Thrombospondin-Related Anonymous Protein (TRAP) Family Expression by Babesia bovis Life Stages within the Mammalian Host and Tick Vector
Abstract
1. Introduction
2. Materials and Methods
2.1. Cattle, Pathogen, and Tick Vector
2.2. Babesia bovis Blood Stages
2.3. Babesia bovis Induced Sexual Stages
2.4. Babesia bovis Kinete Stage Isolation
2.5. RNA Isolation
2.6. Quantitative PCR
2.7. Fixed Immunofluorescence Assay
2.8. Live Immunofluorescence Assay
2.9. Bioinformatic Analysis of TRAP Family Members
2.10. Statistical Analysis
3. Results
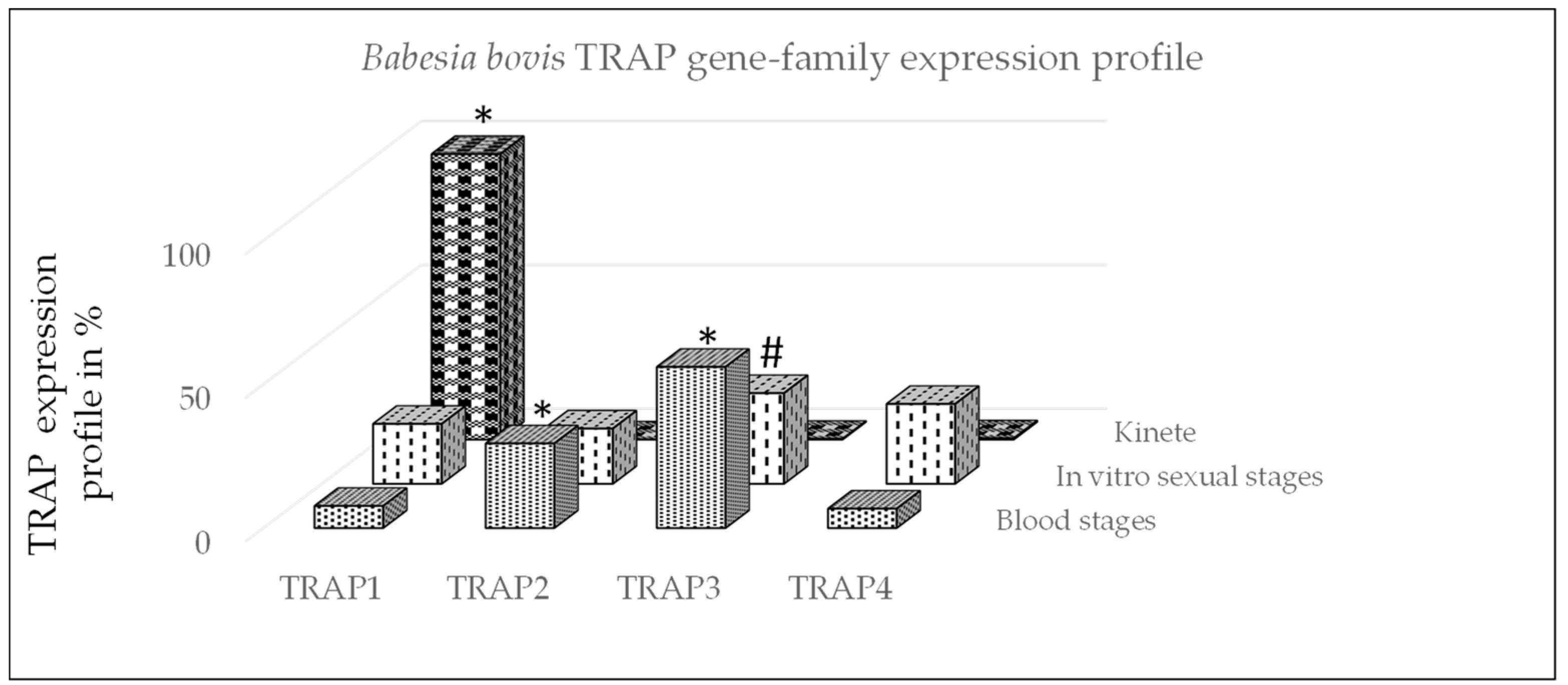
3.1. Quantitative PCR
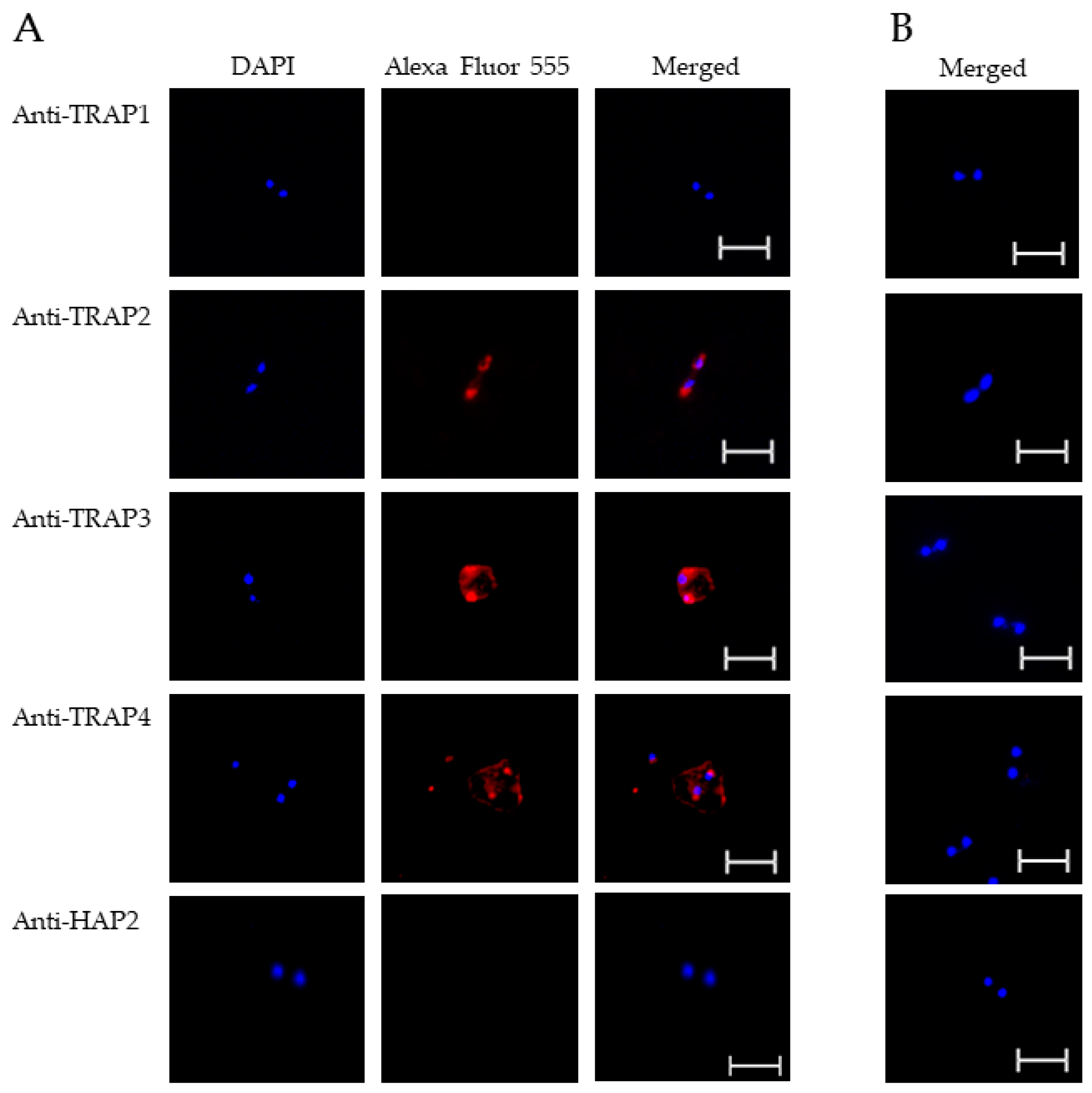
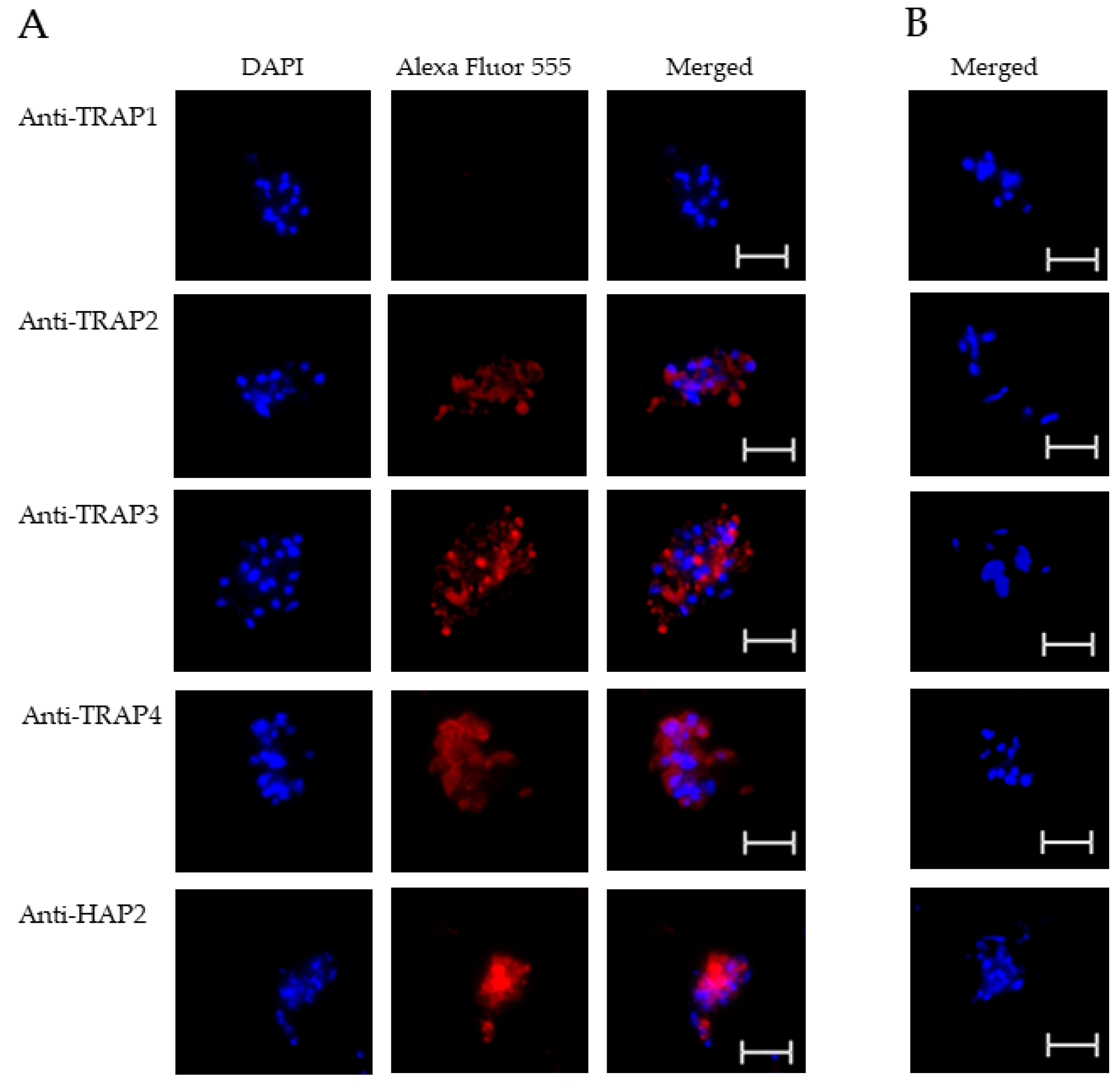
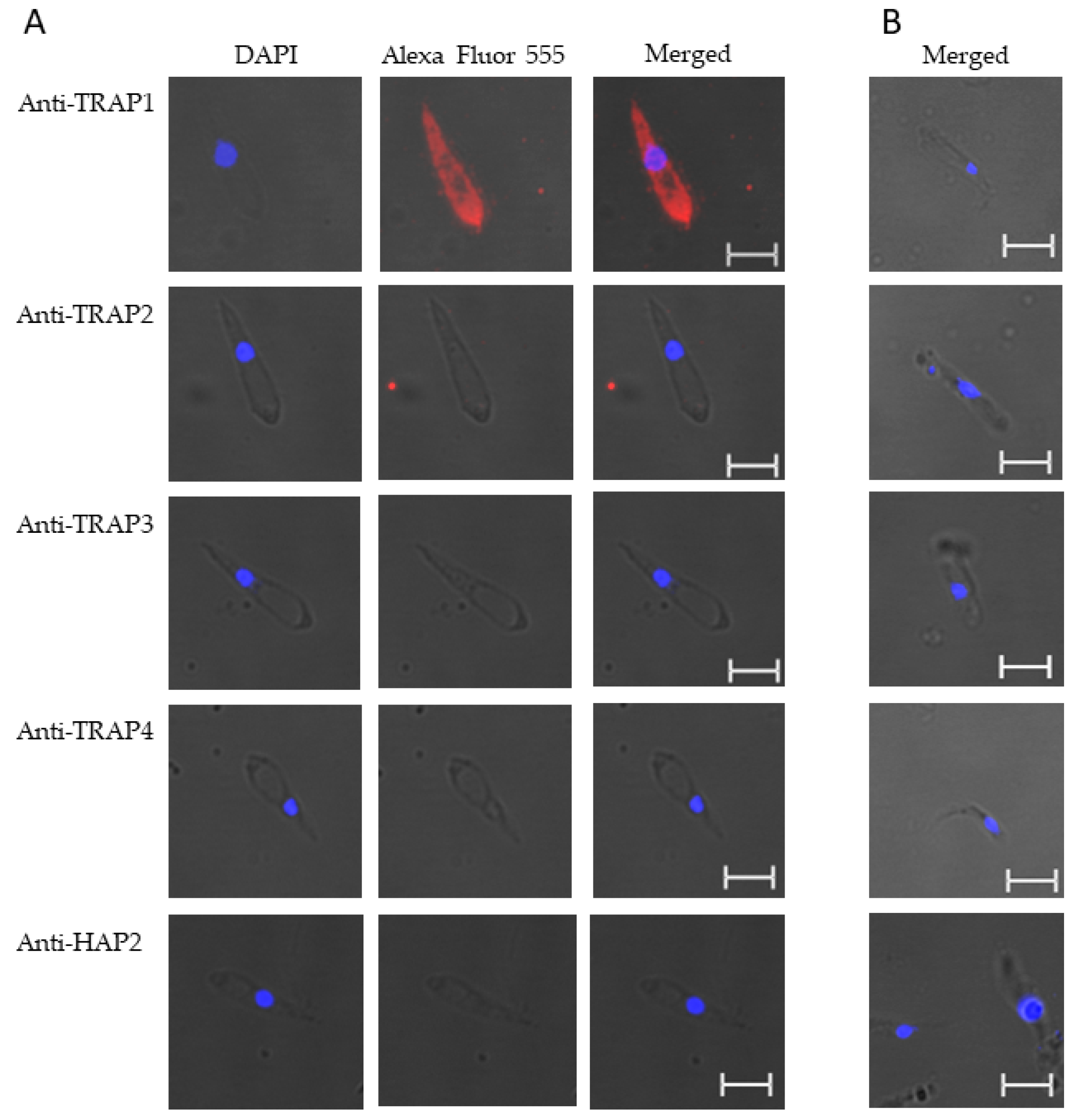
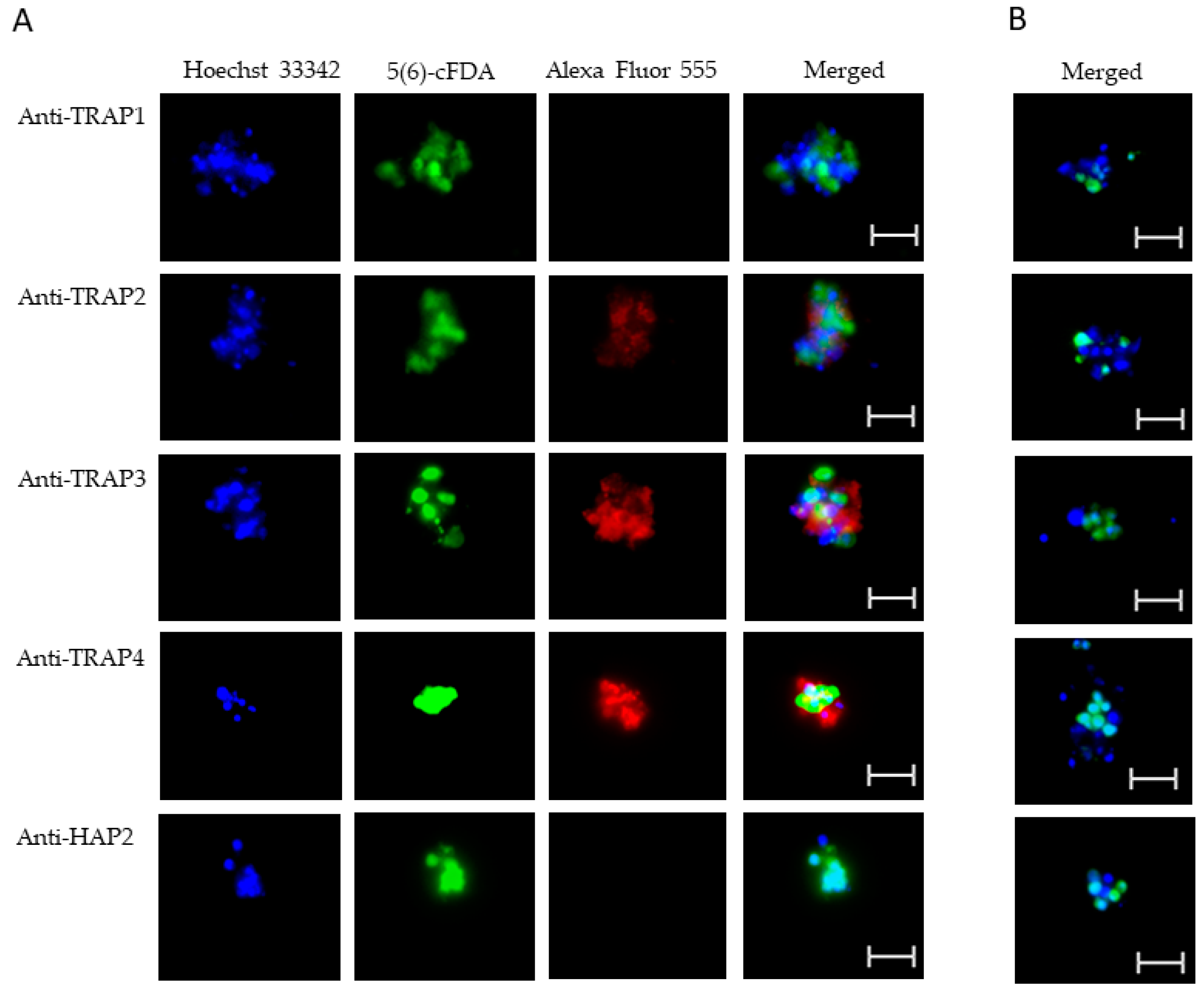
3.2. Expression of TRAP Proteins by B. bovis Blood Stages, Sexual Stages, and Kinetes
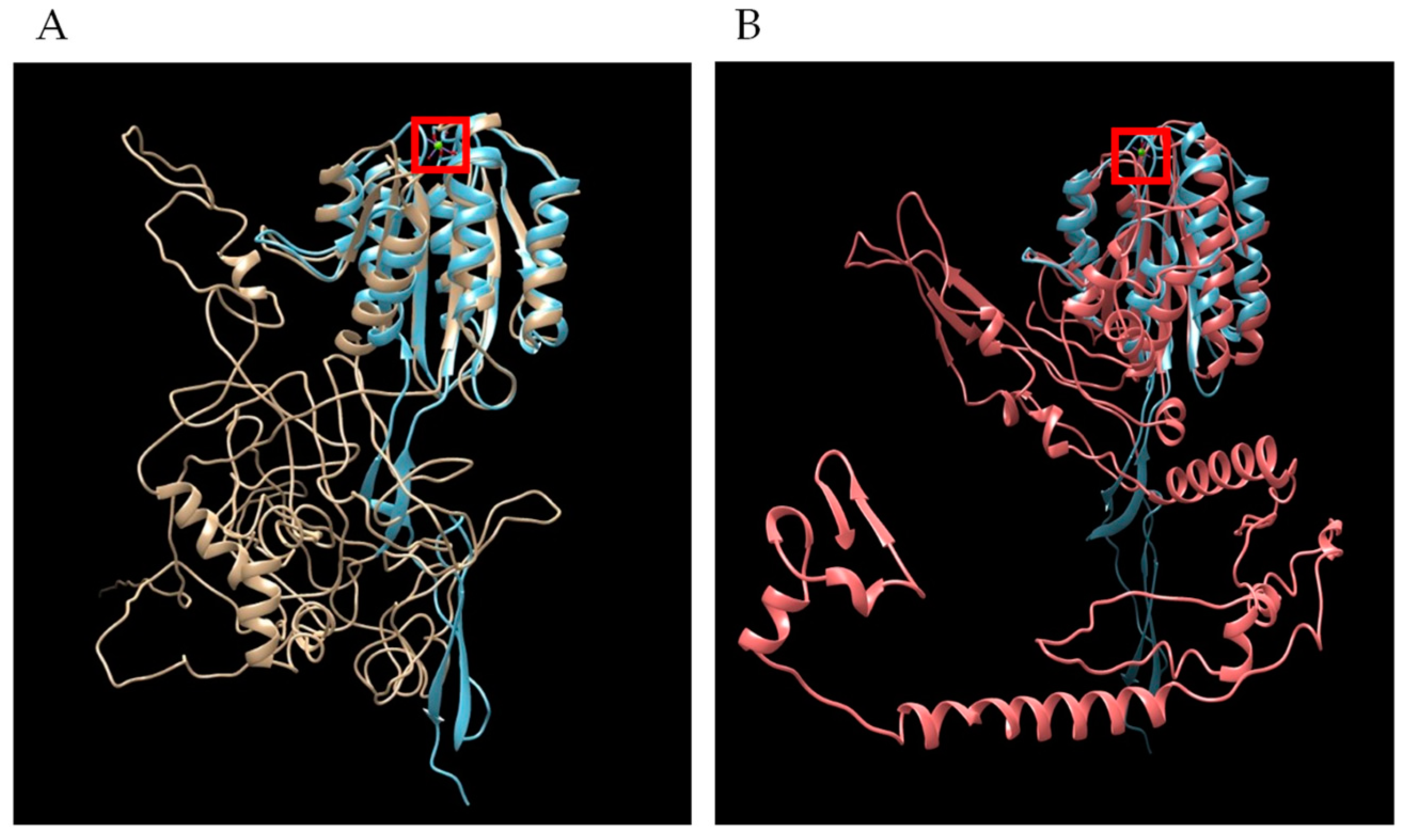
3.3. Bioinformatic Analysis of TRAP Family Members
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beugnet, F.; Moreau, Y. Babesiosis. Rev. Sci. Tech. 2015, 34, 627–639. [Google Scholar] [CrossRef]
- Bock, R.; Jackson, L.; de Vos, A.; Jorgensen, W. Babesiosis of cattle. Parasitology 2004, 129 (Suppl. S1), S247–S269. [Google Scholar] [CrossRef] [PubMed]
- Suarez, C.E.; Noh, S. Emerging perspectives in the research of bovine babesiosis and anaplasmosis. Vet. Parasitol. 2011, 180, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Florin-Christensen, M.; Schnittger, L. Piroplasmids and ticks: A long-lasting intimate relationship. Front. Biosci. (Landmark Ed.) 2009, 14, 3064–3073. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129 (Suppl. S1), S3–S14. [Google Scholar] [CrossRef]
- Gilbert, L. The Impacts of Climate Change on Ticks and Tick-Borne Disease Risk. Annu. Rev. Entomol. 2021, 66, 373–388. [Google Scholar] [CrossRef]
