Altered Nasal Microbiome in Atrophic Rhinitis: A Novel Theory of Etiopathogenesis and Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Assessment
2.2. Clinical Examinations
2.3. Study Treatment
2.4. Histological Assessment
2.5. Immunohistochemistry
2.6. DNA Extraction and 16s rRNA Sequencing
2.7. Microbiome and Statistical Analysis
3. Results
3.1. Effects of Intervention
3.1.1. Clinical Endoscopic Findings Improved with Honey Therapy
3.1.2. Nasal Obstruction, Crust Formation, and Nasal Discharge Improved with Honey Therapy
3.1.3. Honey Therapy Restores Mucosal Glands
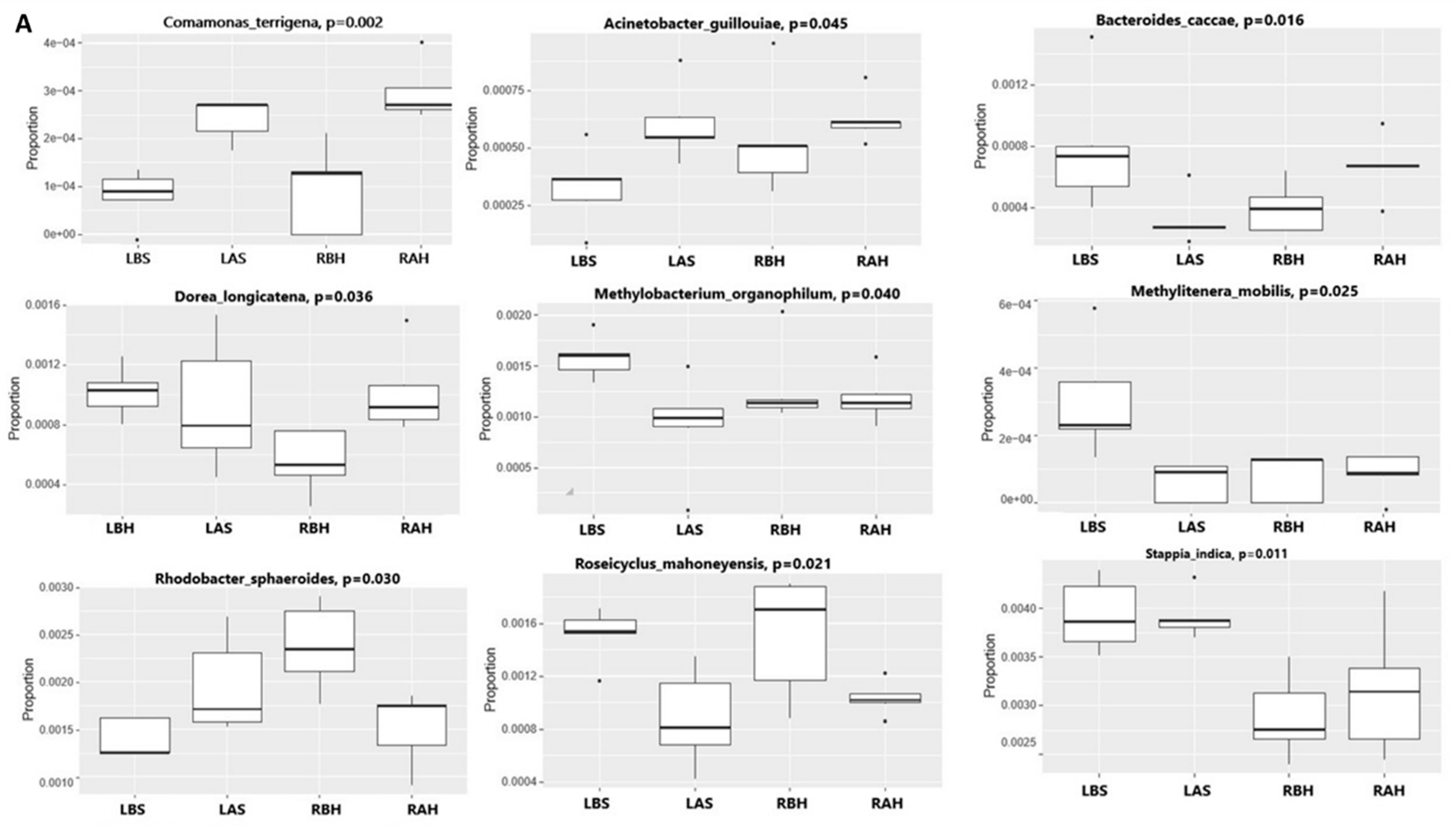
3.1.4. Alteration in Nasal Microbiome
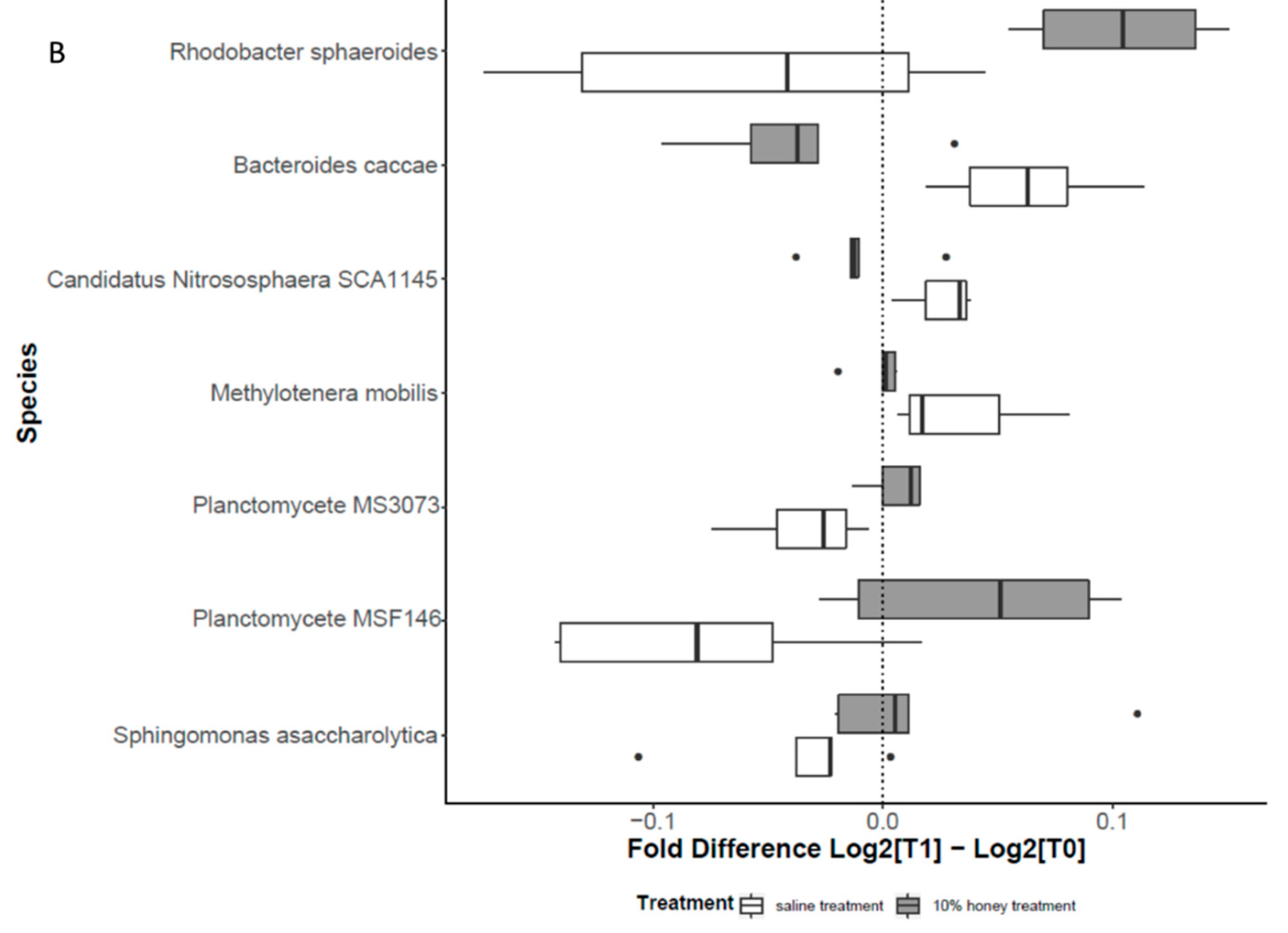
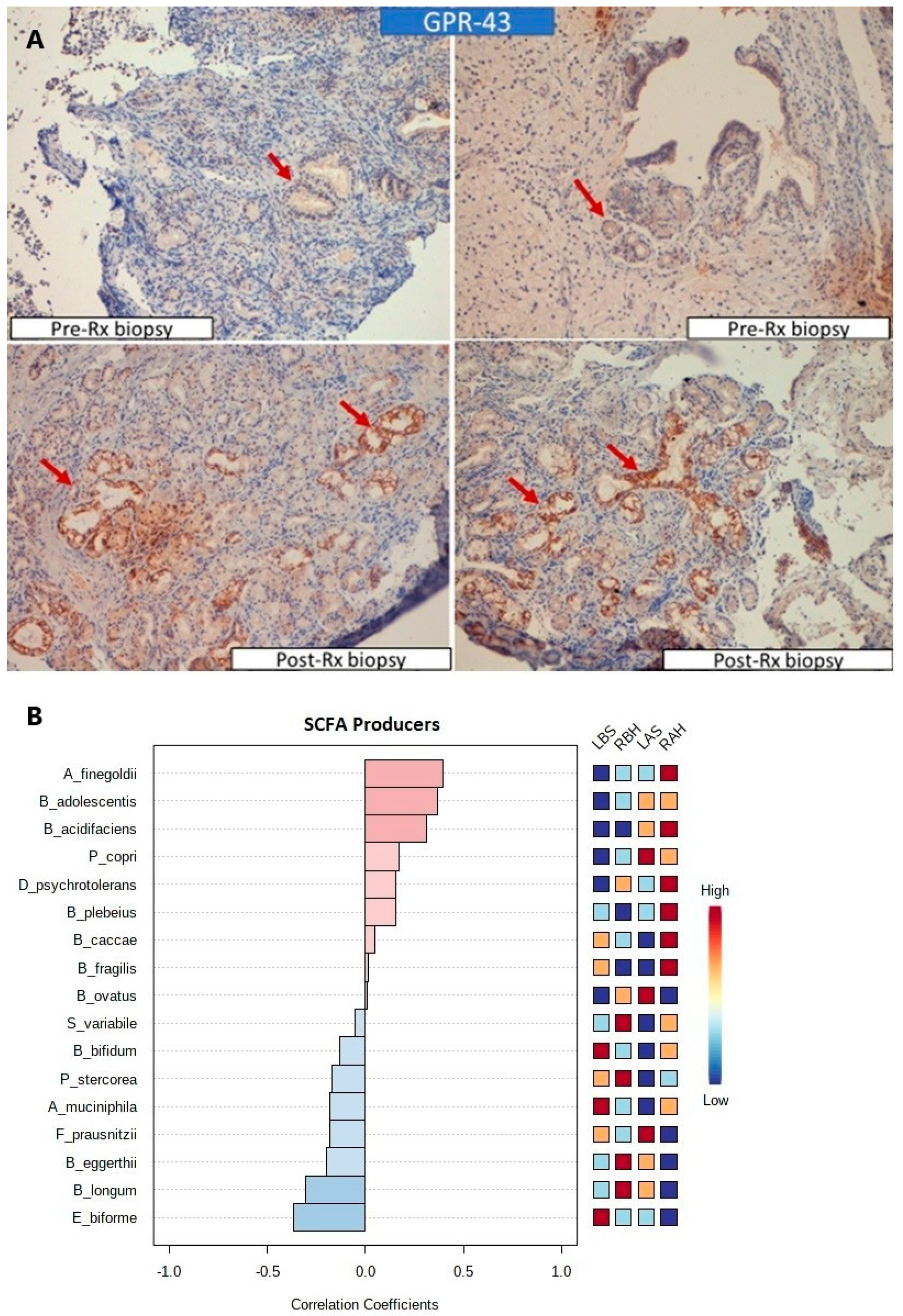
3.1.5. Increased Expression of SCFA Receptors and SCFA Producers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amreliwala, M.S.; Jain, S.K.T.; Raizada, R.M.; Sinha, V.C.V. Atrophic rhinitis: An inherited condition. Indian J. Clin. Pract. 1993, 4, 43–46. [Google Scholar]
- Medina, L.; Benazzo, M.; Bertino, G.; Montecucco, C.M.; Danesino, C.; Martinetti, M.; Mira, E. Clinical, genetic and immunologic analysis of a family affected by ozena. Eur. Arch. Oto-Rhino-Laryngol. 2003, 260, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Borgstein, J.; Sada, E.; Cortes, R. Ciprofloxacin for rhinoscleroma and ozena. Lancet. 1993, 342, 122. [Google Scholar] [CrossRef]
- Honey: A Biologic Wound Dressing—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26061489/ (accessed on 29 May 2022).
- Lay-Flurrie, K. Honey in wound care: Effects, clinical application and patient benefit. Br. J. Nurs. 2008, 17, S30–S36. [Google Scholar] [CrossRef] [PubMed]
