Acute oral toxicity is necessary to prove the safety of probiotic strains for human consumption. Due to the similarity of the physiology of rats and humans, oral toxicity tests using rats can provide evidence for the safety of human consumption [31]. In our oral toxicity test, none of the Lactobacillus strains occurred adverse events in the rats. The antipathogenic effect of the four Lactobacillus strains was investigated with S. aureus, E. faecalis, S. pneumonia, B. cereus, and S. Typhimurium which can cause gastrointestinal disease. In our study, our four Lactobacillus species inhibited the growth of the pathogenic bacteria. These results demonstrate that L. fermentum IDCC 3901, L. gasseri IDCC 3101, L. helveticus IDCC 3801, and L. salivarius IDCC 3551 can be considered safe as probiotic strains. For preclinical studies of these probiotic strains with a medical purpose, more experiments may be required to identify these strains’ acute and subacute toxicity, immunotoxicity, embrotoxicity, etc.
5. Conclusions
This study provides a safety assessment of four different Lactobacillus species, L. fermentum, L. gasseri, L. helveticus, and L. salivarius, through phenotypic and genomic analyses, including whole genome sequencing, MIC test, β-hemolytic and enzyme activity test, carbohydrate utilization test, analysis of biogenic amine and L-/D-lactate production, and acute oral toxicity test in rats. As a result, antibiotic resistances of the four Lactobacillus species were detected; however, neither antibiotic resistance-related genes nor virulence genes were found by genomic analysis. The selected four Lactobacillus species did not exhibit hemolytic activity or β-glucuronidase activity. Neither biogenic amine production nor oral acute toxicity was found in any strains. Moreover, the four Lactobacillus strains exhibited an antipathogenic effect against five pathogenic microorganisms. Through this systematic safety evaluation, we concluded that the four Lactobacillus strains selected and studied in this study may be utilized as a probiotic food supplement. Further in-depth functional study is required to elucidate the health benefits of these strains.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10112218/s1, Figure S1: Functional genes of four Lactobacillus species; Table S1: Biogenic production of four Lactobacillus species; Table S2: L- and D-lactate production of four Lactobacillus species; Table S3. Biogenic production of four Lactobacillus species; Table S4. L- and D-lactate production by four Lactobacillus species.
Author Contributions
Conceptualization, J.Y. and S.-O.S.; investigation, Y.-R.L., W.Y.B., K.-R.B., G.-H.K., and M.-J.K.; data curation, Y.-R.L., W.Y.B., J.Y., and S.-O.S.; writing—original draft preparation, Y.-R.L. and S.-O.S.; writing—review and editing, Y.-R.L., W.Y.B., J.Y., and S.-O.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ildong Bioscience, Co., Ltd in 2022.
Data Availability Statement
Not applicable.
Conflicts of Interest
Authors Won Yeong Bang and Jungwoo Yang are employed by the company IIdong Bioscience Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Araya, M.; Morelli, L.; Reid, G.; Sanders, M.; Stanton, C.; Pineiro, M.; Ben Embarek, P. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; World Health Organization: Geneva: Switzerland; Food and Agriculture Organization of the United Nations: Québec City, QC, Canada, 2002. [Google Scholar]
- Giraffa, G.; Chanishvili, N.; Widyastuti, Y. Importance of lactobacilli in food and feed biotechnology. Res. Microbiol. 2010, 161, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Olson, N.F. The impact of lactic acid bacteria on cheese flavor. FEMS Microbiol. Rev. 1990, 7, 131–147. [Google Scholar] [CrossRef]
- Mohammadi, R.; Sohrabvandi, S.; Mohammad Mortazavian, A. The starter culture characteristics of probiotic microorganisms in fermented milks. Eng. Life Sci. 2012, 12, 399–409. [Google Scholar] [CrossRef]
- Rhee, S.J.; Lee, J.E.; Lee, C.H. Importance of lactic acid bacteria in Asian fermented foods. Microb. Cell Factories 2011, 10 (Suppl. 1), S5. [Google Scholar] [CrossRef] [PubMed]
- Rodas, A.M.; Ferrer, S.; Pardo, I. Polyphasic study of wine Lactobacillus strains: Taxonomic implications. Int. J. Syst. Evol. Microbiol. 2005, 55, 197–207. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, J.; Pan, L.; Zhang, Y. Roles and applications of probiotic Lactobacillus strains. Appl. Microbiol. Biotechnol. 2018, 102, 8135–8143. [Google Scholar] [CrossRef]