- George, J.E. Present and future technologies for tick control. Ann. N. Y. Acad. Sci. 2000, 916, 583–588. [Google Scholar] [CrossRef]
- Mosqueda, J.; Olvera-Ramirez, A.; Aguilar-Tipacamu, G.; Canto, G.J. Current advances in detection and treatment of babesiosis. Curr. Med. Chem. 2012, 19, 1504–1518. [Google Scholar] [CrossRef]
- Rojas-Martínez, C.; Rodríguez-Vivas, R.I.; Millán, J.V.F.; Bautista-Garfias, C.R.; Castañeda-Arriola, R.O.; Lira-Amaya, J.J.; Urióstegui, P.V.; Carrasco, J.J.O.; Martínez, J. Bovine babesiosis: Cattle protected in the field with a frozen vaccine containing Babesia bovis and Babesia bigemina cultured in vitro with a serum-free medium. Parasitol. Int. 2018, 67, 190–195. [Google Scholar] [CrossRef]
- Guerrero, F.D.; Lovis, L.; Martins, J.R. Acaricide resistance mechanisms in Rhipicephalus (Boophilus) microplus. Rev. Bras. Parasitol. Vet. 2012, 21, 1–6. [Google Scholar] [CrossRef]
- Florin-Christensen, M.; Suarez, C.E.; Rodriguez, A.E.; Flores, D.A.; Schnittger, L. Vaccines against bovine babesiosis: Where we are now and possible roads ahead. Parasitology 2014, 141, 1563–1592. [Google Scholar] [CrossRef] [PubMed]
- de Waal, D.T.; Combrink, M.P. Live vaccines against bovine babesiosis. Vet. Parasitol. 2006, 138, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo Ortiz, J.M.; Paoletta, M.S.; Gravisaco, M.J.; López Arias, L.S.; Montenegro, V.N.; de la Fournière, S.A.M.; Valenzano, M.N.; Guillemi, E.C.; Valentini, B.; Echaide, I.; et al. Immunisation of cattle against Babesia bovis combining a multi-epitope modified vaccinia Ankara virus and a recombinant protein induce strong Th1 cell responses but fails to trigger neutralising antibodies required for protection. Ticks. Tick Borne Dis. 2019, 10, 101270. