Comparison of Bacterial Assemblages Associated with Harmful Cyanobacteria under Different Light Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyanobacterial Strains and Seed Cultivation
2.2. Experimental Conditions for Light-Limited Cultivations
2.3. Flow Cytometric Analysis for Live-Cell Density Measurement
2.4. DNA Extraction and Quantification
2.5. Preparation of Libraries for Sequencing Analysis
2.6. Data Analysis
3. Results
3.1. Patterns of Cell Growth of Cyanobacteria
3.2. Variation in Physico-Chemical Factors during Cultivations
3.3. Changes in the Bacterial Community Composition during Cultivations
3.4. Analyses for Bacterial Assemblages Associated with Harmful Cyanobacteria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hallegraeff, G.M. Harmful algal blooms: A global overview. Man. Harmful Mar. Microalgae 2003, 33, 1–22. [Google Scholar]
- Havens, K.E.; James, R.T.; East, T.L.; Smith, V.H. N:P ratios, light limitation, and cyanobacterial dominance in a subtropical lake impacted by non-point source nutrient pollution. Environ. Pollut. 2003, 122, 379–390. [Google Scholar] [CrossRef]
- Schagerl, M.; Müller, B. Acclimation of chlorophyll a and carotenoid levels to different irradiances in four freshwater cyanobacteria. J. Plant Physiol. 2006, 163, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.; Idris, A.; Bukhari, A.; Wahidin, S. Intensity of blue LED light: A potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris. Bioresour. Technol. 2013, 148, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.N.; Oyler, G.A.; Wilkinson, L.; Betenbaugh, M.J. A green light for engineered algae: Redirecting metabolism to fuel a biotechnology revolution. Curr. Opin. Biotechnol. 2008, 19, 430–436. [Google Scholar] [CrossRef]
- Patil, V.; Abate, R.; Wu, W.; Zhang, J.; Lin, H.; Chen, C.; Liang, J.; Sun, L.; Li, X.; Li, Y.; et al. Allelopathic inhibitory effect of the macroalga Pyropia haitanensis (Rhodophyta) on harmful bloom-forming pseudo-Nitzschia species. Mar. Pollut. Bull. 2020, 161, 111752. [Google Scholar] [CrossRef]
- Xiao, M.; Hamilton, D.P.; O’Brien, K.R.; Adams, M.P.; Willis, A.; Burford, M.A. Are laboratory growth rate experiments relevant to explaining bloom-forming cyanobacteria distributions at global scale? Harmful Algae 2010, 92, 101732. [Google Scholar] [CrossRef]
- Louati, I.; Pascault, N.; Debroas, D.; Bernard, C.; Humbert, J.F.; Leloup, J. Structural diversity of bacterial communities associated with bloom-forming freshwater cyanobacteria differs according to the cyanobacterial genus. PLoS ONE 2015, 10, e0140614. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, D.; Zeng, J.; Xu, H.; Huang, R.; Jiao, C.; Guo, L. Variations of bacterial community during the decomposition of Microcystis under different temperatures and biomass. BMC Microbiol. 2019, 19, 207. [Google Scholar] [CrossRef]
- Kim, M.; Shin, B.; Lee, J.; Park, H.Y.; Park, W. Culture-independent and culture-dependent analyses of the bacterial community in the phycosphere of cyanobloom-forming Microcystis aeruginosa. Sci. Rep. 2019, 9, 20416. [Google Scholar] [CrossRef]
- Sakata, T.; Yoshikawa, T.; Nishitarumizu, S. Algicidal activity and identification of an algicidal substance produced by marine Pseudomonas sp. C55a-2. Fish. Sci. 2011, 77, 397–402. [Google Scholar] [CrossRef]
- Wang, X.; Gong, L.; Liang, S.; Han, X.; Zhu, C.; Li, Y. Algicidal activity of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa. Harmful Algae 2005, 4, 433–443. [Google Scholar] [CrossRef]
- Shi, S.S.; Tang, D.; Liu, Y. Effects of an algicidal bacterium Pseudomonas mendocina on the growth and antioxidant system of Aphanizomenon flos-aquae. Curr. Microbiol. 2009, 59, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, R.; Kim, B.H.; Cho, D.H.; Oh, H.M.; Kim, H.S. Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Lea-Smith, D.J.; Biller, S.J.; Davey, M.P.; Cotton, C.A.R.; Perez Sepulveda, B.M.; Turchyn, A.V.; Scanlan, D.J.; Smith, A.G.; Chisholm, S.W.; Howe, C.J. Contribution of cyanobacterial alkane production to the ocean hydrocarbon cycle. Proc. Natl. Acad. Sci. USA 2015, 112, 13591–13596. [Google Scholar] [CrossRef]
- Jung, J.; Seo, Y.; Kim, L.K.R.; Park, H.Y.; Jeon, C.O. Mesorhizobium microcysteis sp. nov., isolated from a culture of Microcystis aeruginosa. Int. J. Syst. Evol. Microbiol. 2021, 71, 004847. [Google Scholar] [CrossRef]
- Chia, M.A.; Jankowiak, J.G.; Kramer, B.J.; Goleski, J.A.; Huang, I.S.; Zimba, P.V.; do Carmo Bittencourt-Oliveira, M.; Gobler, C.J. Succession and toxicity of Microcystis and Anabaena (Dolichospermum) blooms are controlled by nutrient-dependent allelopathic interactions. Harmful Algae 2018, 74, 67–77. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater: Centennial Edition (Standard Methods for the Examination of Water and Wastewater), 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Cunningham, B.R.; John, S.G. The effect of iron limitation on cyanobacteria major nutrient and trace element stoichiometry. Limnol. Oceanogr. 2017, 62, 846–858. [Google Scholar] [CrossRef]
- Nam, G.; Mohamed, M.M.; Jung, J. Novel treatment of Microcystis aeruginosa using chitosan-modified nanobubbles. Environ. Pollut. 2021, 2, 118458. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Oksanen, J. Ordination and Analysis of Dissimilarities: Tutorial with R and Vegan; University Tennessee: Knoxville, TN, USA, 2009. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013; Volume 15. [Google Scholar]
- Roberts, D.W. Labdsv: Ordination and Multivariate Analysis for Ecology. R Package Version 1.4-1. 2010. Available online: http://CRAN.R-project.org/package¼labdsv (accessed on 11 December 2017).
