Bioinoculants—Natural Biological Resources for Sustainable Plant Production
Abstract
1. Introduction
2. Green Agriculture, a Paradigm Shift
3. Role of Bioinoculants in Green Agriculture
3.1. Nutrient Assimilation and Biofortification
3.2. Management of Pests and Pathogens
| Actinobacterial Species | Antibiotic | References |
|---|---|---|
| Streptomyces sp., S. alboniger, S. padanus | Alnumycin, coronamycins, fungichromin, goadsporin, kakadumycins, pamamycin- 607, rhodomycin | [215,216,217] |
| Micromonosporacarbonacea | Everninomicin | [218] |
| Actinoplanesianthinogenes N. sp. | Purpuromycin | [219] |
| Micromonospora inyoensis | Sisomicin | [220] |
| Actinoplanes | Lipiarmycin | [221] |
| Actinomadurasp. | Cationomycin, chandrananimycins, oxanthromicin | [222,223] |
| Actinoplanesteichomyceticus | Teichomycins, teicoplanin | [224,225] |
| Micromonospora echinospora sub-sp. Armeniaca sub-sp. nov. | Clostomicins | [226] |
| A. utahensis | Echinocandin | [227] |
| Actinomaduras piralis | Pyralomicins | [228] |
| Microbispora sp. | Cochinmicins, glucosylquestiomycin | [229,230] |
| Micromonospora lomaivitiensis | Lomaiviticins A and B | [231] |
| Actinoplanesfriuliensis sp. nov. II. | Friulimicins | [232] |
| Microbisporaaerata | Microbiaeratin | [233] |
| Nocardia sp. I. | Nocathiacins | [234] |
| Nocardiamediterranei subsp. kanglensis | Chemomicin A | [235] |
| Nocardiopsis | New thiopeptide antibiotic | [236] |
3.3. Abiotic Stress Management
4. Microbe Based Inoculant
- ○
- Financial gain,
- ○
- Natural wellbeing,
- ○
- Social and monetary value,
- ○
- Protection of condition,
- ○
- Productive utilization of non-renewable assets.
4.1. Bacteria
4.1.1. Azotobactor
4.1.2. Rhizobium
4.1.3. Azospirillum
4.2. Fungi
- ○
- Side effect-based recognition of contagious pathogens,
- ○
- Location of non-indicative pathogens and inactive or tranquil symptoms causing growths,
- ○
- Confirmation or recognizable proof of parasitic pathogens utilizing fitting apparatuses.
4.3. Endophytes
- ○
- The natural endophytic network that ought to contain obstruction skilled attributes, and
- ○
- Restoring of intrinsic endophytic bacterial subpopulations by an approaching bacterium (e.g., a biocontrol operator) [364].
4.3.1. Bacterial Endophytes
4.3.2. Fungal Endocytes
4.4. Effective Microorganisms
5. Next Generation Bioinoculants
Constraint-Based Modelling
6. Future Scope of Bioinoculants in Green Agriculture
- ○
- Extensive exploration for screening multifunctional and predictable microbial strains, which can be utilized in various rhizospheres;
- ○
- Unravelling the benefits of plant-microorganism collaborations and endeavours to make it progressively useful;
- ○
- Evaluation of valuable microbial strains for equipotential under biotic and abiotic stresses;
- ○
- Monitoring of inoculant for endurance and dispersal in rewarded soil for guaranteed execution;
- ○
- Regulated and careful utilization of biofertilizers as far as creation quality and application.
6.1. Improved Root Colonization Ability
6.2. Improvement in Synthesis of Lytic Enzymes
6.3. Improvement in Antibiotic Synthesis
6.4. Improvement in the Synthesis of the Siderophore
6.5. Improvement in Synthesis in Bacteriocins
6.6. Developing Hypovirulence by Hereditary Modification
6.7. Resistance Development against Fungicides by Hereditary Modification
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations. Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights (ST/ESA/SER.A/423). 2019. Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf (accessed on 7 July 2020).
- FAO. Plant Health and Food Security. International Plant Protection Convention, Rome, Italy. 2017. Available online: http://www.fao.org/3/a-i7829e.pdf (accessed on 4 August 2020).
- Lichtfouse, E.; Navarrete, M.; Debaeke, P.; Souchère, V.; Alberola, C.; Ménassieu, J. Agronomy for Sustainable Agriculture, A Review. Agron. Sustain. Dev. 2009, 29, 1–6. [Google Scholar] [CrossRef]
- Maitra, S.; Pine, S. Smart Irrigation for Food Security and Agricultural Sustainability. Ind. J. Nat. Sci. 2020, 10, 20435–20439. [Google Scholar]
- John, D.A.; Babu, G.R. Lessons from the Aftermaths of Green Revolution on Food System and Health. Front. Sustain. Food Syst. 2021, 5, 644559. [Google Scholar] [CrossRef] [PubMed]
- Scherr, S.J.; McNeely, J.A. Biodiversity Conservation and Agricultural Sustainability: Towards a new Paradigm of ‘Ecoagriculture’ Landscapes. Philos. Trans. Royal. Soc. B 2008, 363, 477–494. [Google Scholar] [CrossRef]
- WCED (World Commission on Environment and Development). Our Common Future; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Arora, N.K.; Fatima, T.; Mishra, I.; Verma, M.; Mishra, J.; Mishra, V. Environmental Sustainability: Challenges and Viable Solutions. Environ. Sustain. 2018, 1, 309–350. [Google Scholar] [CrossRef]
- Akinsemolu, A.A. The Role of Microorganisms in Achieving the Sustainable Development Goals. J. Clean Prod. 2018, 182, 139–155. [Google Scholar] [CrossRef]
- Committee on World Food Security. Coming to Terms with Terminology: Food Security, Nutrition Security, Food Security and Nutrition, Food and Nutrition Security. 2012. Available online: http://www.fao.org/fsnforum/sites/default/files/file/Terminology/MD776(CFS_Coming_to_terms_with_Terminology).pdf (accessed on 10 June 2020).