- Pieper, B. Honey-Based Dressings and Wound care: An option for care in the United States. J. Wound Ostomy Cont. Nurs. 2009, 36, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, S.E.; Jenkins, R.E.; Rowlands, R.S.; Purdy, K.J.; Cooper, R.A. Manuka honey inhibits adhesion and invasion of medically important wound bacteria in vitro. Futur. Microbiol. 2013, 8, 1523–1536. [Google Scholar] [CrossRef]
- Ramasamy, B.; Magne, F.; Tripathy, S.; Venugopal, G.; Mukherjee, D.; Balamurugan, R. Association of Gut Microbiome and Vitamin D Deficiency in Knee Osteoarthritis Patients: A Pilot Study. Nutrients 2021, 13, 1272. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? eBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Y.; Jiang, H.; Robbins, G.T.; Nie, D. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int. J. Cancer 2010, 128, 847–856. [Google Scholar] [CrossRef]
- Bindels, L.B.; Dewulf, E.M.; Delzenne, N. GPR43/FFA2: Physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci. 2013, 34, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Kilty, S.J.; Duval, M.; Chan, F.T.; Ferris, W.; Slinger, R. Methylglyoxal: (Active agent of manuka honey) in vitro activity against bacterial biofilms. Int. Forum Allergy Rhinol. 2011, 1, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Young, A. Closure of the nostrils in atrophic rhinitis. J. Laryngol. Otol. 1971, 85, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Saunders, W.H. Atrophic Rhinitis: Results of Surgical Treatment. Arch. Otolaryngol. Head Neck Surg. 1958, 68, 342–345. [Google Scholar] [CrossRef]