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.E.; Bastos, R.G.; Schneider, D.A.; Johnson, W.C.; Adham, F.K.; Davis, W.C.; Laughery, J.M.; Herndon, D.R.; Alzan, H.F.; Ueti, M.W.; et al. The Babesia bovis hap2 gene is not required for blood stage replication, but expressed upon in vitro sexual stage induction. PLoS Negl. Trop. Dis. 2017, 11, e0005965. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tewari, R.; Ning, J.; Blagborough, A.M.; Garbom, S.; Pei, J.; Grishin, N.V.; Steele, R.E.; Sinden, R.E.; Snell, W.J.; et al. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev. 2008, 22, 1051–1068. [Google Scholar] [CrossRef]
- Johnson, W.C.; Taus, N.S.; Reif, K.E.; Bohaliga, G.A.; Kappmeyer, L.S.; Ueti, M.W. Analysis of Stage-Specific Protein Expression during Babesia Bovis Development within Female Rhipicephalus Microplus. J. Proteome Res. 2017, 16, 1327–1338. [Google Scholar] [CrossRef]
- Howell, J.M.; Ueti, M.W.; Palmer, G.H.; Scoles, G.A.; Knowles, D.P. Transovarial transmission efficiency of Babesia bovis tick stages acquired by Rhipicephalus (Boophilus) microplus during acute infection. J. Clin. Microbiol. 2007, 45, 426–431. [Google Scholar] [CrossRef]
- Ueti, M.W.; Johnson, W.C.; Kappmeyer, L.S.; Herndon, D.R.; Mousel, M.R.; Reif, K.E.; Taus, N.S.; Ifeonu, O.O.; Silva, J.C.; Suarez, C.E.; et al. Comparative analysis of gene expression between Babesia bovis blood stages and kinetes allowed by improved genome annotation. Int. J. Parasitol. 2021, 51, 123–136. [Google Scholar] [CrossRef]
- Mosqueda, J.; McElwain, T.F.; Stiller, D.; Palmer, G.H. Babesia bovis merozoite surface antigen 1 and rhoptry-associated protein 1 are expressed in sporozoites, and specific antibodies inhibit sporozoite attachment to erythrocytes. Infect. Immun. 2002, 70, 1599–1603. [Google Scholar] [CrossRef][Green Version]