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous Oxide Emissions from Soils: How well do we understand the Processes and their Controls? Philos. Trans. R. Soc. Lond. Biol. Sci. 2013, 5, 368. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Ahammed, G.J. Combined Inoculation with Multiple Arbuscular mycorrhizal Fungi Improves Growth, Nutrient Uptake and Photosynthesis in Cucumber Seedlings. Front. Microbiol. 2017, 8, 2516. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Boddey, R.M.; Gresshoff, P.M.; Hauggaard-Nielsen, H.; Alves, B.J.; Morrison, M.J. Legumes for Mitigation of Climate Change and the Provision of Feedstock for Biofuels and Biorefineries. A Review. Agron. Sustain. Dev. 2012, 32, 329–364. [Google Scholar] [CrossRef]
- Maitra, S.; Hossain, A.; Brestic, M.; Skalicky, M.; Ondrisik, P.; Gitari, H.; Brahmachari, K.; Shankar, T.; Bhadra, P.; Palai, J.B.; et al. Intercropping–A Low Input Agricultural Strategy for Food and Environmental Security. Agronomy 2021, 11, 343. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, M.; Anand, K.; Saurabh, S.; Kaur, T.; Kour, D.; Yadav, A.N.; Kumar, M. Role and Potential Applications of Plant Growth-Promoting Rhizobacteria for Sustainable Agriculture. In Trends of Microbial Biotechnology for Sustainable Agriculture and Biomedicine Systems: Diversity and Functional Perspectives; Elsevier: Amsterdam, The Netherlands, 2020; pp. 49–60. [Google Scholar] [CrossRef]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Agriculture. In Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Metz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; p. 497. [Google Scholar]
- Morrissey, J.P.; Dow, J.M.; Mark, G.L.; O’Gara, F. Are Microbes at the Root of a Solution to World Food Production? Rational Exploitation of Interactions Between Microbes and Plants Can Help to Transform Agriculture. EMBO Rep. 2004, 5, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Bacteria and ACC Deaminase can Promote Plant Growth and Help to Feed the World. Microbial. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Olubukola, O.; Babalola, O.; Glick, B.R. The use of Microbial Inoculants in African agriculture. Food Agric. Environ. 2012, 10, 540–549. [Google Scholar]
- Lugtenberg, B. Life of Microbes in the Rhizosphere. In Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Kang, S.M.; Khan, A.L.; Waqas, M.; You, Y.H.; Kim, J.H.; Kim, J.G.; Hamayun, M.; Lee, I.J. Plant Growth Promoting Rhizobacteria Reduce Adverse Effects of Salinity and Osmotic Stress by Regulating Phytohormones and Antioxidants in Cucumis Sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Wang, C.; Yang, W.; Wang, C.; Gu, C.; Niu, D.; Liu, H. Induction of Drought Tolerance in Cucumber Plants by a Consortium of Three Plant Growth promoting Rhizobacterium Strains. PLoS ONE 2012, 7, e52565. [Google Scholar] [CrossRef]
- Harika, J.V.; Maitra, S.; Shankar, T.; Bera, M.; Manasa, P. Effect of Integrated Nutrient Management on Productivity, Nutrient Uptake and Economics of Finger Millet (Eleusine coracana L. Gaertn). Int. J. Agric. Environ. Biotech. 2019, 12, 273–279. [Google Scholar] [CrossRef]
- Scagliola, M.; Valentinuzzi, F.; Mimmo, T.; Cesco, S.; Crecchio, C.; Pii, Y. Bioinoculants as Promising Complement of Chemical Fertilizers for a More Sustainable Agricultural Practice. Front. Sustain. Food Syst. 2021, 4, 622169. [Google Scholar] [CrossRef]
- Armada, E.; Portela, G.; Roldán, A.; Azcón, R. Combined Use of Beneficial Soil Microorganism and Agrowaste Residue to Cope with Plant Water Limitation Under Semiarid Conditions. Geoderma 2014, 232–234, 640–648. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications; Hindawi Publishing Corporation Scientifica: London, UK, 2012. [Google Scholar] [CrossRef]
- Mustafa, S.; Kabir, S.; Shabbir, U.; Batool, R. Plant Growth Promoting Rhizobacteria in Sustainable Agriculture: From Theoretical to Pragmatic Approach. Symbiosis 2019, 78, 115–123. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Maji, D.; Chanotiya, C.S.; Kalra, A. 1-Aminocyclopropane-1-carboxylic acid (ACC) Deaminase Containing Rhizobacteria Protect Ocimum Sanctum Plants during Water Logging Stress via Reduced Ethylene Generation. Plant Physiol. Biochem. 2012, 58, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.Z.; Sandhya, V.; Rao, L.V. Isolation and Characterization of Drought Tolerant Acc Deaminase and Exopolysaccharide Producing Fl Uorescent Pseudomonas spp. Ann. Microbiol. 2014, 64, 493–502. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clement, C.; Barka, E.A. Use of Plant Growth Promoting Bacteria for Biocontrol of Diseases: Principles, Mechanisms of Action and Future Prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Herzner, A.M.; Dischinger, J.; Szekat, C.; Josten, M.; Schmitz, S.; Yakéléba, A.; Reinartz, R.; Jansen, A.; Sahl, H.G.; Piel, J.; et al. Expression of the Lantibiotic Mersacidin in Bacillus Amyloliquefaciens FZB42. PLoS ONE 2011, 6, e22389. [Google Scholar] [CrossRef]