- Nielsen, B.C.; Olinder-Nielsen, A.M.; Malmborg, A.S. Successful treatment of ozena with ciprofloxacin. Rhinology 1995, 33, 57–60. [Google Scholar]
- Jaswal, A.; Jana, A.K.; Sikder, B.; Nandi, T.K.; Sadhukhan, S.K.; Das, A. Novel treatment of atrophic rhinitis: Early results. Eur. Arch. Oto-Rhino-Laryngol. 2008, 265, 1211–1217. [Google Scholar] [CrossRef]
- Johnsen, J.; Bratt, B.-M.; Michel-Barron, O.; Glennow, C.; Petruson, B. Pure Sesame Oil vs. Isotonic Sodium Chloride Solution as Treatment for Dry Nasal Mucosa. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 1353–1356. [Google Scholar] [CrossRef]
- Lohiya, A.; Abdulkader, R.S.; Rath, R.S.; Jacob, O.; Chinnakali, P.; Goel, A.D.; Agrawal, S. Prevalence and patterns of drug resistant pulmonary tuberculosis in India—A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2020, 22, 308–316. [Google Scholar] [CrossRef]
- Hashemian, F.; Baghbanian, N.; Majd, Z.; Rouini, M.-R.; Jahanshahi, J.; Hashemian, F. The effect of thyme honey nasal spray on chronic rhinosinusitis: A double-blind randomized controlled clinical trial. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 1429–1435. [Google Scholar] [CrossRef]
- Roberts, A.E.L.; Maddocks, S.E.; Cooper, R.A. Manuka honey is bactericidal against Pseudomonas aeruginosa and results in differential expression of oprF and algD. Microbiology 2012, 158, 3005–3013. [Google Scholar] [CrossRef]