- Kim, E.J.; Kim, J.S.; Lee, I.H.; Rhee, H.J.; Lee, J.K. Superoxide generation by chlorophyllide a reductase of Rhodobacter sphaeroides. J. Biol. Chem. 2008, 283, 3718–3730. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lee, M.K.; Kim, M.S.; Lee, J.K. Molecular hydrogen production by nitrogenase of Rhodobacter sphaeroides and by Fe-only hydrogenase of Rhodospirillum rubrum. Int. J. Hydrog. Energy 2008, 33, 1516–1521. [Google Scholar] [CrossRef]
- Balkwill, D.L.; Fredrickson, J.K.; Romine, M.F. Sphingomonas and Related Genera (No. PNNL-SA-39356); Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2003.
- Chen, H.; Jogler, M.; Rohde, M.; Klenk, H.P.; Busse, H.J.; Tindall, B.J.; Spröer, C.; Overmann, J.; Overmann, J. Sphingobium limneticum sp. nov. and Sphingobium boeckii sp. nov., two freshwater planktonic members of the family Sphingomonadaceae, and reclassification of Sphingomonas suberifaciens as Sphingobium suberifaciens comb. nov. Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 2, 735–743. [Google Scholar] [CrossRef]
- Kulichevskaya, I.S.; Suzina, N.E.; Liesack, W.; Dedysh, S.N. Bryobacter aggregatus gen. nov., sp. nov., a peat-inhabiting, aerobic chemo-organotroph from subdivision 3 of the Acidobacteria. Int. J. Syst. Evol. Microbiol. 2010, 60, 301–306. [Google Scholar] [CrossRef]
- Vandamme, P.; Coenye, T. Taxonomy of the genus Cupriavidus: A tale of lost and found. Int. J. Syst. Evol. Microbiol. 2004, 54, 2285–2289. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Rensing, C.; Zhao, K.; Johnstone, L.; Wang, G. Genome sequence of the facultative anaerobic arsenite-oxidizing and nitrate-reducing bacterium Acidovorax sp. strain NO1. J. Bacteriol. 2012, 194, 1635–1636. [Google Scholar] [CrossRef]