- Raza, W.; Yuan, J.; Ling, N.; Huang, Q.; Shen, Q. Production of Volatile Organic Compounds by an Antagonistic Strain Paenibacillus Polymyxa WR-2 in the Presence of Root Exudates and Organic Fertilizer and their Antifungal Activity Against Fusarium oxysporum f. sp. niveum. Biol. Control 2015, 80, 89–95. [Google Scholar] [CrossRef]
- Hartman, W.H.; Richardson, C.J. Differential Nutrient Limitation of Soil Microbial Biomass and Metabolic Quotients (Qco2): Is There a Biological Stoichiometry of Soil Microbes. PLoS ONE 2013, 8, e57127. [Google Scholar] [CrossRef] [PubMed]
- Baez-Rogelio, A.; Morales-García, Y.E.; Quintero-Hernández, V.; Muñoz-Rojas, J. Next Generation of Microbial Inoculants for Agriculture and Bioremediation. Microb. Biotechnol. 2017, 10, 19–21. [Google Scholar] [CrossRef]
- Nobbe, F.; Hiltner, L. Inoculation of the Soil for Cultivating Leguminous Plants. US. Patent 1896,570,813, 9 August 1895. [Google Scholar]
- Prashar, P.; Shah, S. Impact of fertilizers and pesticides on soil microflora in agriculture. In Bambara Groundnut for Food Security in the Changing African Climate; Lichtfouse, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 19, pp. 331–362. [Google Scholar]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium Solubilizing Bacteria (KSB): Mechanisms, Promotion of Plant Growth, and Future Prospects—A Review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Adams, D.G.; Duggan, P.S. Cyanobacteria-bryophytes Symbioses. J. Exp. Bot. 2008, 59, 1047–1058. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Dubey, R.K.; Tripathi, V.; Gupta, V.K.; Singh, H.B. Plant Growth-Promoting Microorganisms for Environmental Sustainability. Trends Biotechnol. 2016, 34, 847–850. [Google Scholar] [CrossRef]
- Batra, P.; Barkodia, M.; Ahlawat, U.; Sansanwal, R.; Wati, L. Effect of Compatible and Incompatible Endophytic Bacteria on Growth of Chickpea Plant. Def. Life Sci. J. 2020, 5, 45–48. [Google Scholar] [CrossRef]
- Gray, E.J.; Smith, D.L. Intracellular and Extracellular Pgpr: Commonalities and Distinctions in the Plant-Bacterium Signaling Processes. Soil. Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for Beneficial Microorganisms Inocula Used as Biofertilizers. Sci. World J. 2012, 491206. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J.A. Use of Commercial Bio-Inoculants to Increase Agricultural Production through Improved Phosphrous Acquisition. Appl. Soil. Ecol. 2015, 86, 41–54. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial Inoculants: Reviewing the past, Discussing the Present and Previewing an Outstanding Future for the use of Benefcial Bacteria in Agriculture. AMB Exp. 2019, 9, 205. [Google Scholar] [CrossRef]
- Koohafkan, P.; Altieri, M.A.; Gimenez, E.H. Green Agriculture: Foundations For Biodiverse, Resilient and Productive Agricultural Systems. Int. J. Agric. Sust. 2012, 10, 61–75. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. Biodiversity and Pest Management in Agroecosystems; Haworth Press: New York, NY, USA, 2004. [Google Scholar]
- Uphoff, N. Agroecological Innovations: Increasing Food Production with Participatory Development; Earthscan: London, UK, 2002. [Google Scholar]
- Toledo, V.M.; Barrera-Bassals, N. La Memoria Biocultural: La Importancia Ecologica de las Sabidurias Tradicionales; ICARIA Editorial: Barcelona, Spain, 2009. [Google Scholar]
- Pretty, J.; Sutherland, W.J.; Ashby, J.; Auburn, J.; Baulcombe, D.; Bell, M.; Bentley, J.; Bickersteth, S.; Brown, K.; Burke, J.; et al. The top 100 Questions of Importance to the Future of Global Agriculture. Int. J. Agric. Sust. 2011, 9, 1–20. [Google Scholar] [CrossRef]
- Herren, H.R.; Bassi, A.M.; Tan, Z.; Binns, W.P. Green Jobs for a Revitalized Food and Agriculture Sector; Natural Resources Management and Environment Department Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; p. 4. [Google Scholar]
- Lovo, S.; Bezabih, M.; Singer, G. Green Agricultural Policies and Poverty Reduction, Policy Brief; The Grantham Research Institute on Climate Change and the Environment, London and Global Green Growth Institute: Seoul, Korea, 2015; p. 24. Available online: https://gregorsinger.com/files/papers/GRI_LSE-Agriculture-GGGI-policy.pdf (accessed on 30 August 2021).