- Halstead, F.D.; Webber, M.A.; Rauf, M.; Burt, R.; Dryden, M.; Oppenheim, B.A. In vitro activity of an engineered honey, medical-grade honeys, and antimicrobial wound dressings against biofilm-producing clinical bacterial isolates. J. Wound Care 2016, 25, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, R.; Barbaro, E.; Zangrando, R.; Rizzoli, A.; Malagnini, V.; Gambaro, A.; Fontana, P.; Capodaglio, G. Carbohydrate determination in honey samples by ion chromatography–mass spectrometry (HPAEC-MS). Anal. Bioanal. Chem. 2020, 412, 5217–5227. [Google Scholar] [CrossRef] [PubMed]
- Swallow, K.W.; Low, N.H. Analysis and quantitation of the carbohydrates in honey using high-performance liquid chromatography. J. Agric. Food Chem. 1990, 38, 1828–1832. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef]
- Dimitri-Pinheiro, S.; Soares, R.; Barata, P. The Microbiome of the Nose—Friend or Foe? Allergy Rhinol. 2020, 11, 2152656720911605. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.T.; Hamilos, D.L. The Nasal and Sinus Microbiome in Health and Disease. Curr. Allergy Asthma Rep. 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Thomas, S.; Izard, J.; Walsh, E.; Batich, K.; Chongsathidkiet, P.; Clarke, G.; Sela, D.A.; Muller, A.J.; Mullin, J.M.; Albert, K.; et al. The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-Microbiologists. Cancer Res. 2017, 77, 1783–1812. [Google Scholar] [CrossRef]
- Hardy, B.L.; Dickey, S.W.; Plaut, R.D.; Riggins, D.P.; Stibitz, S.; Otto, M.; Merrell, D.S. Corynebacterium pseudodiphtheriticum Exploits Staphylococcus aureus Virulence Components in a Novel Polymicrobial Defense Strategy. mBio 2019, 10, e02491-18. [Google Scholar] [CrossRef]
