A Retrospective Study of the Proportion of Women at High and Low Risk of Intrauterine Infection Meeting Sepsis Criteria
Abstract
:1. Introduction
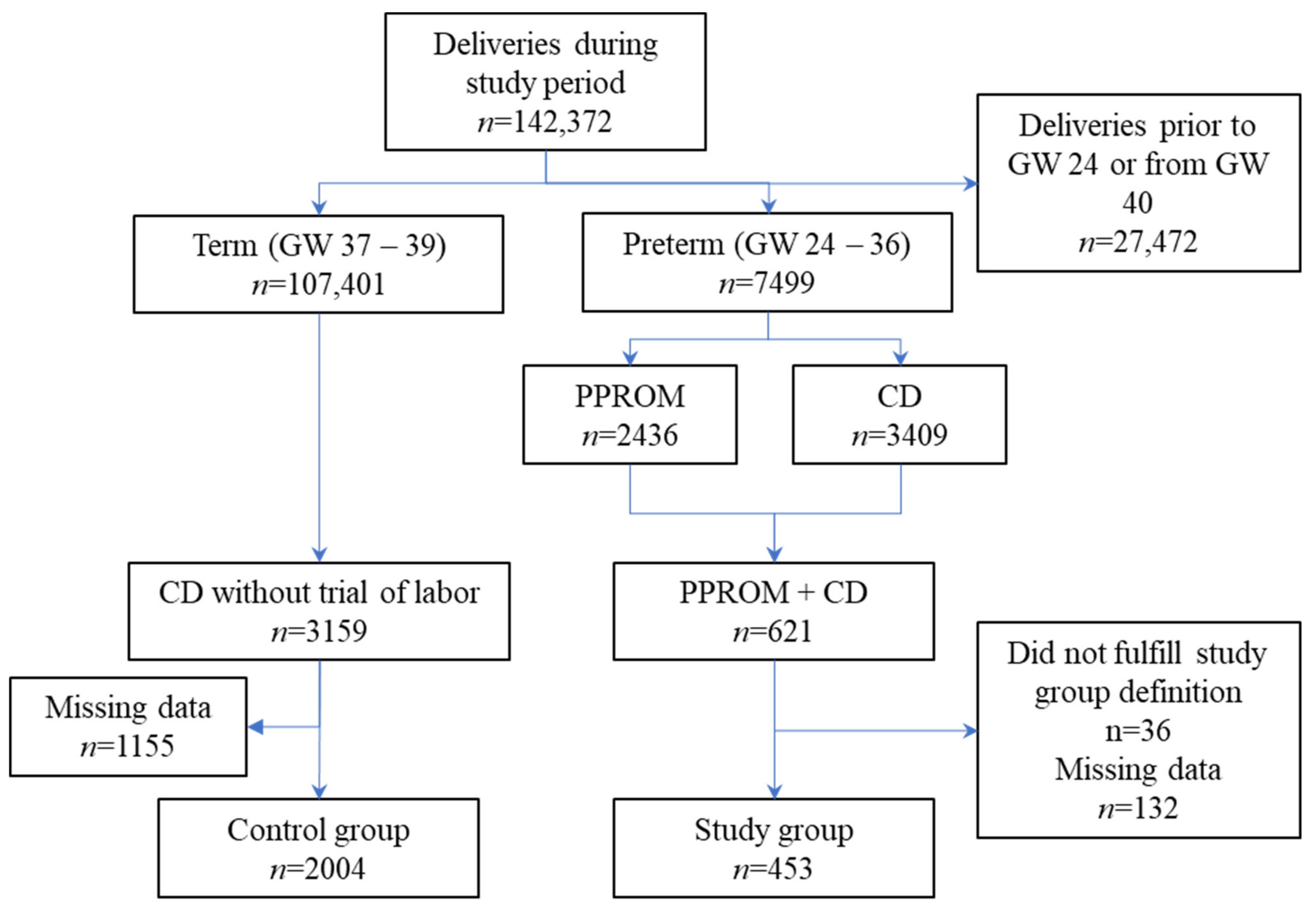
2. Materials and Methods
3. Results
3.1. Assessment of Reporting Bias
3.2. Description of the Study Population
3.3. Comparison between Study and Control Groups—Vital Signs and Complete Blood Count
3.4. Rates of Vital Sign Documentation
3.5. Comparison between Study and Control Groups—Fulfillment of SIRS and qSOFA Criteria
3.6. Comparison between Study and Control Groups—Rate of Diagnosis of Intrauterine Infection
3.7. Comparison between Study and Control Groups—Cultures
3.7.1. Sampling Rates
3.7.2. Culture Results
3.7.3. Sensitivity Analysis—Urinary Cultures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levy, M.M.; Evans, L.E.; Rhodes, A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018, 44, 925–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, M.M.; Dellinger, R.P.; Townsend, S.R.; Linde-Zwirble, W.T.; Marshall, J.C.; Bion, J.; Schorr, C.; Artigas, A.; Ramsay, G.; Beale, R.; et al. The Surviving Sepsis Campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010, 36, 222–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creanga, A.A. Maternal Mortality in the United States: A Review of Contemporary Data and Their Limitations. Clin. Obs. Gynecol. 2018, 61, 296–306. [Google Scholar] [CrossRef]
- Creanga, A.A.; Syverson, C.; Seed, K.; Callaghan, W.M. Pregnancy-Related Mortality in the United States, 2011–2013. Obs. Gynecol. 2017, 130, 366–373. [Google Scholar] [CrossRef]
- Blagoeva Atanasova, V.; Arevalo-Serrano, J.; Antolin Alvarado, E.; Garcia-Tizon Larroca, S. Maternal mortality in Spain and its association with country of origin: Cross-sectional study during the period 1999–2015. BMC Public Health 2018, 18, 1171. [Google Scholar] [CrossRef]
- Singh, S.; McGlennan, A.; England, A.; Simons, R. A validation study of the CEMACH recommended modified early obstetric warning system (MEOWS). Anaesthesia 2012, 67, 12–18. [Google Scholar] [CrossRef]
- Mhyre, J.M.; D’Oria, R.; Hameed, A.B.; Lappen, J.R.; Holley, S.L.; Hunter, S.K.; Jones, R.L.; King, J.C.; D’Alton, M.E. The maternal early warning criteria: A proposal from the national partnership for maternal safety. Obs. Gynecol. 2014, 124, 782–786. [Google Scholar] [CrossRef] [Green Version]