- Van Baarlen, P.; Wells, J.M.; Kleerebezem, M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends. Immunol. 2013, 34, 208–215. [Google Scholar] [CrossRef]
- Williams, N.T. Probiotics. Am. J. Health-Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef]
- Bang, W.Y.; Kim, H.; Chae, S.A.; Yang, S.Y.; Ban, O.H.; Kim, T.Y.; Kwon, H.S.; Jung, Y.H.; Yang, J. A Quadruple Coating of Probiotics for Enhancing Intestinal Adhesion and Competitive Exclusion of Salmonella typhimurium. J. Med. Food 2022, 25, 213–218. [Google Scholar] [CrossRef]
- Min Cheol, K.; Jin Seok, M.; Young-Hoo, K.; Min-Goo, K.; Tae-Yoon, K.; Ha-Young, P. Analysis of Quadruple Coating for Improvement in Improving Gastrointestinal Stability and Survivability of Probiotics. KSBB J. 2019, 34, 107–113. [Google Scholar] [CrossRef]
- Yeon, S.-W.; You, Y.S.; Kwon, H.-S.; Yang, E.H.; Ryu, J.-S.; Kang, B.H.; Kang, J.-H. Fermented milk of Lactobacillus helveticus IDCC3801 reduces beta-amyloid and attenuates memory deficit. J. Funct. Foods 2010, 2, 143–152. [Google Scholar] [CrossRef]
- Connolly, E.; Abrahamsson, T.; Bjorksten, B. Safety of D(−)-lactic acid producing bacteria in the human infant. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2005, 33, D325–D328. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [PubMed]
- Campedelli, I.; Mathur, H.; Salvetti, E.; Clarke, S.; Rea, M.C.; Torriani, S.; Ross, R.P.; Hill, C.; O’Toole, P.W. Genus-Wide Assessment of Antibiotic Resistance in Lactobacillus spp. Appl. Env. Microbiol. 2019, 85, e01738-18. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jin, Y.H. Intestinal bacterial beta-glucuronidase activity of patients with colon cancer. Arch. Pharmacal Res. 2001, 24, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef]
- Kandler, O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 1983, 49, 209–224. [Google Scholar] [CrossRef]
- Petersen, C. D-lactic acidosis. Nutr. Clin. Pr. 2005, 20, 634–645. [Google Scholar] [CrossRef]
- Georgieva, R.; Yocheva, L.; Tserovska, L.; Zhelezova, G.; Stefanova, N.; Atanasova, A.; Danguleva, A.; Ivanova, G.; Karapetkov, N.; Rumyan, N.; et al. Antimicrobial activity and antibiotic susceptibility of Lactobacillus and Bifidobacterium spp. intended for use as starter and probiotic cultures. Biotechnol. Biotechnol. Equip. 2015, 29, 84–91. [Google Scholar] [CrossRef]
- Cortesia, C.; Vilchèze, C.; Bernut, A.; Contreras, W.; Gómez, K.; Waard, J.d.; Jacobs, W.R.; Kremer, L.; Takiff, H. Acetic Acid, the Active Component of Vinegar, Is an Effective Tuberculocidal Disinfectant. mBio 2014, 5, e00013–e00014. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Szkaradkiewicz, A.K. Characteristic of bacteriocines and their application. Pol. J. Microbiol. 2013, 62, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Gorbach, S.L. Microbiology of the Gastrointestinal Tract. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Dasaraju, P.V.; Liu, C. Infections of the respiratory system. In Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; pp. 312–344. [Google Scholar]
- Klare, I.; Konstabel, C.; Werner, G.; Huys, G.; Vankerckhoven, V.; Kahlmeter, G.; Hildebrandt, B.; Muller-Bertling, S.; Witte, W.; Goossens, H. Antimicrobial susceptibilities of Lactobacillus, Pediococcus and Lactococcus human isolates and cultures intended for probiotic or nutritional use. J. Antimicrob. Chemother. 2007, 59, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Salminen, M.K.; Rautelin, H.; Tynkkynen, S.; Poussa, T.; Saxelin, M.; Valtonen, V.; Jarvinen, A. Lactobacillus bacteremia, species identification, and antimicrobial susceptibility of 85 blood isolates. Clin. Infect. Dis. 2006, 42, e35–e44. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chae, S.; Lee, M.; Yang, S.-Y.; Ban, O.H.; Jung, Y.H.; Yang, J. Genomic and Toxicity Studies on Bifidobacterium longum IDCC 4101 and Bifidobacterium bifidum IDCC 4201 Isolated from Feces of Breast-Fed Infants. Food Suppl. Biomater. Health 2021, 1, e37. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, W.; Lukasiewicz, M.; Puppel, K. Biogenic amines: Formation, action and toxicity—A review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Ban, O.H.; Jung, Y.H.; Yang, J.; Kim, Y. Genomic characterization and probiotic potential of Lactobacillus casei IDCC 3451 isolated from infant faeces. Lett. Appl. Microbiol. 2021, 72, 578–588. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).