- Rawls, M.; Ellis, A.K. The microbiome of the nose. Ann. Allergy Asthma Immunol. 2019, 122, 17–24. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Bogan, B.W.; Sullivan, W.R.; Kayser, K.J.; Derr, K.; Aldrich, H.C.; Paterek, J.R. Alkanindiges illinoisensis gen. nov., sp. nov., an obligately hydrocarbonoclastic, aerobic squalane-degrading bacterium isolated from oilfield soils. Int. J. Syst. Evol. Microbiol. 2003, 53, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Yamamoto, S.; Hino, S.; Harayama, S. Population Dynamics of Phenol-Degrading Bacteria in Activated Sludge Determined by gyrB -Targeted Quantitative PCR. Appl. Environ. Microbiol. 1998, 64, 1203–1209. [Google Scholar] [CrossRef]
- Meerbergen, K.; Willems, K.A.; Dewil, R.; Van Impe, J.; Appels, L.; Lievens, B. Isolation and screening of bacterial isolates from wastewater treatment plants to decolorize azo dyes. J. Biosci. Bioeng. 2018, 125, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Basis of Virulence in Community-Associated Methicillin-Resistant Staphylococcus aureus. Annu. Rev. Microbiol. 2010, 64, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.A.J.W.; Wertheim, H.F.L. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection 2005, 33, 3–8. [Google Scholar] [CrossRef]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial Community Variation in Human Body Habitats Across Space and Time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef]
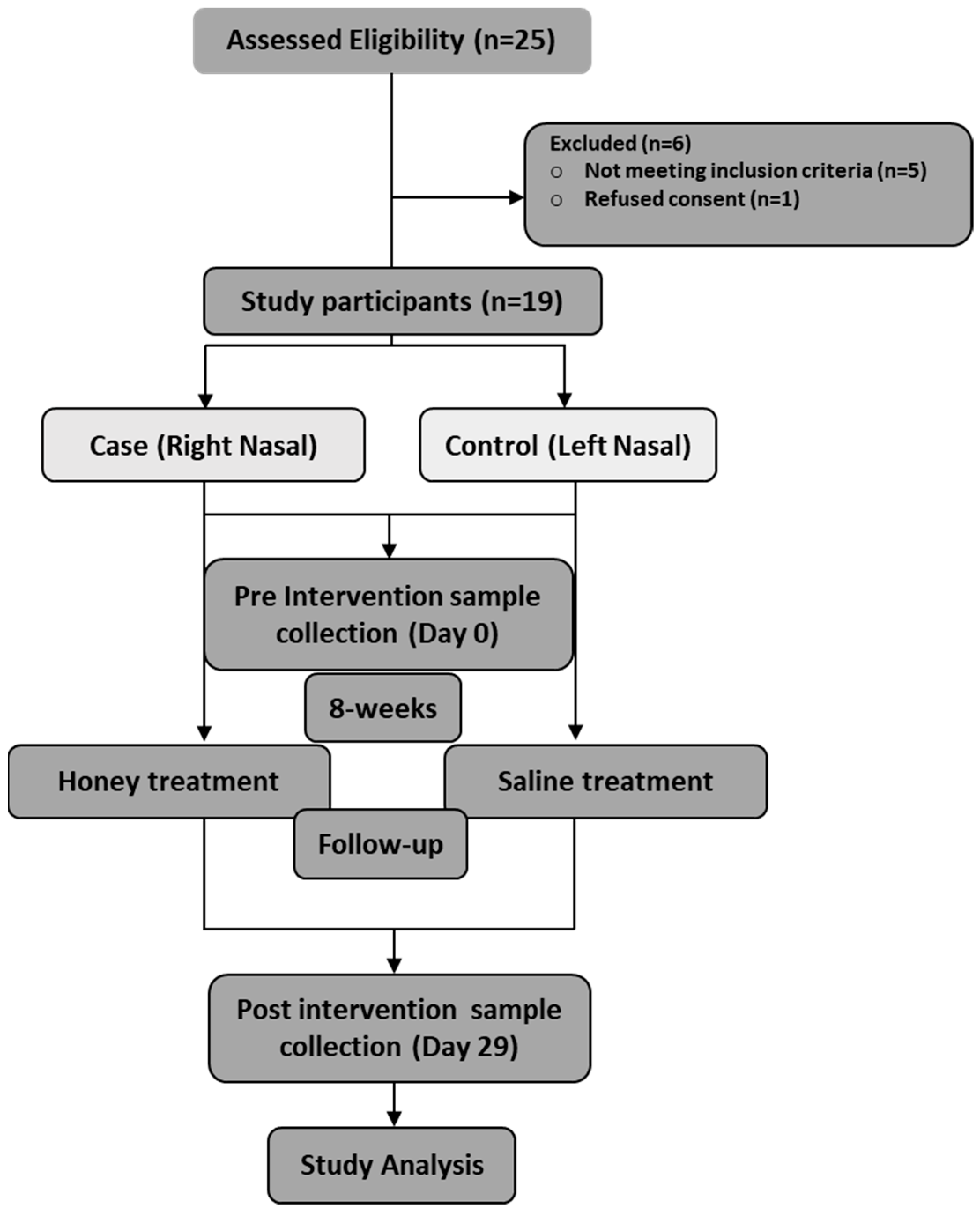

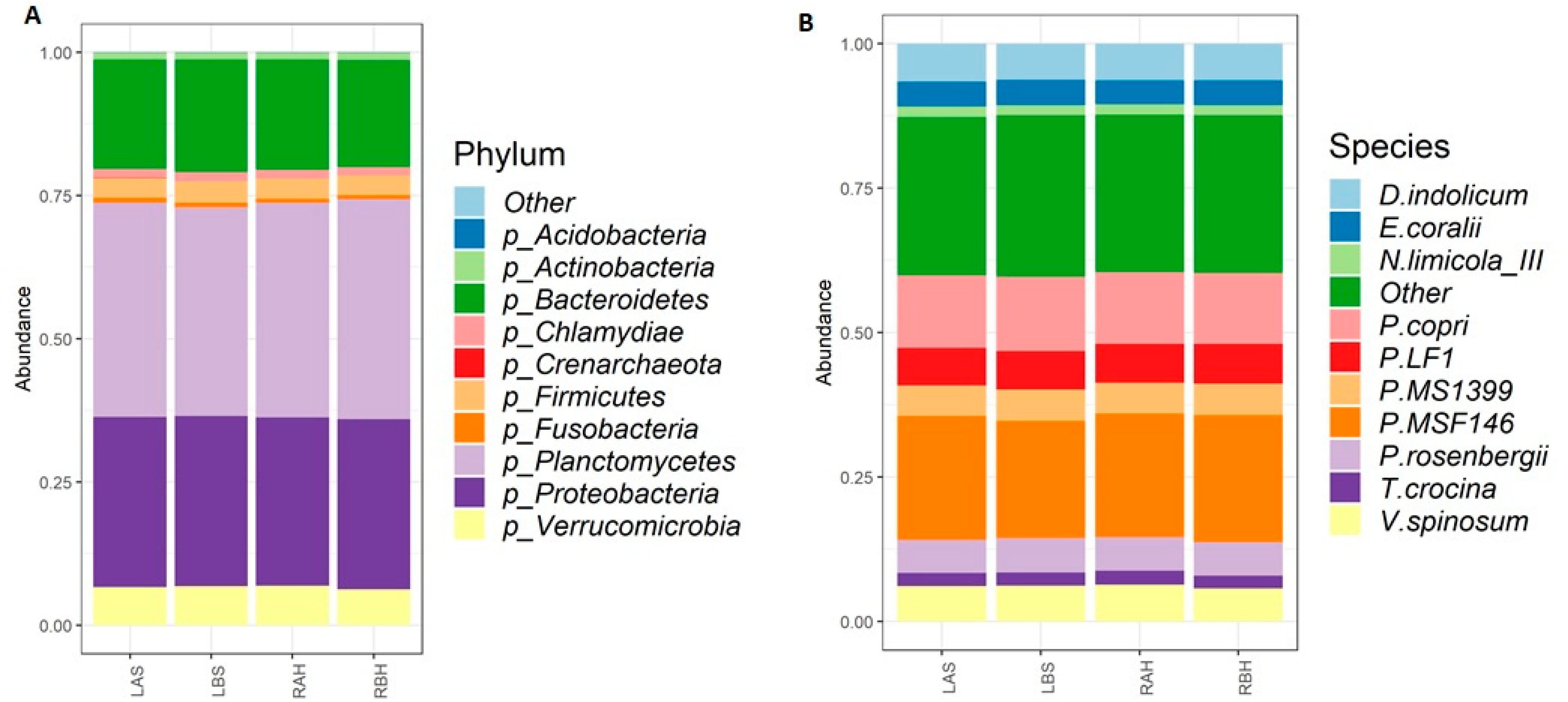
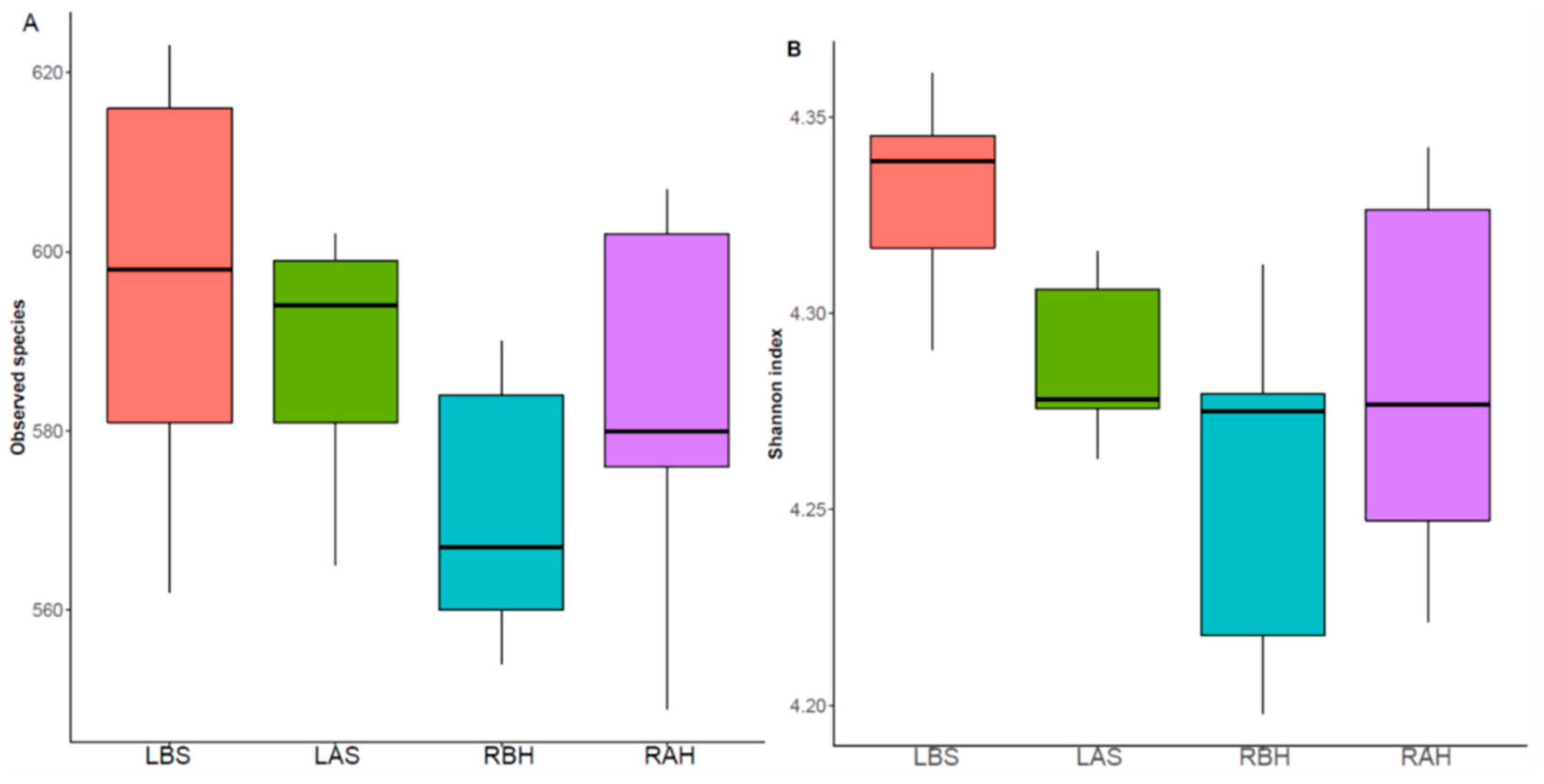
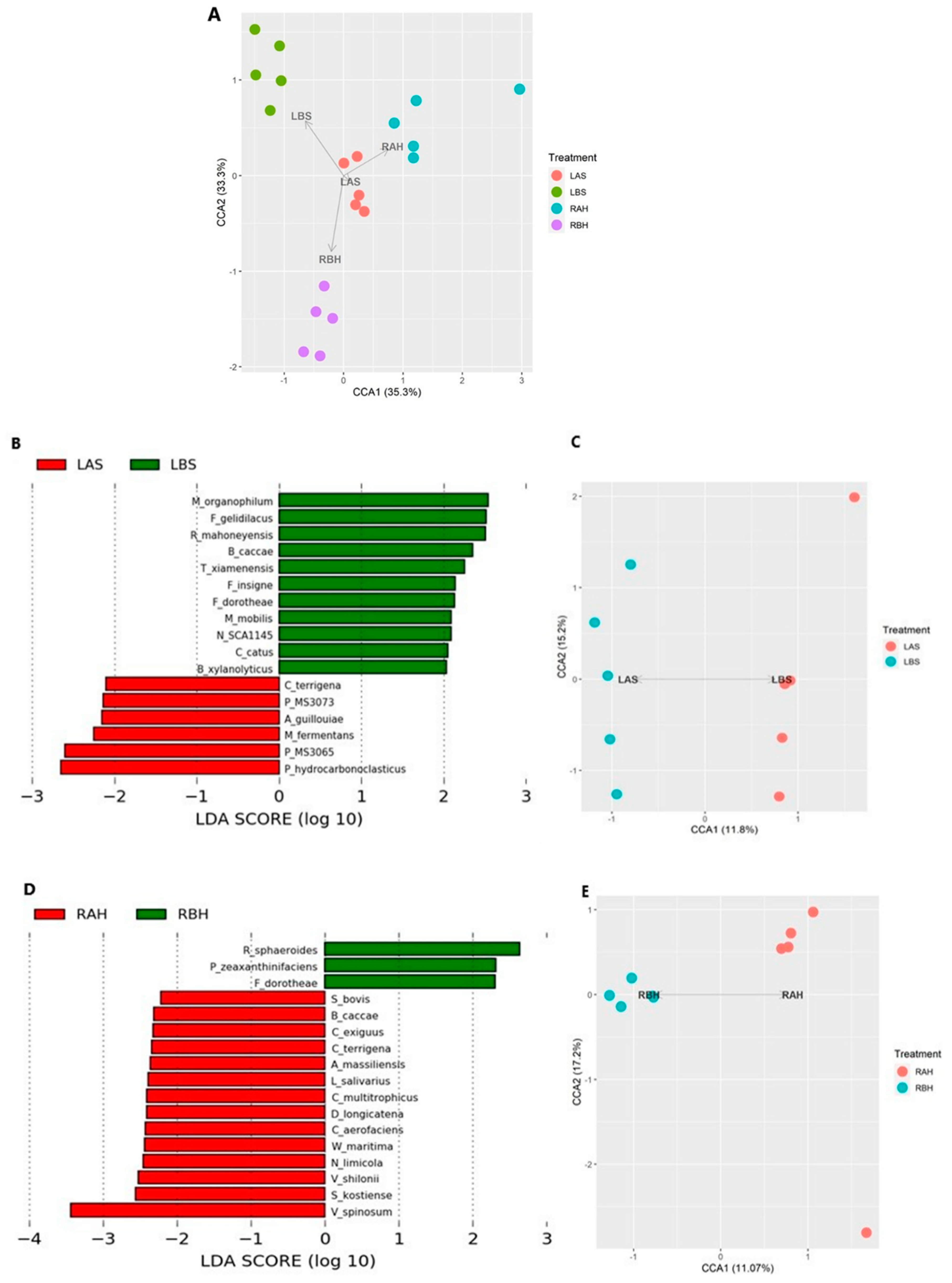
| Serial No | Symptom | Number (n) of Patients Who Presented with Clinical Symptoms, n (%) |
|---|---|---|
| 1 | Nasal obstruction | 19 (100%) |