- Isaacs, R.A.; Wee, M.Y.; Bick, D.E.; Beake, S.; Sheppard, Z.A.; Thomas, S.; Hundley, V.; Smith, G.B.; van Teijlingen, E.; Thomas, P.W.; et al. A national survey of obstetric early warning systems in the United Kingdom: Five years on. Anaesthesia 2014, 69, 687–692. [Google Scholar] [CrossRef]
- Balk, R.A.; Bone, R.C. The septic syndrome. Definition and clinical implications. Crit Care Clin. 1989, 5, 1–8. [Google Scholar]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Higgins, R.D.; Saade, G.; Polin, R.A.; Grobman, W.A.; Buhimschi, I.A.; Watterberg, K.; Silver, R.M.; Raju, T.N.K.; Chorioamnionitis Workshop, P. Evaluation and Management of Women and Newborns with a Maternal Diagnosis of Chorioamnionitis: Summary of a Workshop. Obs. Gynecol 2016, 127, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Arora, P.; Bagga, R.; Kalra, J.; Kumar, P.; Radhika, S.; Gautam, V. Mean gestation at delivery and histological chorioamnionitis correlates with early-onset neonatal sepsis following expectant management in pPROM. J. Obs. Gynaecol 2015, 35, 235–240. [Google Scholar] [CrossRef]
- Navathe, R.; Schoen, C.N.; Heidari, P.; Bachilova, S.; Ward, A.; Tepper, J.; Visintainer, P.; Hoffman, M.K.; Smith, S.; Berghella, V.; et al. Azithromycin vs erythromycin for the management of preterm premature rupture of membranes. Am. J. Obs. Gynecol. 2019, 221, 144.e141–144.e148. [Google Scholar] [CrossRef] [Green Version]
- Yudin, M.H.; van Schalkwyk, J.; Eyk, N.V.; Infectious Diseases, C.; Maternal Fetal Medicine, C. Antibiotic therapy in preterm premature rupture of the membranes. J. Obs. Gynaecol. Can. 2009, 31, 863–867. [Google Scholar] [CrossRef]
- Gibbs, R.S.; Duff, P. Progress in pathogenesis and management of clinical intraamniotic infection. Am. J. Obs. Gynecol. 1991, 164, 1317–1326. [Google Scholar] [CrossRef]
- Kamath, B.D.; Todd, J.K.; Glazner, J.E.; Lezotte, D.; Lynch, A.M. Neonatal outcomes after elective cesarean delivery. Obs. Gynecol. 2009, 113, 1231–1238. [Google Scholar] [CrossRef] [Green Version]
- Lappen, J.R.; Keene, M.; Lore, M.; Grobman, W.A.; Gossett, D.R. Existing models fail to predict sepsis in an obstetric population with intrauterine infection. Am. J. Obs. Gynecol. 2010, 203, P573.E1–573.E5. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bertozzi-Villa, A.; Coggeshall, M.S.; Shackelford, K.A.; Steiner, C.; Heuton, K.R.; Gonzalez-Medina, D.; Barber, R.; Huynh, C.; Dicker, D.; et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 980–1004. [Google Scholar] [CrossRef] [Green Version]