- Gaffar, F.R.; Yatsuda, A.P.; Franssen, F.F.; de Vries, E. A Babesia bovis merozoite protein with a domain architecture highly similar to the thrombospondin-related anonymous protein (TRAP) present in Plasmodium sporozoites. Mol. Biochem. Parasitol. 2004, 136, 25–34. [Google Scholar] [CrossRef]
- Paoletta, M.S.; Wilkowsky, S.E. Thrombospondin Related Anonymous Protein Superfamily in Vector-Borne Apicomplexans: The Parasite’s Toolkit for Cell Invasion. Front. Cell. Infect. Microbiol. 2022, 12, 831592. [Google Scholar] [CrossRef] [PubMed]
- Morahan, B.J.; Wang, L.; Coppel, R.L. No TRAP, no invasion. Trends Parasitol. 2009, 25, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Koksal, A.C.; Lu, C.; Springer, T.A. Shape change in the receptor for gliding motility in Plasmodium sporozoites. Proc. Natl. Acad. Sci. USA 2012, 109, 21420–21425. [Google Scholar] [CrossRef] [PubMed]
- Terkawi, M.A.; Ratthanophart, J.; Salama, A.; AbouLaila, M.; Asada, M.; Ueno, A.; Alhasan, H.; Guswanto, A.; Masatani, T.; Yokoyama, N.; et al. Molecular characterization of a new Babesia bovis thrombospondin-related anonymous protein (BbTRAP2). PLoS ONE 2013, 8, e83305. [Google Scholar] [CrossRef] [PubMed]
- Mosqueda, J.; Falcon, A.; Antonio Alvarez, J.; Alberto Ramos, J.; Oropeza-Hernandez, L.F.; Figueroa, J.V. Babesia bigemina sexual stages are induced in vitro and are specifically recognized by antibodies in the midgut of infected Boophilus microplus ticks. Int. J. Parasitol. 2004, 34, 1229–1236. [Google Scholar] [CrossRef]
- Levy, M.G.; Ristic, M. Babesia bovis: Continuous cultivation in a microaerophilous stationary phase culture. Science 1980, 207, 1218–1220. [Google Scholar] [CrossRef]
- Bohaliga, G.A.R.; Johnson, W.C.; Taus, N.S.; Hussein, H.E.; Bastos, R.G.; Suarez, C.E.; Scoles, G.A.; Ueti, M.W. Identification of proteins expressed by Babesia bigemina kinetes. Parasit. Vectors 2019, 12, 271. [Google Scholar] [CrossRef]