- Velichko, N.; Chernyaeva, E.; Averina, S.; Gavrilova, O.; Lapidus, A.; Pinevich, A. Consortium of the ‘bichlorophyllous’ cyanobacterium Prochlorothrix hollandica and chemoheterotrophic partner bacteria: Culture and metagenome-based description. Environ. Microbiol. Rep. 2015, 7, 623–633. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Lee, T.K.; Woo, S.G.; Baek, G.S.; Park, J. In-depth characterization of wastewater bacterial community in response to algal growth using Pyrosequencing. J. Microbiol. Biotechnol. 2013, 23, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.; Herrero, A. Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem. Soc. Trans. 2005, 33, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Lea, P.J.; Miflin, B.J. Photosynthetic ammonia assimilation. In Photosynthesis II: Encyclopedia of Plant Physiology (New Series); Springer: Berlin, Germany, 1979; Volume 6, pp. 444–456. [Google Scholar] [CrossRef]
- Patel, A.; Tiwari, S.; Prasad, S.M. Effect of time interval on arsenic toxicity to paddy field cyanobacteria as evident by nitrogen metabolism, biochemical constituent, and exopolysaccharide content. Biol. Trace Elem. Res. 2021, 199, 2031–2046. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Shi, W.; Takatani, N.; Aichi, M.; Maeda, S.I.; Watanabe, S.; Yoshikawa, H.; Omata, T. Regulation of nitrate assimilation in cyanobacteria. J. Exp. Bot. 2011, 62, 1411–1424. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, M.; Yu, J.; Zhang, J.; Zhang, R.; Zhang, L.; Chen, G. Differences in growth, pigment composition and photosynthetic rates of two phenotypes Microcystis aeruginosa strains under high and low iron conditions. Biochem. Syst. Ecol. 2014, 55, 112–117. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of light intensity and quality on growth rate and composition of Chlorella vulgaris. Plants 2019, 9, 31. [Google Scholar] [CrossRef]
- Hesse, K.; Dittmann, E.; Börner, T. Consequences of impaired microcystin production for light-dependent growth and pigmentation of Microcystis aeruginosa PCC 7806. FEMS Microbiol. Ecol. 2001, 37, 39–43. [Google Scholar] [CrossRef]
- Becerro, M.A.; Paul, V.J. Effects of depth and light on secondary metabolites and cyanobacterial symbionts of the sponge Dysidea granulosa. Mar. Ecol. Prog. Ser. 2004, 280, 115–128. [Google Scholar] [CrossRef]