| 2 | Foul smell | 15 (78.94%) |
| 3 | Impaired smell sensation | 9 (47.36%) |
| 4 | Nasal crust | 17 (89.47%) |
| 5 | Nasal discharge | 12 (63.16%) |
| 6 | Maggots | 1 (5.26%) |
| 7 | Epiphora | 1 (5.26%) |
| 8 | Epistaxis | 3 (15.79%) |
| 9 | Headache | 5 (26.31%) |
| Endoscopic Feature | Cases | Controls | Cases vs. Control Pre-Treatment Score | Cases vs. Control Post-Treatment Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | MD 95% CI | p-Value | Pre-Treatment | Post-Treatment | MD 95% CI | p-Value | MD | p-Value | MD | p-Value | |
| Crusts | 1.84 ± 0.38 | 0.21 ± 0.42 | 1.63 SE: 0.13 CI: 1.37–1.89 | 0.0001 | 1.73 ± 0.45 | 1.63 ± 0.49 | 0.1 SE: 0.15 CI: −0.21–0.41 | 0.52 | 0.11 SE: 0.14 CI: −0.16–0.38 | 0.419 | 1.5 SE: 0.05 CI: −1.43–1.62 | 0.0001 |
| Nasal cavity roominess | 1.42 ± 0.507 | 1.37 ± 0.496 | 0.05 SE: 0.163 CI: −0.28–0.38 | 0.764 | 1.47 ± 0.513 | 1.47 ± 0.513 | 0 SE: 0.166 CI: −0.39–0.34 | 1 | 0.050 SE: 0.165 CI: −0.3–0.39 | 0.764 | 0.050 SE −0.053 CI: −0.06–0.16 | 0.35 |
| Atrophic turbinates | 0.26 ± 0.45 | 0.21 ± 0.42 | 0.052 SE: 0.14 CI: −0.24–0.34 | 0.71 | 0.32 ± 0.48 | 0.32 ± 0.48 | 0 SE: 0.16 CI: −0.32–0.32 | 1 | 0.05 SE: 0.151 CI: −0.25–0.36 | 0.73 | 0.05 SE: 0.05 CI: −0.05–0.15 | 0.28 |
| Nasal mucosa congestion | 1.68 ± 0.48 | 1.05 ± 0.23 | 0.630 SE: 0.12 CI: 0.38–0.88 | 0.0001 | 1.73 ± 0.45 | 1.68 ± 0.48 | 0.05 SE: 0.15 CI: −0.26–0.36 | 0.743 | 0.05 SE: 0.15 CI: −0.26–0.36 | 0.74 | 0.58 SE: 0.05 CI: −0.48–0.67 | 0.0001 |
| Nasal discharge | 1.05 ± 0.91 | 0.16 ± 0.37 | 0.89 SE: 0.23 CI: 0.44–1.35 | 0.003 | 1.05 ± 0.91 | 1 ± 0.88 | 0.05 SE: 0.291 CI: −0.54–0.64 | 0.865 | 0 SE: 0.29 CI: −0.59–0.59 | 1 | 0.843 SE: 0.09 CI: 0.67–1.01 | 0.0001 |