- Gaieski, D.F.; Mikkelsen, M.E.; Band, R.A.; Pines, J.M.; Massone, R.; Furia, F.F.; Shofer, F.S.; Goyal, M. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit. Care Med. 2010, 38, 1045–1053. [Google Scholar] [CrossRef]
- Kramer, H.M.; Schutte, J.M.; Zwart, J.J.; Schuitemaker, N.W.; Steegers, E.A.; van Roosmalen, J. Maternal mortality and severe morbidity from sepsis in the Netherlands. Acta Obs. Gynecol. Scand. 2009, 88, 647–653. [Google Scholar] [CrossRef]
- Bauer, M.E.; Bateman, B.T.; Bauer, S.T.; Shanks, A.M.; Mhyre, J.M. Maternal sepsis mortality and morbidity during hospitalization for delivery: Temporal trends and independent associations for severe sepsis. Anesth. Analg. 2013, 117, 944–950. [Google Scholar] [CrossRef]
- Karat, C.; Madhivanan, P.; Krupp, K.; Poornima, S.; Jayanthi, N.V.; Suguna, J.S.; Mathai, E. The clinical and microbiological correlates of premature rupture of membranes. Indian J. Med. Microbiol. 2006, 24, 283–285. [Google Scholar] [CrossRef]
- Horowitz, E.; Yogev, Y.; Ben-Haroush, A.; Samra, Z.; Feldberg, D.; Kaplan, B. Urine culture at removal of indwelling catheter after cesarean section. Int. J. Gynaecol. Obs. 2004, 85, 276–278. [Google Scholar] [CrossRef]
- Atacag, T.; Yayci, E.; Guler, T.; Suer, K.; Yayci, F.; Deren, S.; Cetin, A. Asymptomatic bacteriuria screened by catheterized samples at pregnancy term in women undergoing cesarean delivery. Clin. Exp. Obs. Gynecol. 2015, 42, 590–594. [Google Scholar]
- Nasr, A.M.; ElBigawy, A.F.; Abdelamid, A.E.; Al-Khulaidi, S.; Al-Inany, H.G.; Sayed, E.H. Evaluation of the use vs nonuse of urinary catheterization during cesarean delivery: A prospective, multicenter, randomized controlled trial. J. Perinatol. 2009, 29, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Bauer, M.E.; Bauer, S.T.; Rajala, B.; MacEachern, M.P.; Polley, L.S.; Childers, D.; Aronoff, D.M. Maternal physiologic parameters in relationship to systemic inflammatory response syndrome criteria: A systematic review and meta-analysis. Obs. Gynecol. 2014, 124, 535–541. [Google Scholar] [CrossRef]
- Mackintosh, N.; Watson, K.; Rance, S.; Sandall, J. Value of a modified early obstetric warning system (MEOWS) in managing maternal complications in the peripartum period: An ethnographic study. BMJ Qual. Saf. 2014, 23, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Sela, H.Y.; Weiniger, C.F.; Hersch, M.; Smueloff, A.; Laufer, N.; Einav, S. The pregnant motor vehicle accident casualty: Adherence to basic workup and admission guidelines. Ann. Surg. 2011, 254, 346–352. [Google Scholar] [CrossRef]