- Erenler, R.; Karan, T.; Altuner, Z. Growth and metabolite production of Chroococcus minutus under different temperature and light conditions. J. New Results Sci. 2017, 6, 47–52. [Google Scholar]
- Park, J.; Dinh, T.B. Contrasting effects of monochromatic LED lighting on growth, pigments and photosynthesis in the commercially important cyanobacterium Arthrospira maxima. Bioresour. Technol. 2019, 291, 121846. [Google Scholar] [CrossRef]
- Vijaya, V.; Anand, N. Blue light enhance the pigment synthesis in cyanobacterium Anabaena ambigua rao (nostacales). J. Agric. Biol. Sci. 2009, 4, 3. [Google Scholar]
- Gutu, A.; Kehoe, D.M. Emerging perspectives on the mechanisms, regulation, and distribution of light color acclimation in cyanobacteria. Mol. Plant 2012, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liotenberg, S.; Campbell, D.; Rippka, R.; Houmard, J.; de Marsac, N.T. Effect of the nitrogen source on phycobiliprotein synthesis and cell reserves in a chromatically adapting filamentous cyanobacterium. Microbiology 1996, 142, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Hemlata, T.; Fatma, T. Screening of cyanobacteria for phycobiliproteins and effect of different environmental stress on its yield. Bull. Environ. Contam. Toxicol. 2009, 83, 509–515. [Google Scholar] [CrossRef]
- Häubner, N.; Sylvander, P.; Vuori, K.; Snoeijs, P. Abiotic stress modifies the synthesis of alpha-tocopherol and beta-carotene in phytoplankton species. J. Phycol. 2014, 50, 753–759. [Google Scholar] [CrossRef]
- Rebolloso-Fuentes, M.M.; Navarro-Pérez, A.; García-Camacho, F.; Ramos-Miras, J.J.; Guil-Guerrero, J.L. Biomass nutrient profiles of the microalga Nannochloropsis. J. Agric. Food Chem. 2001, 49, 2966–2972. [Google Scholar] [CrossRef]
- Boyd, E.S.; Peters, J.W. New insights into the evolutionary history of biological nitrogen fixation. Front. Microbiol. 2013, 4, 201. [Google Scholar] [CrossRef]