- Berg, G.; Rybakova, D.; Grube, M.; Koberl, M. The Plant Microbiome Explored: Implications for Experimental Botany. J. Exp. Bot. 2016, 67, 995–1002. [Google Scholar] [CrossRef]
- Smith, D.L.; Gravel, V.; Yergeau, E. Editorial: Signaling in the Phytomicrobiome. Front. Plant Sci. 2017, 8, 611. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Turner, T.R.; James, E.K.; Poole, P.S. The Plant Microbiome. Genome. Biol. 2013, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Lebeis, S.L. The Potential for Give and Take in Plant-Microbiome Relationships. Front. Plant Sci. 2014, 5, 287. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Subramanian, S.; Lamont, J.R.; Bywater-Ekegard, M. Signaling in the phytomicrobiome: Breadth and potential. Front. Plant Sci. 2015, 6, 709. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, D.; Mhamdi, R. Microbial Inoculants and their Impact on Soil Microbial Communities: A Review. Biomed. Res. Int. 2013, 863240. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.S.; Sadowsky, M.J. Secretion Systems and Signal Exchange Between Nitrogen-Fixing Rhizobia and Legumes. Front. Plant Sci. 2015, 6, 491. [Google Scholar] [CrossRef]
- Leach, J.E.; Triplett, L.R.; Argueso, C.T.; Trivedi, P. Communication in the Phytobiome. Cell 2017, 169, 587–596. [Google Scholar] [CrossRef]
- Massalha, H.; Korenblum, E.; Tholl, D.; Aharoni, A. Small Molecules Below-Ground: The Role of Specialized Metabolites in the Rhizosphere. Plant J. 2017, 90, 788–807. [Google Scholar] [CrossRef]
- Uchida, R. Essential Nutrients for Plant Growth: Nutrient Functions and Deficiency Symptoms, Chapter 3. In Plant Nutrient Management in Hawaii’s Soils, Approaches for Tropical and Subtropical Agriculture; Silva, J.A., Uchida, R., Eds.; College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa: Honolulu, HI, USA, 2000; pp. 31–55. Available online: https://www.ctahr.hawaii.edu/oc/freepubs/pdf/pnm3.pdf (accessed on 13 July 2020).
- White, P.J.; Brown, P.H. Plant Nutrition for Sustainable Development and Global Health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef]
- Polacco, J.C. Nitrogen Metabolism in Soybean Tissue Culture: II. Urea Utilization and Urease Synthesis Require Ni. Plant Physiol. 1977, 59, 827–830. [Google Scholar] [CrossRef]
- Eskew, D.L.; Welch, R.M.; Cary, E.E. Nickel: An Essential Micronutrient for Legumes and Possibly all Higher Plants. Science 1983, 222, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Gerendás, J.; Sattelmacher, B. Influence of Ni Supply on Growth and Nitrogen Metabolism of Brassica Napus L. Grown with NH4NO3 or Urea As N Source. Ann. Bot. 1999, 83, 65–71. [Google Scholar] [CrossRef]
- Fabiano, C.C.; Tezotto, T.; Favarin, J.L.; Polacco, J.C.; Mazzafera, P. Essentiality of Nickel in Plants: A Role in Plant Stresses. Front. Plant Sci. 2015, 6, 754. [Google Scholar] [CrossRef]
- Grusak, M.A.; Broadley, M.R.; White, P.J. Plant Macro- and Micronutrient Minerals; John Wiley & Sons, Ltd.: Chichester, UK, 2016. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and Fungi can contribute to Nutrients Bioavailability and Aggregate Formation in Degraded Soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Vessey, J.K. Plant Growth Promoting Rhizobacteria as Biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Stamenković, S.; Beškoski, V.; Karabegović, I.; Lazić, M.; Nikolić, N. Microbial fertilizers: A Comprehensive Review of Current Findings and Future Perspectives. Span. J. Agric. Res. 2018, 16, e09R01. [Google Scholar] [CrossRef]
- Patil, M.G.; Sayyed, R.Z.; Chaudhari, A.B.; Chincholkar, S.B. Phosphate Solubilizing Microbes: A Potential Bioinoculant for Efficient use of Phosphate Fertilizers. Bioinoculants for Sustainable Agriculture and Forestry. Sci. Pub. 2002, l107, 118. [Google Scholar]
- Dey, R.; Pal, K.K.; Bhatt, D.M.; Chauhan, S.M. Growth Promotion and Yield Enhancement of Peanut (Arachis Hypogaea L) by Application of Plant Growth Promoting Rhizobacteria. Microbiol. Res. 2004, 159, 371–394. [Google Scholar] [CrossRef]
- Ponmurugan, P.; Gopi, C. Distribution Pattern and Screening of Phosphate Solubilizing Bacteria Isolated from Different Food and Forage Crops. J. Agron. 2006, 5, 600–604. [Google Scholar]
- Afzal, A.; Asghari, B. Rhizobium and Phosphate Solubilizing Bacteria Improve the Yield and Phosphorus Uptake in Wheat. (Triticum aestivum L.). Int. J. Agric. Biotec. 2008, 10, 85–88. [Google Scholar]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil Beneficial Bacteria and Their Role in Plant Growth Promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Kammar, S.C.; Gundappagol, R.C.; Santhosh, G.P.; Shubha, S.; Ravi, M.V. Influence of Potassium Solubilizing Bacteria on Growth and Yield of Sunflower (Helianthus annuus L.). Environ. Ecol. 2016, 34, 33–37. [Google Scholar]