- Ellefsen, S.; Stensløkken, K.O. Gene-family profiling: A normalization-free real-time RT-PCR approach with increased physiological resolution. Physiol. Genom. 2010, 42, 1–4. [Google Scholar] [CrossRef]
- Goff, W.L.; McElwain, T.F.; Suarez, C.E.; Johnson, W.C.; Brown, W.C.; Norimine, J.; Knowles, D.P. Competitive enzyme-linked immunosorbent assay based on a rhoptry-associated protein 1 epitope specifically identifies Babesia bovis-infected cattle. Clin. Diagn. Lab. Immunol. 2003, 10, 38–43. [Google Scholar] [CrossRef]
- Laughery, J.M.; Knowles, D.P.; Schneider, D.A.; Bastos, R.G.; McElwain, T.F.; Suarez, C.E. Targeted surface expression of an exogenous antigen in stably transfected Babesia bovis. PLoS ONE 2014, 9, e97890. [Google Scholar] [CrossRef][Green Version]
- Kumar, H.; Tolia, N.H. Getting in: The structural biology of malaria invasion. PLoS Pathog. 2019, 15, e1007943. [Google Scholar] [CrossRef] [PubMed]
- Tiono, A.B.; Nébié, I.; Anagnostou, N.; Coulibaly, A.S.; Bowyer, G.; Lam, E.; Bougouma, E.C.; Ouedraogo, A.; Yaro, J.B.B.; Barry, A.; et al. First field efficacy trial of the ChAd63 MVA ME-TRAP vectored malaria vaccine candidate in 5–17 months old infants and children. PLoS ONE 2018, 13, e0208328. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Song, G.; Beale, K.; Yan, J.; Garst, E.; Feng, J.; Lund, E.; Catteruccia, F.; Springer, T.A. Design and assessment of TRAP-CSP fusion antigens as effective malaria vaccines. PLoS ONE 2020, 15, e0216260. [Google Scholar] [CrossRef] [PubMed]