- Blessberger, H.; Kammler, J.; Domanovits, H.; Schlager, O.; Wildner, B.; Azar, D.; Schillinger, M.; Wiesbauer, F.; Steinwender, C. Perioperative beta-blockers for preventing surgery-related mortality and morbidity. Cochrane Database Syst. Rev. 2018, 3, CD004476. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, D.S.; Gould, J.B.; Profit, J. Perinatal Risk Factors and Outcome Coding in Clinical and Administrative Databases. Pediatrics 2019, 143, e20181487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greksova, K.; Parrak, V.; Chovancova, D.; Stencl, P.; Oravec, J.; Marsik, L.; Sysak, R.; Fuchs, D.; Peskova, Z.; Borovsky, M. Procalcitonin, neopterin and C-reactive protein in diagnostics of intrauterine infection and preterm delivery. Bratisl. Lek. Listy. 2009, 110, 623–626. [Google Scholar] [PubMed]
- Oh, K.J.; Park, K.H.; Kim, S.N.; Jeong, E.H.; Lee, S.Y.; Yoon, H.Y. Predictive value of intra-amniotic and serum markers for inflammatory lesions of preterm placenta. Placenta 2011, 32, 732–736. [Google Scholar] [CrossRef]
- Etyang, A.K.; Omuse, G.; Mukaindo, A.M.; Temmerman, M. Maternal inflammatory markers for chorioamnionitis in preterm prelabour rupture of membranes: A systematic review and meta-analysis of diagnostic test accuracy studies. Syst. Rev. 2020, 9, 141. [Google Scholar] [CrossRef]

| Characteristic | PPROM n = 453 (%) | Control n = 2004 (%) | p |
|---|---|---|---|
| Maternal age (mean ± CI) | 31.5 ± 6.7 | 33.8 ± 5.3 | 0.001 |
| >35 year | 127 (28.0) | 750 (37.4) | 0.001 |
| Member of minority | 70 (15.5) | 220 (11.0) | 0.001 |
| Completed secondary education | 411 (90.7) | 1934 (96.5) | 0.005 |
| Gestation number | 3.2 ± 2.5 | 3.9 ± 2.6 | 0.001 |
| Previous cesareans (mean ± CI) | 0.4 ± 0.8 | 1.1 ± 1.2 | 0.001 |
| Any previous cesarean | 121 (26.7) | 1077 (59.9) | 0.001 |
| Previous abortion (mean ± CI) | 0.7 ± 1.3 | 0.7 ± 1.1 | 0.531 |
| In vitro fertilization | 126 (27.8) | 319 (15.9) | 0.001 |
| Twin pregnancy | 160 (35.3) | 200 (10.0) | 0.001 |
| Gestational diabetes | 46 (10.2) | 269 (13.4) | 0.060 |
| Hypertension | 18 (4.0) | 69 (3.4) | 0.581 |
| Hypothyroidism | 29 (6.4) | 117 (5.8) | 0.647 |
| Characteristic | DAY | PPROM | n | Control | n | p |
|---|---|---|---|---|---|---|
| Temperature | Admission | 36.73 ± 0.4 | 453 | 36.68 ± 0.3 | 2004 | 0.005 |
| POD0 | 36.72 ± 0.5 | 365 | 36.46 ± 0.5 | 1104 | 0.001 | |
| POD1 | 36.82 ± 0.5 | 453 | 36.81 ± 0.4 | 2004 | 0.367 | |
| POD2 | 36.69 ± 0.4 | 453 | 36.65 ± 0.4 | 2004 | 0.147 | |