- Korir, H.; Mungai, N.W.; Thuita, M.; Hamba, Y.; Masso, C. Co-inoculation Effect of Rhizobia and Plant Growth Promoting Rhizobacteria on Common Bean Growth in a Low Phosphorus Soil. Front. Plant. Sci. 2017, 8, 141. [Google Scholar] [CrossRef]
- Leggett, M.; Diaz-Zorita, M.; Koivunen, M.; Bowman, R.; Pesek, R.; Stevenson, C.; Leister, T. Soybean Response Ti Inoculation with Bradyrhizobium Japonicum in the Unites States and Argentina. Agron. J. 2017, 109, 1031–1038. [Google Scholar] [CrossRef]
- Rosa, P.A.L.; Mortinho, E.S.; Jalal, A.; Galindo, F.S.; Buzetti, S.; Fernandes, G.C.; Barco Neto, M.; Pavinato, P.S.; Teixeira Filho, M.C.M. Inoculation With Growth-Promoting Bacteria Associated With the Reduction of Phosphate Fertilization in Sugarcane. Front. Environ. Sci. 2020, 8, 32. [Google Scholar] [CrossRef]
- Bourion, V.K.; Heulin-Gotty, V.; Aubert, P.; Tisseyre, M.; Chabert-Martinello, M.; Pervent, C.; Delaitre, D.; Vile, M.; Siol, G.; Duc, B.; et al. Co-Inoculation of a Pea Core-Collection with Diverse Rhizobial Strains Shows Competitiveness for Nodulation and Efficiency of Nitrogen Fixation are Distinct Traits in the Interaction. Front. Plant Sci. 2018, 8, 2249. [Google Scholar] [CrossRef]
- Galindo, F.S.; Filho, M.C.M.T.; Buzetti, S.; Pagliari, P.H.; Santini, J.M.K.; Alves, C.J.; Megda, M.M.; Nogueira, T.A.R.; Reotti, M.; Arf, O. Maize Yield Response to Nitrogen Rates and Sources Associated with Azospirillum Brasilense. Agron. J. 2019, 111, 1985–1997. [Google Scholar] [CrossRef]
- Galindo, F.S.; Rodrigues, W.L.; Biagini, A.L.C.; Fernandes, G.C.; Baratella, E.B.; Junior, C.A.d.; Buzetti, S.; Filho, M.C.M. Assessing Forms of Application of Azospirillum Brasilense Associated With Silicon Use on Wheat. Agronomy 2019, 9, 678. [Google Scholar] [CrossRef]
- Zeffa, D.M.; Perini, L.J.; Silva, M.B.; de Sousa, N.V.; Scapim, C.A.; Oliveira, A.L.M.D.; Amaral Júnior, A.T.D.; Azeredo Goncalves, L.S. Azospirillum Brasilense Promotes Increases in Growth and Nitrogen Use Efficiency of Maize Genotypes. PLoS ONE 2019, 14, e0215332. [Google Scholar] [CrossRef]
- Zeffa, D.M.; Fantin, L.H.; Koltun, A.; de Oliveira, A.L.; Nunes, M.P.; Canteri, M.G.; Gonçalves, L.S. Effects of Plant Growth-Promoting Rhizobacteria on Co-Inoculation with Bradyrhizobium in Soybean Crop: A Meta-Analysis of Studies from 1987 to 2018. PeerJ 2020, 8, e7905. [Google Scholar] [CrossRef]
- Bouguyon, E.; Brun, F.; Meynard, D.; Kubeš, M.; Pervent, M.; Leran, S.; Lacombe, B.; Krouk, G.; Guiderdoni, E.; Zažímalová, E.; et al. Multiple Mechanisms of Nitrate Sensing by Arabidopsis Nitrate Transceptor NRT1.1. Nat. Plants 2015, 1, 15015. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Morrison, E.; Chanton, J.P.; Ogram, A. Methanogens are Major Contributors to Nitrogen Fixation in Soils of the Florida Everglades. Appl. Environ. Microbiol. 2018, 84, 7. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, R.; Nigam, V.; Mohan, M.; Sasmal, D.; Ghosh, P. Screening of Nitrogen Fixers From Rhizospehric Bacterial Isolates Associated with Important Desert Plants. Appl. Ecol. Environ. Res. 2009, 6, 101–109. [Google Scholar] [CrossRef]
- Kuan, K.B.; Othman, R.; Abdul Rahim, K.; Shamsuddin, Z.H. Plant Growth-Promoting Rhizobacteria Inoculation to Enhance Vegetative Growth, Nitrogen Fixation and Nitrogen Remobilisation of Maize Under Greenhouse Conditions. PLoS ONE 2016, 11, e0152478. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of Plant Growth Promoting Rhizobacteria for Sustainable Development in Agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Sulieman, S.; Tran, L.S.P. Symbiotic nitrogen fixation in legume nodules: Metabolism and regulatory mechanisms. Int. J. Mol. Sci. 2014, 15, 19389–19393. [Google Scholar] [CrossRef] [PubMed]
- Mus, F.; Crook, M.B.; Garcia, K.; Garcia Costas, A.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.H.; Oldroyd, G.E.; Poole, P.S.; et al. Symbiotic Nitrogen Fixation and the Challenges to its Extension to Nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef]
- Oke, V.; Long, S.R. Bacteroid formation in the Rhizobium–legume symbiosis. Curr. Opin. Microbiol. 1999, 2, 641–646. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.A. Future of Bio-Fertilizers in Indian Agriculture: An Overview. Int. J. Agric. Food Res. 2014, 3, 1–23. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global Inputs of Biological Nitrogen Fixation in Agricultural Systems. Plant Soil. 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Maróti, G.; Kondorosi, E. Nitrogen-Fixing Rhizobium-Legume Symbiosis: Are Polyploidy and Host Peptide-Governed Symbiont Differentiation General Principles of Endosymbiosis. Front. Microbiol. 2014, 5, 326. [Google Scholar] [PubMed]
- Saikia, S.P.; Jain, V.; Srivastava, G.C. Nitrogen Fixation in Nodules of Maize (Zea mays) Roots by Introduced Free-Living Diazo-Troph. Indian J. Agric. Sci. 2004, 74, 213–214. [Google Scholar]
- Dawson, T.L. It must be Green: Meeting Society’s Environmental Concerns. Color. Technol. 2008, 124, 67–78. [Google Scholar] [CrossRef]