- Paerl, H.W.; Millie, D.F. Physiological ecology of toxic aquatic cyanobacteria. Phycologia 1996, 35, 160–167. [Google Scholar] [CrossRef]
- Cai, H.; Jiang, H.; Krumholz, L.R.; Yang, Z. Bacterial community composition of size-fractioned aggregates within the phycosphere of cyanobacterial blooms in a eutrophic freshwater lake. PLoS ONE 2014, 9, e102879. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Yang, D.; Park, H.Y.; Park, W. Seasonal dynamics of the bacterial communities associated with cyanobacterial blooms in the Han River. Environ. Pollut. 2020, 266, 115198. [Google Scholar] [CrossRef]
- Laanbroek, H.J.; Keijzer, R.M.; Verhoeven, J.T.; Whigham, D.F. The distribution of ammonia-oxidizing Betaproteobacteria in stands of black mangroves (Avicennia germinans). Front. Microbiol. 2012, 3, 153. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Morikawa, T.; Wasai, S.; Kasahara, Y.; Koshiba, T.; Yamazaki, K.; Fujiwara, T.; Tokunaga, T.; Minamisawa, K. Identification of nitrogen-fixing Bradyrhizobium associated with roots of field-grown sorghum by metagenome and proteome analyses. Front. Microbiol. 2019, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Stahl, D.A.; Urbance, J.W. The division between fast- and slow-growing species corresponds to natural relationships among the mycobacteria. J. Bacteriol. 1990, 172, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Brown-Elliott, B.A.; Wallace, R.J. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 2002, 15, 716–746. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, F.; Pasek, S.; Schenowitz, C.; Dossat, C.; Barbe, V.; Rottman, M.; Macheras, E.; Heym, B.; Herrmann, J.L.; Daffé, M.; et al. Non mycobacterial virulence genes in the genome of the emerging pathogen Mycobacterium abscessus. PLoS ONE 2009, 4, e5660. [Google Scholar] [CrossRef]
- Giannuzzi, L. Cyanobacteria Growth Kinetics; IntechOpen: London, UK, 2019. [Google Scholar]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Nalewajko, C.; Murphy, T.P. Effects of temperature, and availability of nitrogen and phosphorus on the abundance of Anabaena and Microcystis in Lake Biwa, Japan: An experimental approach. Limnology 2001, 2, 45–48. [Google Scholar] [CrossRef]