| Improved | Not Improved | p-Value | |
|---|---|---|---|
| Nasal obstruction (n = 19) | |||
| Right | 18 | 1 | 0.003 |
| Left | 9 | 10 | |
| Foul smell (n = 15) | |||
| Right | 9 | 6 | 0.06 |
| Left | 3 | 12 | |
| Impaired smell sensation (n = 9) | |||
| Right | 6 | 3 | 0.637 |
| Left | 4 | 5 | |
| Crusts (n = 17) | |||
| Right | 16 | 1 | 0.00001 |
| Left | 2 | 15 | |
| Nasal discharge (n = 12) | |||
| Right | 12 | 0 | 0.0013 |
| Left | 4 | 8 | |
| Epistaxis (n = 3) | |||
| Right | 3 | 0 | 0.4 |
| Left | 1 | 2 | |
| Headache (n = 5) | |||
| Right | 1 | 4 | 1 |
| Left | 1 | 4 | |
| Test/Case Side (Right) | Control Side (Left Side) | Cases vs. Control Pre-Treatment | Cases vs. Control Post-Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | MD 95% CI | p-Value | Pre-Treatment | Post-Treatment | MD 95% CI | p-Value | MD | p-Value | MD | p-Value | |
| Cumulative symptom score | 4.32 ± 1.92 | 0.84 ± 1.13 | 3.47 SE: 0.51 CI: 2.4–4.5 | 0.0001 | 4.26 ± 1.88 | 3.47 ± 2.01 | 0.79 SE: 0.63 CI: 0.49–2.07 | 0.22 | 0.053 SE: 0.66 CI: 1.19–1.3 | 0.93 | 2.685 SE: 0.18 CI: 2.3–3.06 | 0.0001 |
| Improved | Not Improved | p-Value | |
|---|---|---|---|
| Basement membrane thinning | |||
| Right (19) | 7 | 12 | 0.12 |
| Left (19) | 2 | 17 | |
| Granulation tissue | |||
| Right (15) | 14 | 1 | 0.005 |
| Left (19) | 2 | 17 | |
| Fibrosis | |||
| Right (9) | 7 | 2 | 0.13 |
| Left (7) | 2 | 5 | |
| Mucus glands | |||
| Right (19) | 15 | 4 | 0.0001 |
| Left (19) | 1 | 18 | |
| Bacterial colonies | |||
| Right (17) | 15 | 2 | 0.01 |