- Santi, C.; Bogusz, D.; Franche, C. Biological Nitrogen Fixation in Non-Legume Plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Patten, C.L.; Holguin, G.; Penrose, D.M. Biochemical and Genetic Mechanisms Used by Plant Growth Promoting Bacteria; Imperial College Press: London, UK, 1999. [Google Scholar]
- Kloepper, J.W.; Lifshitz, R.; Zablotowicz, R.M. Free-Living Bacterial Inocula for Enhancing Crop Productivity. Trends Technol. 1989, 7, 39–44. [Google Scholar] [CrossRef]
- Brady, N.C.; Well, R.R. The Nature and Properties of Soils, 13th ed.; Pearson Education Pvt. Ltd.: Bengaluru, India, 2002; p. 735. [Google Scholar]
- Wang, Y.; Yang, Z.; Kong, Y.; Li, X.; Li, W.; Du, H.; Zhang, C. GmPAP12 Is Required for Nodule Development and Nitrogen Fixation under Phosphorus Starvation in Soybean. Front. Plant Sci. 2020, 11, 450. [Google Scholar] [CrossRef]
- Taliman, N.A.; Dong, Q.; Echigo, K.; Raboy, V.; Saneoka, H. Effect of Phosphorus Fertilization on the Growth, Photosynthesis, Nitrogen Fixation, Mineral Accumulation, Seed Yield, and Seed Quality of a Soybean Low-Phytate Line. Plants 2019, 8, 119. [Google Scholar] [CrossRef]
- Kadam, D.V.; Indulkar, B.S.; Kadam, L.S.; Jadhav, V.S.; Jadhav, P.N. Effect of Phosphorus and Zinc and Quality of Groundnut (Arachis hypogaea L.) in Inceptisol. Int. J. Pure App. Biosci. 2018, 6, 105–110. [Google Scholar]
- Ray, K.; Banerjee, H.; Dutta, S.; Hazra, A.K.; Majumdar, K. Macronutrients Influence Yield and Oil Quality of Hybrid Maize (Zea mays L.). PLoS ONE 2019, 14, e0216939. [Google Scholar] [CrossRef]
- Whitelaw, M.A. Growth Promotion of Plants Inoculated With Phosphate-Solubilizing Fungi. Adv. Agron. 2000, 69, 100–151. [Google Scholar]
- Rodriguez, H.; Fraga, R. Phosphate Solubilizing Bacteria and their Role in Plant Growth Promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Hajjam, Y.; Cherkaoui, S. The Influence of Phosphate Solubilizing Microorganisms on Symbiotic Nitrogen Fixation: Perspectives for Sustainable Agriculture. J. Mater. 2017, 8, 801–808. [Google Scholar]
- Dhull, S.; Gera, R.; Singh, H.S.; Kakar, R. Phosphate Solubilization Activity of Rhizobial Strains Isolated from Root Nodule of Cluster Bean Plant Native to Indian Soils. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 255–266. [Google Scholar] [CrossRef]
- Sharon, J.A.; Hathwaik, L.T.; Glenn, G.M.; Imam, S.H.; Lee, C.C. Isolation of efficient phosphate solubilizing bacteria capable of enhancing tomato plant growth. J. Soil Sci. Plant Nut. 2016, 16, 525–536. [Google Scholar] [CrossRef]
- Subbarao, N.S. Phosphate solubilizing microorganisms. In Biofertilizers in Agriculture and Forestry; Regional Biofert Dev Centre: Hissar, India, 1998; pp. 133–142. [Google Scholar]
- Kucey, R.M.N.; Janzen, H.H.; Legget, M.E. Microbial Mediated Increases in Plant-Available Phosphorus. Adv. Agron. 1989, 42, 199–228. [Google Scholar]
- Sridevi, M.; Mallaiah, K.V. Phosphate solubilization by Rhizobium strains. Ind. J. Microbiol. 2009, 49, 98–102. [Google Scholar] [CrossRef]
- Anand, K.; Kumari, B.; Mallick, M.A. Phosphate Solubilizing Microbes: An Effective and Alternative Approach as Biofertilizers. Int. J. Pharm. Pharm. Sci. 2016, 8, 37–40. [Google Scholar]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate Solubilizing Microbes, Sustainable Approach for Managing Phosphorus Deficiency in Agricultural Soils. Springer Plus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Baykov, A.A.; Malinen, A.M.; Luoto, H.H.; Lahti, R. Pyrophosphate-Fueled Na+ and H+ Transport in Prokaryotes. Microbiol. Mol. Biol. Rev. 2013, 77, 267–276. [Google Scholar] [CrossRef]
- Yan, F.; Zhu, Y.; Muller, C.; Zörb, C.; Schubert, S. Adaptation of H+-Pumping and Plasma Membrane H+ Atpase Activity in Proteoid Roots of White Lupin Under Phosphate Deficiency. Plant Physiol. 2002, 129, 50–63. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Nacoon, S.; Jogloy, S.; Riddech, N.; Mongkolthanaruk, W.; Kuyper, T.; Boonlue, S. Interaction Between Phosphate Solubilizing Bacteria and Arbuscular Mycorrhizal Fungi on Growth Promotion and Tuber Inulin Content of Helianthus tuberosus L. Sci. Rep. 2020, 10, 4916. [Google Scholar] [CrossRef]
- Salam, E.A.; Alatar, A.; El-Sheikh, M.A. Inoculation with Arbuscular Mycorrhizal Fungi Alleviates Harmful Effects of Drought Stress on Damask Rose. Saudi. J. Biol. Sci. 2017, 25, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Nanjundappa, A.; Bagyaraj, D.J.; Saxena, A.K.; Kumar, M.; Chakdar, H. Interaction between Arbuscular Mycorrhizal Fungi and Bacillus Spp. in Soil Enhancing Growth of Crop Plants. Fungal Biol. Biotechnol. 2019, 6, 23. [Google Scholar] [CrossRef]
- Pal, D.; Sinha, S.N. Isolation and Characterization of Phosphate Solubilizing Bacterium Pseudomonas aeruginosa KUPSB12 with Antibacterial Potential from River Ganga, India. Ann. Agrarian. Sci. 2017, 15, 130–136. [Google Scholar] [CrossRef]