| Pulse | Admission | 92.7 ± 14.4 | 453 | 88.2 ± 11.4 | 2004 | 0.001 |
| POD0 | 84.8 ± 12.5 | 453 | 83.2 ± 10.8 | 2004 | 0.06 | |
| POD1 | 90.6 ± 11.5 | 453 | 88.8 ± 9.2 | 2004 | 0.029 | |
| POD2 | 88.9 ± 11 | 453 | 87.8 ± 9.6 | 2004 | 0.34 | |
| Mean arterial pressure | Admission | 90.3 ± 10.9 | 453 | 85.6 ± 9.3 | 2004 | 0.001 |
| POD0 | 76.8 ± 10.5 | 453 | 74.3 ± 9.5 | 2004 | 0.001 | |
| POD1 | 73.6 ± 9.5 | 453 | 70.8 ± 8.2 | 2004 | 0.001 | |
| POD2 | 78.2 ± 10 | 453 | 76.8 ± 9.1 | 2004 | 0.008 | |
| Leukocyte count (×103) | Admission | 10.3 ± 9.2 | 452 | 9.2 ± 2.2 | 1997 | 0.001 |
| POD0 | 13.4 ± 11.7 | 310 | 11.7 ± 3.3 | 1617 | 0.001 | |
| POD1 | 12.2 ± 11.4 | 187 | 11.4 ± 3 | 587 | 0.007 | |
| POD2 | 11.4 ± 9.7 | 56 | 9.7 ± 2.6 | 138 | 0.005 |
| Body Site | Cultures Obtained | Positive Cultures | ||||
|---|---|---|---|---|---|---|
| PPROM (n = 453) | Control (n = 2004) | p | PPROM (n = 453) | Control (n = 2004) | p | |
| Urine | 414 (91.4) | 2004 (100.0) | 0.001 | 25 (5.5) | 60 (3.0) | 0.008 |
| Cervix | 135 (29.8) | 38 (1.9) | 0.001 | 19 (4.2) | 4 (0.2) | 0.001 |
| Placenta | 124 (27.4) | 22 (1.1) | 0.001 | 33 (7.3) | 1 (0.0) | 0.001 |
| Blood | 34 (7.5) | 34 (1.7) | 0.001 | 4 (0.9) | 3 (0.1) | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sela, H.Y.; Seri, V.; Zimmerman, F.S.; Cortegiani, A.; Levin, P.D.; Smueloff, A.; Einav, S. A Retrospective Study of the Proportion of Women at High and Low Risk of Intrauterine Infection Meeting Sepsis Criteria. Microorganisms 2022, 10, 82. https://doi.org/10.3390/microorganisms10010082
Sela HY, Seri V, Zimmerman FS, Cortegiani A, Levin PD, Smueloff A, Einav S. A Retrospective Study of the Proportion of Women at High and Low Risk of Intrauterine Infection Meeting Sepsis Criteria. Microorganisms. 2022; 10(1):82. https://doi.org/10.3390/microorganisms10010082
Chicago/Turabian StyleSela, Hen Y., Vered Seri, Frederic S. Zimmerman, Andrea Cortegiani, Philip D. Levin, Arnon Smueloff, and Sharon Einav. 2022. "A Retrospective Study of the Proportion of Women at High and Low Risk of Intrauterine Infection Meeting Sepsis Criteria" Microorganisms 10, no. 1: 82. https://doi.org/10.3390/microorganisms10010082
APA StyleSela, H. Y., Seri, V., Zimmerman, F. S., Cortegiani, A., Levin, P. D., Smueloff, A., & Einav, S. (2022). A Retrospective Study of the Proportion of Women at High and Low Risk of Intrauterine Infection Meeting Sepsis Criteria. Microorganisms, 10(1), 82. https://doi.org/10.3390/microorganisms10010082