- Alzan, H.F.; Knowles, D.P.; Suarez, C.E. Comparative Bioinformatics Analysis of Transcription Factor Genes Indicates Conservation of Key Regulatory Domains among Babesia bovis, Babesia microti, and Theileria equi. PLoS Negl. Trop. Dis. 2016, 10, e0004983. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; He, J.; Yu, L.; Liu, Q.; Sun, Y.; Nie, Z.; Guo, J.; Zhao, Y.; Li, M.; Luo, X.; et al. Identification of a novel thrombospondin-related anonymous protein (BoTRAP2) from Babesia orientalis. Parasit. Vectors 2019, 12, 200. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Huyen, N.X.; Wibowo, P.E.; Seuseu, F.J.; Aboulaila, M.; Ueno, A.; Goo, Y.K.; Yokoyama, N.; Xuan, X.; Igarashi, I. Spherical body protein 4 is a new serological antigen for global detection of Babesia bovis infection in cattle. Clin. Vaccine Immunol. 2011, 18, 337–342. [Google Scholar] [CrossRef]
- Montenegro, V.N.; Paoletta, M.S.; Jaramillo Ortiz, J.M.; Suarez, C.E.; Wilkowsky, S.E. Identification and characterization of a Babesia bigemina thrombospondin-related superfamily member, TRAP-1: A novel antigen containing neutralizing epitopes involved in merozoite invasion. Parasit. Vectors 2020, 13, 602. [Google Scholar] [CrossRef]
- Chenet, S.M.; Branch, O.H.; Escalante, A.A.; Lucas, C.M.; Bacon, D.J. Genetic diversity of vaccine candidate antigens in Plasmodium falciparum isolates from the Amazon basin of Peru. Malar. J. 2008, 7, 93. [Google Scholar] [CrossRef]
- Ueti, M.W.; Johnson, W.C.; Kappmeyer, L.S.; Herndon, D.R.; Mousel, M.R.; Reif, K.E.; Taus, N.S.; Ifeonu, O.O.; Silva, J.C.; Suarez, C.E.; et al. Transcriptome dataset of Babesia bovis life stages within vertebrate and invertebrate hosts. Data Brief. 2020, 33, 106533. [Google Scholar] [CrossRef]
- Baker, R.P.; Wijetilaka, R.; Urban, S. Two Plasmodium rhomboid proteases preferentially cleave different adhesins implicated in all invasive stages of malaria. PLoS Pathog. 2006, 2, e113. [Google Scholar] [CrossRef]

| Gene ID | Protein Name | Sequence | TM |