- Prajapati, K.; Modi, H.A. The importance of Potassium in Plant Growth—A Review. Indian J. Plant Sci. 2012, 1, 177–186. [Google Scholar]
- Cakmak, I. The Role of Potassium in Alleviating Detrimental Effects of Abiotic Stresses in Plants. J. Plant. Nutr. Soil. Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- O’Neill, S.D.; Spanswick, R.M. Characterization of Native and Reconstituted Plasma Membrane H+ -ATPase from the Plasma Membrane of Beta vulgaris. J. Mem. Biol. 1984, 79, 245–256. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, I.D.; Moumita, A.A.C.; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Bahadur, I. Potassium Solubilization by Bacterial Strain in Waste Mica. J. Bot. 2015, 43, 235–237. [Google Scholar] [CrossRef]
- Ruiz, J.L.; Salas, M.D.C. Evaluation of Organic Substrates and Microorganisms as Bio-Fertilisation Tool in Container Crop Production. Agronomy 2019, 9, 705. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P. Does a Rhizospheric Microorganism Enhance K+ Availability in Agricultural Soils? Microbiol. Res. 2014, 169, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.; Silva, L.R.; Ramirez-Bahena, M.H.; Peix, A. Diversity of Potassium-Solubilizing Microorganisms and Their Interactions with Plants. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 1–331. [Google Scholar]
- Haro, R.; Benito, B. The Role of Soil Fungi In K+ Plant Nutrition. Int. J. Mol. Sci. 2019, 20, 3169. [Google Scholar] [CrossRef]
- Rana, A.; Saharan, B.; Joshi, M. Identification of Multi-Trait PGPR Isolates and Evaluating Their Potential as Inoculants for Wheat. Ann. Microbiol. 2011, 61, 893–900. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishizawa, N.K. Iron Uptake, Translocation, and Regulation in Higher Plants. Ann. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef]
- Jin, C.W.; Ye, Y.Q.; Zheng, S.J. An Underground Tale: Contribution of Microbial Activity to Plant Iron Acquisition via Ecological Processes. Ann. Bot. 2014, 113, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Shenker, M.; Chen, Y. Increasing Iron Availability to Crops: Fertilizers, Organo-Fertilizers, and Biological Approaches. Soil Sci. Plant Nut. 2005, 51, 1–17. [Google Scholar] [CrossRef]
- Schalk, I.J.; Hannauer, M.; Braud, A. New roles for Bacterial Siderophores in Metal Transport and Tolerance. Environ. Microbiol. 2011, 13, 2844–2854. [Google Scholar] [CrossRef]
- Jin, C.W.; Li, G.X.; Yu, X.H.; Zheng, S.J. Plant Fe Status Affects the Composition of Siderophore-Secreting Microbes in the Rhizosphere. Ann. Bot. 2010, 105, 835–841. [Google Scholar] [CrossRef]
- Mishra, P.K.; Bisht, S.C.; Mishra, S.; Selvakumar, G.; Bisht, J.K.; Gupta, H.S. Co-inoculation of Rhizobium Leguminosarum-PR1 With a Cold Tolerant Pseudomonas sp. Improves Iron Acquisition, Nutrient Uptake and Growth of Field Pea (Pisum sativum L.). J. Plant Nutr. 2012, 35, 243–256. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Xie, X.; Kim, M.S.; Dowd, S.E.; Paré, P.W. A Soil Bacterium Regulates Plant Acquisition of Iron via Deficiency-Inducible Mechanisms. Plant J. 2009, 58, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Camares, O.; Veisseire, P. Effect of Zinc and Influence of Acremonium Lolii on Growth Parameters, Chlorophyll A Fluorescence and Antioxidant Enzyme Activities of Ryegrass (Lolium perenne L. cv Apollo). J. Exp. Bot. 2000, 51, 945–953. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in Plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Tsonev, T.; Lidon, F.J.C. Zinc in Plants—An Overview. Emir. J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- Disante, K.B.; Fuentes, D.; Cortina, J. Response to Drought of Zn-Stressed Quercus Suber L. Seedlings. Env. Exp. Bot. 2010, 70, 96–103. [Google Scholar] [CrossRef]
- Peck, A.W.; McDonald, G.K. Adequate Zinc Nutrition Alleviates the Adverse Effects of Heat Stress in Bread Wheat. Plant Soil. 2010, 337, 355–374. [Google Scholar] [CrossRef]
- Tavallali, V.; Rahemi, M.; Eshghi, S.; Kholdebarin, B.; Ramezanian, A. Zinc alleviates salt stress and increases antioxidant enzyme activity in the leaves of pistachio (Pistacia vera L. ‘Badami’) seedlings, Turk. J. Agric. Forest. 2010, 34, 349–359. [Google Scholar]
- López-Pazos, S.A.; Cortazar, J.E.; Cerón, J. Cry1B and Cry3A are Active against Hypothenemus Hampei Ferrari (coleoptera Scolytidae). J. Invertebr. Pathol. 2009, 101, 242–245. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological Functions of Mineral Micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl), Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar]
- Coleman, J.E. Zinc Proteins: Enzymes, Storage Proteins, Transcription Factors, and Replication Proteins. Annu. Rev. Biochem. 1992, 61, 897–946. [Google Scholar] [CrossRef]