| Left (17) | 7 | 10 | |
| Right Nasal Cavity | Left Nasal Cavity | Right Pre-Treatment vs. Left Pre-Treatment | Improvement in Right vs. Improvement in Left | |||||
|---|---|---|---|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | p-Value | Pre-Treatment | Post-Treatment | p-Value | p-Value | p-Value | |
| Median | 0 | 15 | 0.001 | 1 | 1 | 0.32 | 0.16 | 0.001 |
| IQR | 0 | 0–2 | 0–2 | 0–2 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, S.; Magne, F.; Venugopal, G.; Purkait, S.; Mutha, N.V.R.; Maiti, R.; Sharma, P.; Ramadass, B. Altered Nasal Microbiome in Atrophic Rhinitis: A Novel Theory of Etiopathogenesis and Therapy. Microorganisms 2022, 10, 2092. https://doi.org/10.3390/microorganisms10112092
Sarkar S, Magne F, Venugopal G, Purkait S, Mutha NVR, Maiti R, Sharma P, Ramadass B. Altered Nasal Microbiome in Atrophic Rhinitis: A Novel Theory of Etiopathogenesis and Therapy. Microorganisms. 2022; 10(11):2092. https://doi.org/10.3390/microorganisms10112092
Chicago/Turabian StyleSarkar, Saurav, Fabien Magne, Giriprasad Venugopal, Suvendu Purkait, Naresh V. R. Mutha, Rituparna Maiti, Prity Sharma, and Balamurugan Ramadass. 2022. "Altered Nasal Microbiome in Atrophic Rhinitis: A Novel Theory of Etiopathogenesis and Therapy" Microorganisms 10, no. 11: 2092. https://doi.org/10.3390/microorganisms10112092
APA StyleSarkar, S., Magne, F., Venugopal, G., Purkait, S., Mutha, N. V. R., Maiti, R., Sharma, P., & Ramadass, B. (2022). Altered Nasal Microbiome in Atrophic Rhinitis: A Novel Theory of Etiopathogenesis and Therapy. Microorganisms, 10(11), 2092. https://doi.org/10.3390/microorganisms10112092