|---|---|---|---|
| BBOV_II002650 | TRAP1 | F-5′ACC ACT TAC TCA ACT CCA ACT3′ R-5′GTA CCG GCC ATC CAA TCA A3′ | 55 °C 55 °C |
| BBOV_II002890 | TRAP2 | F-5′GTC ATG AGT ATT CCC AGC CTT C3′ R-5′TCA CTT CCT TCC GAT GCT TTC3′ | 55 °C 55 °C |
| BBOV_II002630 | TRAP3 | F-5′AAC CTA CCC AAA CCG GAA AT3′ R-5′AGT CGT TGT TAC TTG TCT CCT C3′ | 55.5 °C 55.5 °C |
| BBOV_II002870 | TRAP4 | F-5′TTT GGA TAC CGC TGT GCT AC 3′ R-5′CGG CTA GTC AAG GTC GTT AAA3′ | 55.5 °C 55.5 °C |
| Peptides | |||
|---|---|---|---|
| TRAP1 | ADKGVGSPKGKQC (28–40) | ESEDYEGEKQNDESNARSTSNTTK (571–594) | KKNKTPNETESGDYTGADESAE (616–638) |
| TRAP2 | DRELSKVKVESEWYKPK (393–409) | ESERDSPVKNESTISSEIPK (899–910) | RGYIRLNRATREQSDIDENT (981–1000) |
| TRAP3 | EASDKSAGAPSEDKSAESTSATE (752–774) | TNEHVETPAGTVESTESTSEEPTPVA (782–807) | KPEIETPSHEVAPTVDEHQN (944–963) |
| TRAP4 | EHESTSLSRGPRPTEDQISQLPK (42–64) | ESSYRSRRLQSVEKHNEQQTGSQET (360–384) | NSGTHHPPHHRKGANGSGKK (462–481) |
| Babesia bovis | |||
|---|---|---|---|
| Blood Stages | In Vitro Sexual Stages | Kinetes | |
| TRAP1 | 104.16 (4.3) b | 104.32 (3.6) b,c | 105.74 (4.8) b |
| TRAP2 | 104.74 (4.3) c | 104.27 (3.6) b | 101.83 (4.8) a |
| TRAP3 | 105.02 (4.3) a | 104.52 (3.6) a | 103.09 (4.8) a |
| TRAP4 | 104.10 (4.3) b | 104.43 (3.6) a,c | 103.35 (4.8) a |
| Cysteine Residues Involved in MIDAS Domain Formation | Residues Involved in Mg2+ Binding | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. falcip TRAP | C43 | C205 | C212 | C235 | C253 | C257 | C281 | C286 | D54 | S56 | S58 | T131 | D162 |
| P. vivax TRAP | C39 | C201 | C208 | C231 | C249 | C254 | C277 | C282 | D50 | S52 | S54 | T127 | D158 |
| B. bovis TRAP1 | C40 | C200 | C206 | C229 | C248 | C252 | C283 | C297 | D51 | S53 | S55 | T128 | D158 |
| B. bovis TRAP2 | − | + | + | + | + | + | + | + | − | − | − | − | − |