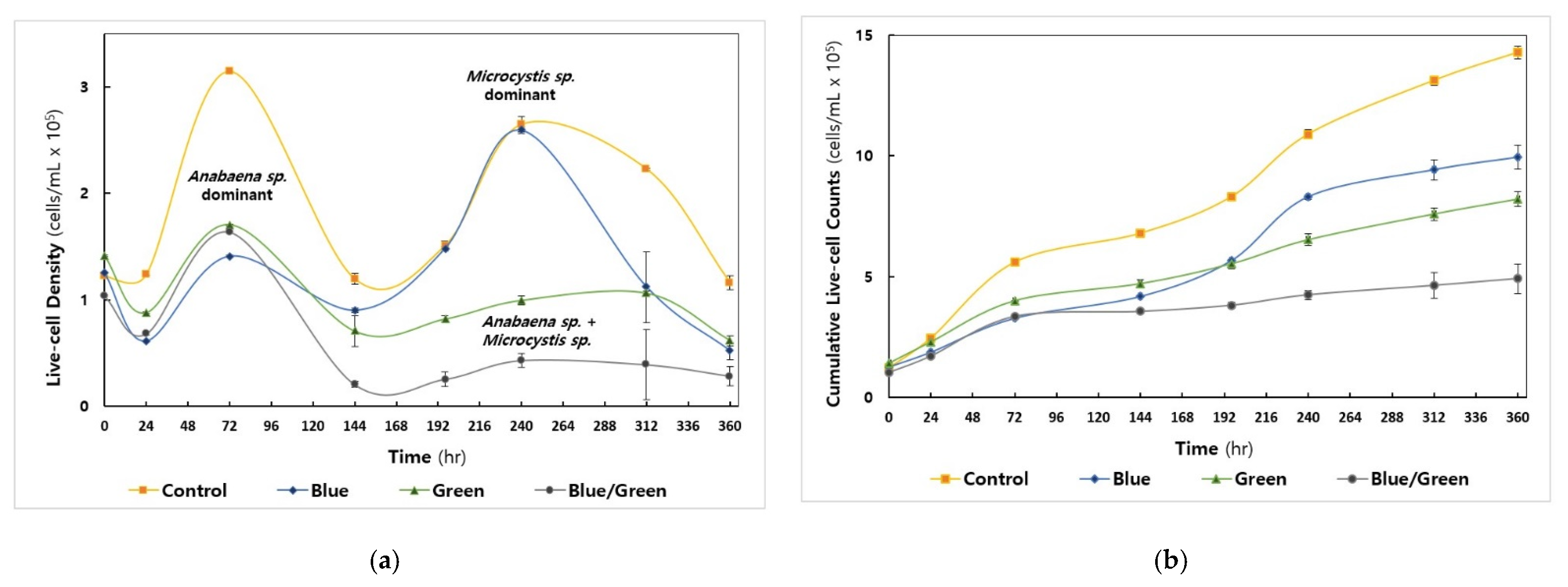
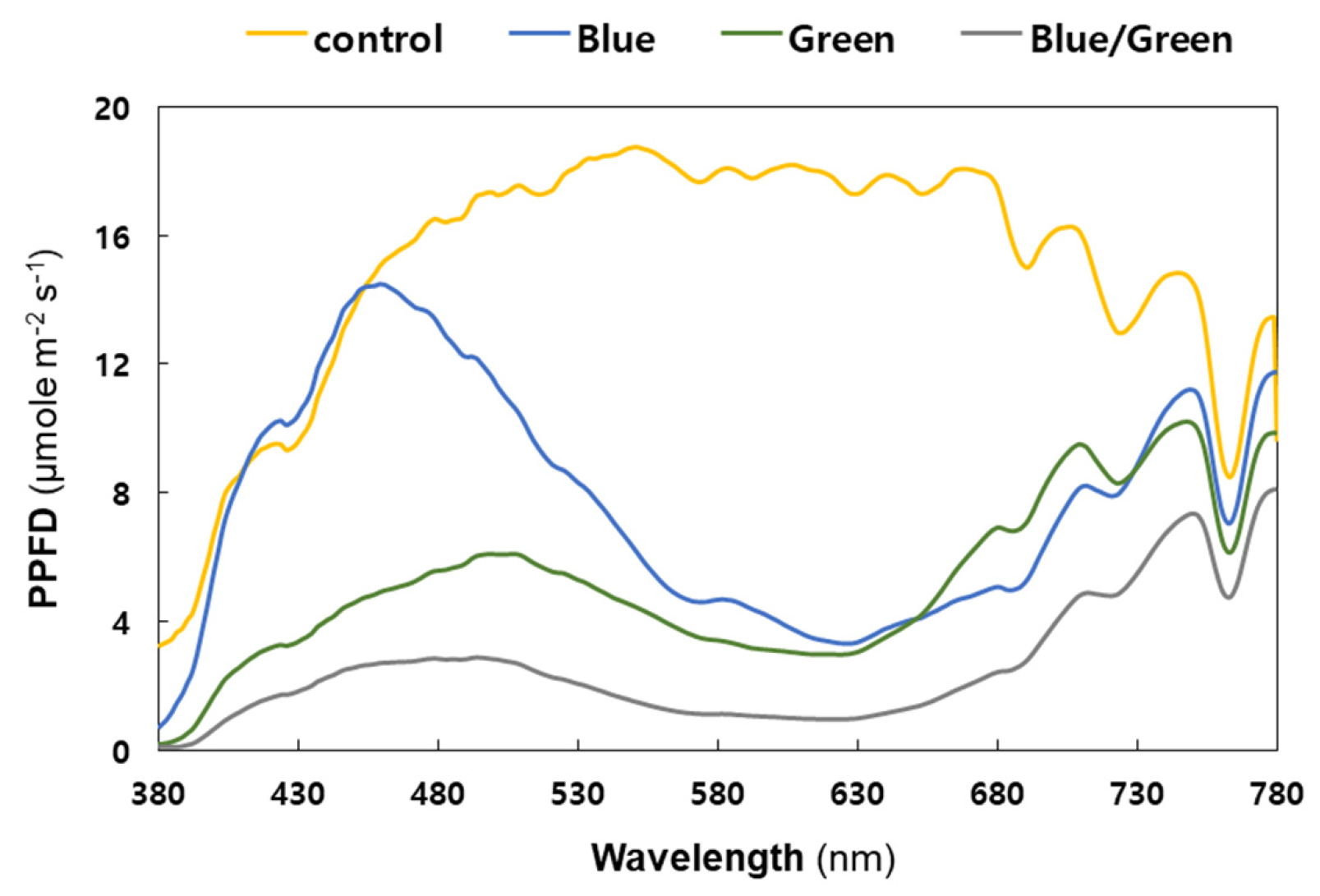
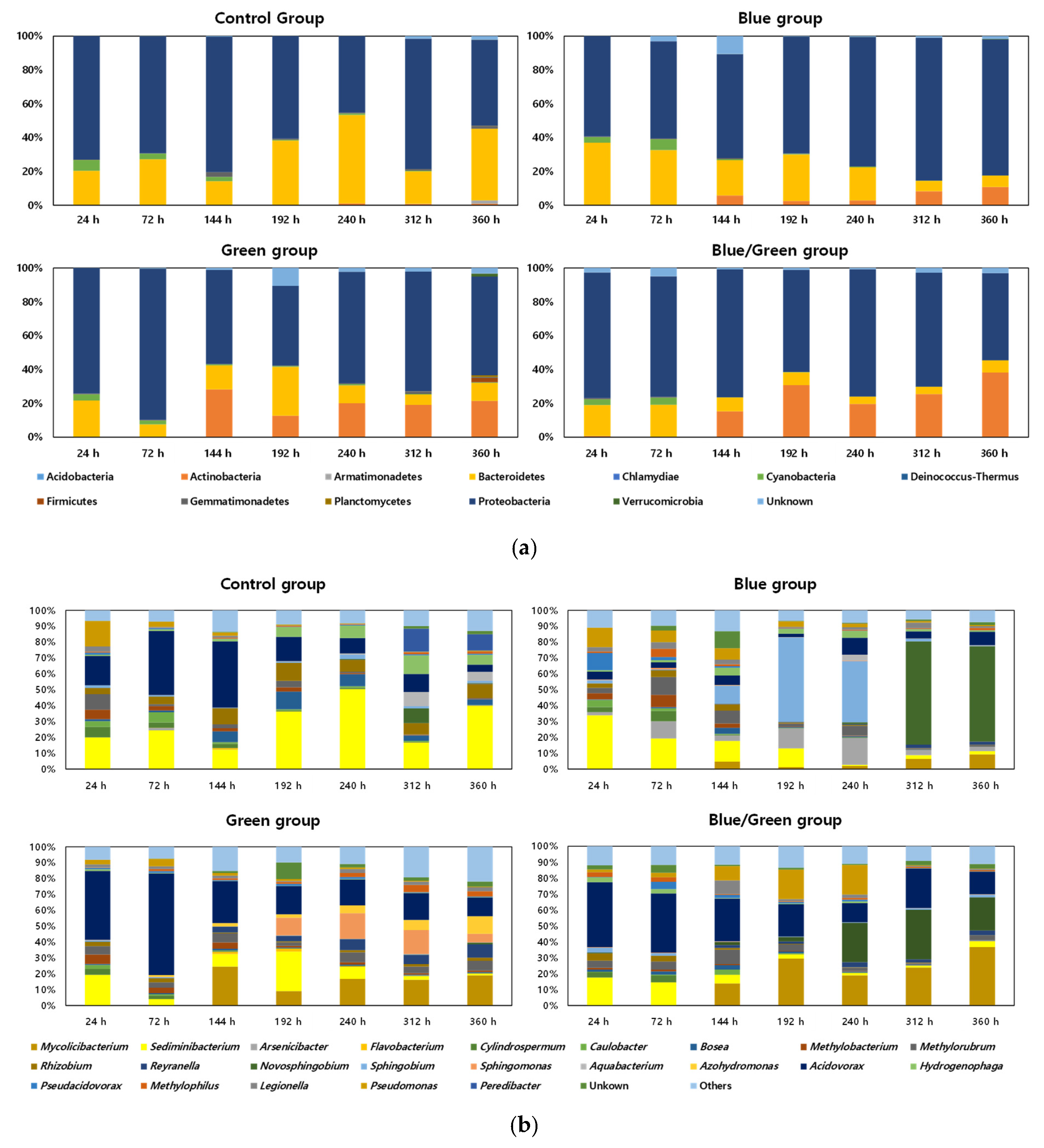
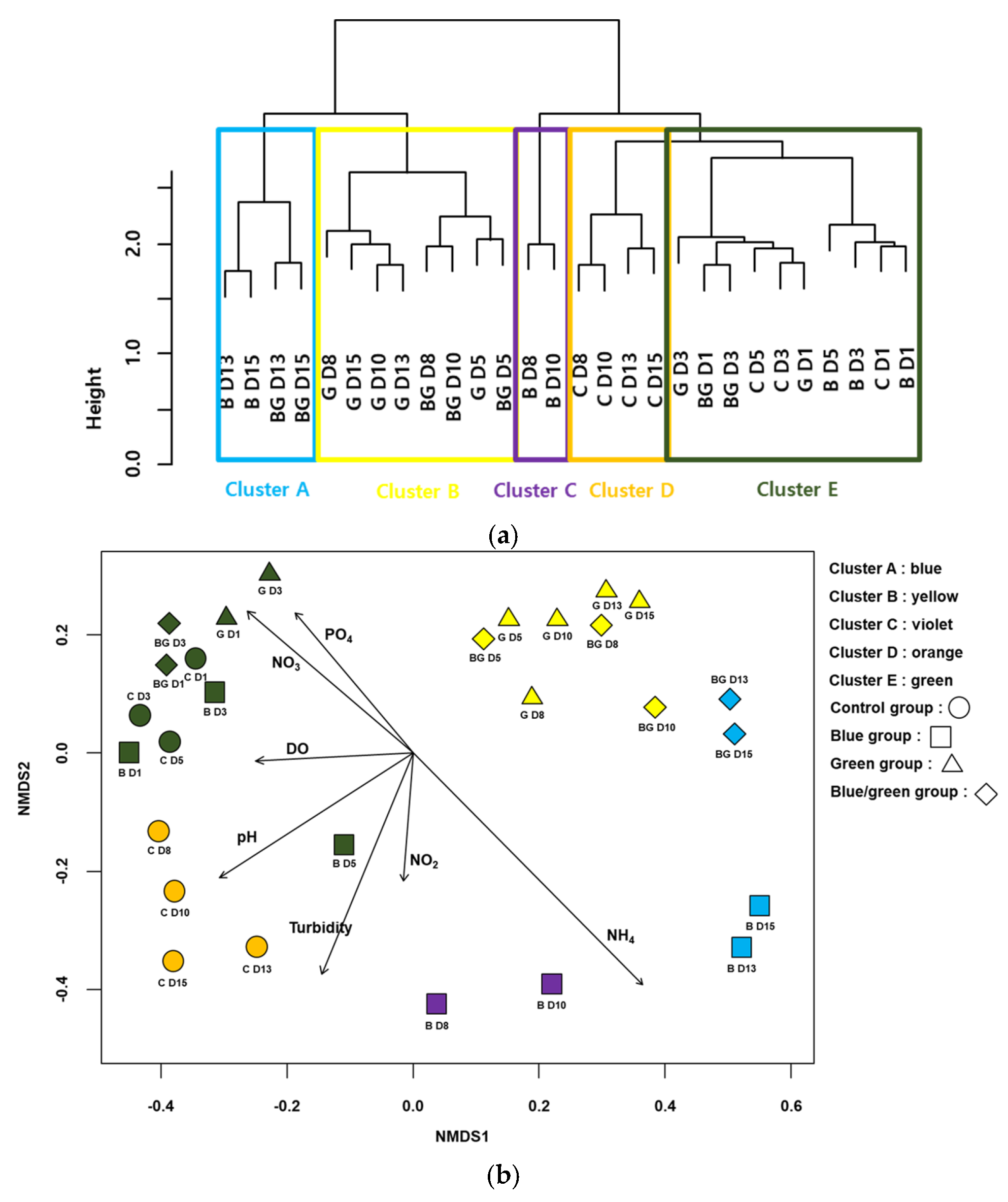
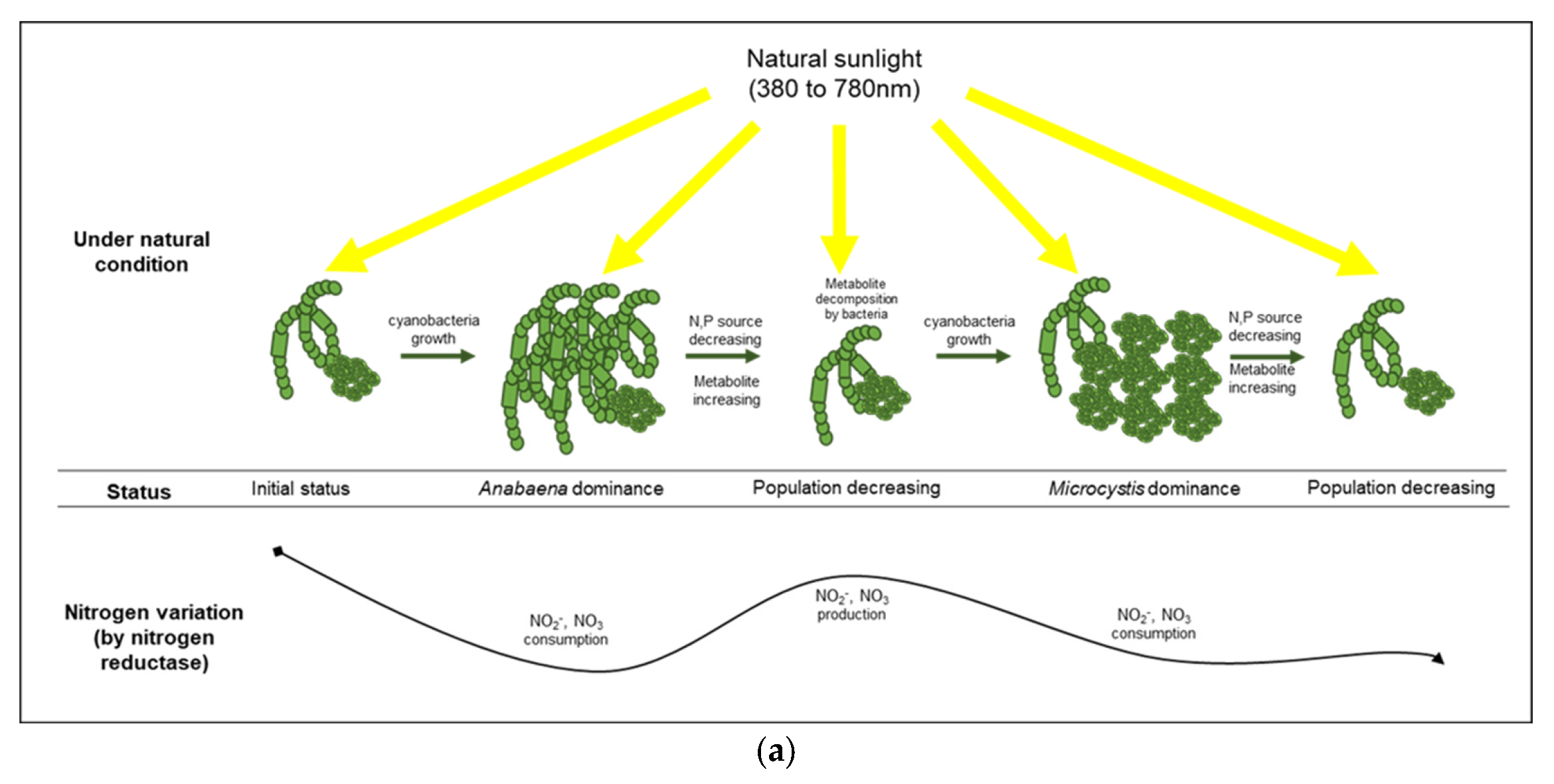
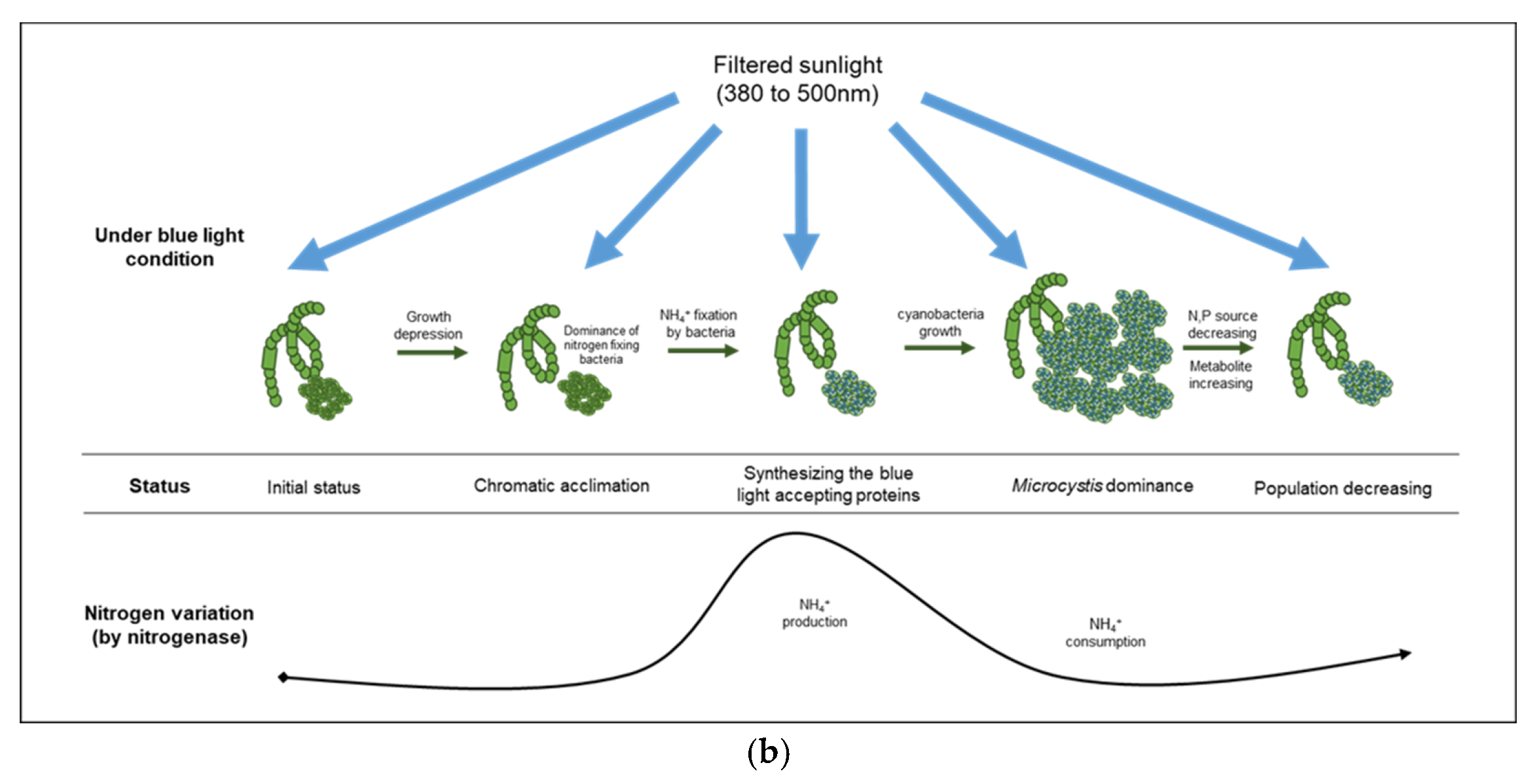
| Factors | Conditions | 0 h | 24 h | 72 h | 144 h | 196 h | 240 h | 312 h | 360 h |
|---|---|---|---|---|---|---|---|---|---|
| BOD (mg/L) | Control | 0.3 | 0.4 | 2.8 | 3.2 | 5.6 | 6.8 | 6.4 | 6.4 |
| Blue | 0.3 | 0.8 | 0.8 | 1.6 | 2.8 | 2.4 | 3.2 | 0.2 | |
| Green | 0.3 | 0.8 | 3.6 | 2.4 | 2.4 | 2.4 | 4.4 | 0.2 | |
| Blue/Green | 0.3 | 0.4 | 4.0 | 2.0 | 2.0 | 3.6 | 3.6 | 0.4 | |
| COD (mg/L) | Control | 1.0 | 1.6 | 4.4 | 6.0 | 8.8 | 6.8 | 9.2 | 7.2 |
| Blue | 1.0 | 2.8 | 2.4 | 5.6 | 3.6 | 3.6 | 4.4 | 5.6 | |
| Green | 1.0 | 3.2 | 4.8 | 5.6 | 3.6 | 3.2 | 6.0 | 6.0 | |
| Blue/Green | 1.0 | 3.6 | 1.6 | 5.2 | 2.4 | 4.8 | 5.6 | 5.6 | |
| TN (mg/L) | Control | 50.00 | 49.18 | 38.70 | 34.47 | 52.59 | 41.00 | 38.99 | 28.34 |
| Blue | 50.00 | 46.67 | 54.30 | 38.32 | 47.55 | 48.87 | 54.42 | 35.28 | |
| Green | 50.00 | 55.66 | 57.82 | 37.59 | 42.37 | 48.51 | 57.94 | 43.12 | |
| Blue/Green | 50.00 | 49.50 | 37.98 | 39.98 | 37.74 | 38.55 | 38.24 | 37.94 | |
| TP (mg/L) | Control | 1.00 | 0.98 | 0.75 | 0.60 | 0.87 | 0.63 | 0.74 | 0.53 |
| Blue | 1.00 | 1.00 | 0.96 | 0.67 | 0.74 | 0.78 | 0.96 | 0.76 | |
| Green | 1.00 | 1.18 | 1.13 | 0.65 | 0.69 | 1.01 | 1.12 | 1.00 | |
| Blue/Green | 1.00 | 1.05 | 0.68 | 0.72 | 0.48 | 0.60 | 0.70 | 1.14 | |
| NO3-N (mg/L) | Control | 52.85 | 55.57 | 52.44 | 21.33 | 27.64 | 20.48 | 23.97 | 30.08 |
| Blue | 52.85 | 55.70 | 47.26 | 35.77 | 21.71 | 19.24 | 22.33 | 36.29 | |
| Green | 52.85 | 57.35 | 38.62 | 30.97 | 29.12 | 27.23 | 18.97 | 28.09 | |
| Blue/Green | 52.85 | 53.51 | 50.38 | 30.97 | 25.31 | 18.42 | 28.53 | 25.00 | |
| NO2-N (mg/L) | Control | 0.02 | 0.02 | 0.05 | 0.14 | 0.27 | 0.18 | 0.18 | 0.23 |
| Blue | 0.02 | 0.00 | 0.03 | 0.06 | 0.04 | 0.04 | 0.06 | 0.12 | |
| Green | 0.02 | 0.01 | 0.04 | 0.03 | 0.04 | 0.04 | 0.04 | 0.04 | |
| Blue/Green | 0.02 | 0.00 | 0.05 | 0.14 | 0.11 | 0.07 | 0.15 | 0.12 | |
| NH3-N (mg/L) | Control | 0.08 | 0.00 | 0.00 | 0.00 | 0.04 | 0.07 | 0.18 | 0.14 |
| Blue | 0.08 | 0.00 | 0.00 | 0.00 | 0.39 | 0.10 | 0.22 | 0.35 | |
| Green | 0.08 | 0.00 | 0.00 | 0.04 | 0.06 | 0.15 | 0.14 | 0.15 | |
| Blue/Green | 0.08 | 0.00 | 0.00 | 0.01 | 0.19 | 0.15 | 0.26 | 0.15 | |
| PO4-P (mg/L) | Control | 1.01 | 0.93 | 0.69 | 0.34 | 0.37 | 0.38 | 0.38 | 0.39 |
| Blue | 1.01 | 0.98 | 0.67 | 0.42 | 0.37 | 0.29 | 0.38 | 0.58 | |
| Green | 1.01 | 1.10 | 0.60 | 0.55 | 0.55 | 0.38 | 0.26 | 0.34 | |
| Blue/Green | 1.01 | 1.11 | 0.74 | 0.56 | 0.54 | 0.29 | 0.56 | 0.48 |
| Group | Indicator Species | IndVal | Freq | p |
|---|---|---|---|---|
| A | Nordella | 1.00 | 4 | 0.001 |
| Rhodobacter | 0.97 | 7 | 0.001 | |
| Aestuariisphingobium | 0.94 | 11 | 0.001 | |
| Novosphingobium | 0.86 | 28 | 0.002 | |
| Lentimicrobium | 0.75 | 3 | 0.004 | |
| Sphingorhabdus | 0.75 | 3 | 0.011 | |
| Herbaspirillum | 0.74 | 4 | 0.013 | |
| Geothrix | 0.50 | 2 | 0.043 | |
| Pelomonas | 0.49 | 4 | 0.047 | |
| Neochlamydia | 0.48 | 3 | 0.041 | |
| Tsukamurella | 0.46 | 3 | 0.047 | |
| B | Sphingomonas | 0.91 | 28 | 0.002 |
| Azohydromonas | 0.72 | 13 | 0.023 | |
| Simkania | 0.63 | 5 | 0.019 | |
| Reyranella | 0.60 | 24 | 0.008 | |
| Mucilaginibacter | 0.58 | 6 | 0.034 | |
| Gordonia | 0.57 | 20 | 0.003 | |
| Zoogloea | 0.54 | 23 | 0.017 | |
| C | Sphingobium | 0.90 | 28 | 0.003 |
| Flavobacterium | 0.87 | 23 | 0.006 | |
| Spirosoma | 0.85 | 14 | 0.003 | |
| D | Bryobacter | 0.97 | 6 | 0.001 |
| Roseomonas | 0.97 | 9 | 0.001 | |
| Lentilitoribacter | 0.95 | 8 | 0.001 | |
| Hymenobacter | 0.92 | 7 | 0.001 | |
| Blastomonas | 0.92 | 10 | 0.001 | |
| Peredibacter | 0.75 | 6 | 0.032 | |
| Fimbriimonas | 0.75 | 4 | 0.012 | |