| B. bovis TRAP3 | − | + | + | + | + | + | + | + | − | + | − | − | − |
| B. bovis TRAP4 | − | + | + | + | − | − | + | + | + | + | + | + | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masterson, H.E.; Taus, N.S.; Johnson, W.C.; Kappmeyer, L.; Capelli-Peixoto, J.; Hussein, H.E.; Mousel, M.R.; Hernandez-Silva, D.J.; Laughery, J.M.; Mosqueda, J.; et al. Thrombospondin-Related Anonymous Protein (TRAP) Family Expression by Babesia bovis Life Stages within the Mammalian Host and Tick Vector. Microorganisms 2022, 10, 2173. https://doi.org/10.3390/microorganisms10112173
Masterson HE, Taus NS, Johnson WC, Kappmeyer L, Capelli-Peixoto J, Hussein HE, Mousel MR, Hernandez-Silva DJ, Laughery JM, Mosqueda J, et al. Thrombospondin-Related Anonymous Protein (TRAP) Family Expression by Babesia bovis Life Stages within the Mammalian Host and Tick Vector. Microorganisms. 2022; 10(11):2173. https://doi.org/10.3390/microorganisms10112173
Chicago/Turabian StyleMasterson, Hayley E., Naomi S. Taus, Wendell C. Johnson, Lowell Kappmeyer, Janaina Capelli-Peixoto, Hala E. Hussein, Michelle R. Mousel, Diego J. Hernandez-Silva, Jacob M. Laughery, Juan Mosqueda, and et al. 2022. "Thrombospondin-Related Anonymous Protein (TRAP) Family Expression by Babesia bovis Life Stages within the Mammalian Host and Tick Vector" Microorganisms 10, no. 11: 2173. https://doi.org/10.3390/microorganisms10112173
APA StyleMasterson, H. E., Taus, N. S., Johnson, W. C., Kappmeyer, L., Capelli-Peixoto, J., Hussein, H. E., Mousel, M. R., Hernandez-Silva, D. J., Laughery, J. M., Mosqueda, J., & Ueti, M. W. (2022). Thrombospondin-Related Anonymous Protein (TRAP) Family Expression by Babesia bovis Life Stages within the Mammalian Host and Tick Vector. Microorganisms, 10(11), 2173. https://doi.org/10.3390/microorganisms10112173