| Gemmobacter | 0.72 | 27 | 0.001 | |
| Bosea | 0.64 | 28 | 0.005 | |
| Devosia | 0.60 | 28 | 0.001 | |
| Sphingopyxis | 0.59 | 20 | 0.004 | |
| Rhizobium | 0.59 | 28 | 0.001 | |
| Chryseolinea | 0.57 | 24 | 0.022 | |
| Phreatobacter | 0.56 | 13 | 0.018 | |
| Hydrogenophaga | 0.55 | 28 | 0.018 | |
| Ensifer | 0.54 | 28 | 0.002 | |
| Sediminibacterium | 0.52 | 28 | 0.001 | |
| Exiguobacterium | 0.50 | 2 | 0.038 | |
| Sediminicoccus | 0.50 | 2 | 0.031 | |
| Simplicispira | 0.50 | 2 | 0.027 | |
| E | Cupriavidus | 0.95 | 23 | 0.010 |
| Porphyrobacter | 0.93 | 15 | 0.001 | |
| Bradyrhizobium | 0.78 | 28 | 0.001 | |
| Cylindrospermum | 0.74 | 28 | 0.001 | |
| Brevundimonas | 0.70 | 27 | 0.001 | |
| Limnobacter | 0.65 | 15 | 0.001 | |
| Caulobacter | 0.63 | 27 | 0.006 | |
| Ralstonia | 0.61 | 26 | 0.020 | |
| Methylobacterium | 0.57 | 28 | 0.003 | |
| Aeromicrobium | 0.50 | 5 | 0.041 | |
| Acidovorax | 0.38 | 28 | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Lee, C.S.; Cho, J.-Y.; Bae, M.-J.; Kim, E.-J. Comparison of Bacterial Assemblages Associated with Harmful Cyanobacteria under Different Light Conditions. Microorganisms 2022, 10, 2150. https://doi.org/10.3390/microorganisms10112150
Yang T, Lee CS, Cho J-Y, Bae M-J, Kim E-J. Comparison of Bacterial Assemblages Associated with Harmful Cyanobacteria under Different Light Conditions. Microorganisms. 2022; 10(11):2150. https://doi.org/10.3390/microorganisms10112150
Chicago/Turabian StyleYang, Taehui, Chang Soo Lee, Ja-Young Cho, Mi-Jung Bae, and Eui-Jin Kim. 2022. "Comparison of Bacterial Assemblages Associated with Harmful Cyanobacteria under Different Light Conditions" Microorganisms 10, no. 11: 2150. https://doi.org/10.3390/microorganisms10112150
APA StyleYang, T., Lee, C. S., Cho, J.-Y., Bae, M.-J., & Kim, E.-J. (2022). Comparison of Bacterial Assemblages Associated with Harmful Cyanobacteria under Different Light Conditions. Microorganisms, 10(11), 2150. https://doi.org/10.3390/microorganisms10112150