- Iratkar, A.G.; Giri, J.D.; Kadam, M.M.; Giri, J.N.; Dabhade, M.B. Distribution of DTPA Extractable Micronutrients and their Relationship with Soil Properties in soil of Parsori Watershed of Nagpur District of Maharashtra. Asian J. Soil Sci. 2014, 9, 297–299. [Google Scholar]
- Yang, X.W.; Tian, X.H.; Lu, X.C.; Cao, Y.X.; Chen, Z.H. Impacts of Phosphorus and Zinc Levels on Phosphorus and Zinc Nutrition and Phytic Acid Concentration in Wheat (Triticum aestivum L.). J. Sci. Food Agri. 2011, 91, 2322–2328. [Google Scholar] [CrossRef]
- Rengel, Z. Availability of Mn, Zn and Fe in the Rhizosphere. J. Soil Sci. Plant Nutr. 2015, 15, 397–409. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc Solubilizing Bacillus Spp. Potential Candidates for Biofortification in Maize. Microbial. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.; Shahid, I.; Baig, D.N.; Rizwan, M.; Malik, K.A.; Mehnaz, S. Contribution of Zinc Solubilizing Bacteria in Growth Promotion and Zinc Content of Wheat. Front. Microbiol. 2017, 8, 2593. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Inoculation of Zinc Solubilizing Bacillus Aryabhattai Strains for Improved Growth, Mobilization and Biofortification of Zinc In Soybean And Wheat Cultivated In Vertisols Of Central India. Agric. Ecosyst. Environ. Appl. Soil Ecol. 2014, 73, 87–96. [Google Scholar] [CrossRef]
- Hussain, A.; Arshad, M.; Zahir, Z.A.; Asghar, M. Prospects of Zinc Solubilizing Bacteria for Enhancing Growth of Maize. Pak. J. Agric. Sci. 2015, 52, 915–922. [Google Scholar]
- Deepak, J.; Geeta, N.; Sachin, V.; Anita, S. Enhancement of Wheat Growth and Zn Content in Grains by Zinc Solubilizing Bacteria. Int. J. Agric. Environ. Biotechnol. 2013, 6, 363–370. [Google Scholar] [CrossRef]
- Naz, I.; Ahmad, H.; Khokhar, S.N.; Khan, K.; Shah, A.H. Impact of Zinc Solubilizing Bacteria on Zinc Contents of Wheat. Am. Euras. J. Agric. Environ. Sci. 2016, 16, 449–454. [Google Scholar] [CrossRef]
- Fasim, F.; Ahmed, N.; Parsons, R.; Gadd, G.M. Solubilization of Zinc Salts By A Bacterium Isolated from the Air Environment of a Tannery. FEMS. Microbiol. Lett. 2002, 213, 1–6. [Google Scholar] [CrossRef]
- Abaid-Ullah, M.; Nadeem, M.; Hassan, M.; Ganter, J.; Muhammad, B.; Nawaz, K.; Shah, A.S.; Hafeez, F.Y. Plant Growth Promoting Rhizobacteria: An Alternate Way to Improve Yield and Quality of Wheat (Triticum aestivum). Int. J. Agric. Biol. 2015, 17, 51–60. [Google Scholar]
- Saravanan, V.S.; Madhaiyan, M.; Thangaraju, M. Solubilization of Zinc Compounds by the Diazotrophic, Plant Growth Promoting Bacterium Gluconacetobacter diazotrophicus. Chemosphere 2007, 66, 1794–1798. [Google Scholar] [CrossRef]
- Saravanan, V.S.; Kumar, M.R.; Sa, T.M. Microbial Zinc Solubilization and Their Role on Plants, Chapter 3. In Bacteria in Agrobiology: Plant Nutrient Management; Maheshwari, D.K., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Pawar, A.; Ismail, S.; Mundhe, S.; Patil, V.D. Solubilization of Insoluble Zinc Compounds by Different Microbial Isolates in Vitro Condition. Int. J. Trop. Agric. 2015, 33, 865–869. [Google Scholar]
- Othman, N.M.I.; Othman, R.; Saud, H.M.; Wahab, P.E.M. Effects of Root Colonization by Zinc-Solubilizing Bacteria on Rice Plant (Oryza sativa MR219) Growth. Agric. Nat. Res. 2017, 51, 532–537. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- Xie, L.; Bi, Y.; Ma, S.; Shang, J.; Hu, Q.; Christie, P. Combined inoculation with dark septate endophytes and arbuscular mycorrhizal fungi: Synergistic or competitive growth effects on maize? BMC Plant Biol. 2021, 21, 498. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- .Ficke, A.; Cowger, C.; Bergstrom, G.; Brodal, G. Understanding Yield Loss and Pathogen Biology to Improvement Disease Management: Septoria Nodorum Blotch- A case study In Wheat. Plant. Dis. 2018, 102, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Wang, M.C.; Gong, M.; Zang, H.B.; Hua, X.M.; Yao, J.; Pang, Y.J.; Yang, Y.H. Effect of Methamidophos and Urea Application on Microbial Communities in Soils as Determined by Microbial Biomass and Community Level Physiological Profiles. J. Environ. Sci. Health B 2006, 41, 399–413. [Google Scholar] [CrossRef]
- Miller, G.T. Sustaining the Earth; Brooks/Cole: Monterey County, CA, USA, 2004; ISBN 9780534400880. [Google Scholar]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of Pesticides Use in Agriculture: Their Benefits and Hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bowmer, K.H. Ecosystem Effects from Nutrient and Pesticide Pollutants: Catchment Care as a Solution. Resources 2013, 2, 439–456. [Google Scholar] [CrossRef]
- Hussain, S.; Siddique, T.; Saleem, M.; Arshad, M.; Khalid, A. Impact of Pesticides on Soil Microbial Diversity, Enzymes, and Biochemical Reactions. Adv. Agron. 2009, 102, 159–200. [Google Scholar]
- Lo, C.C. Effect of Pesticides on Soil Microbial Community. J. Environ. Sci. Health. Part B 2010, 45, 348–359. [Google Scholar] [CrossRef]
- Chagnon, M.; Kreutzweiser, D.; Mitchell, E.A.; Morrissey, C.A.; Noome, D.A.; Van der Sluijs, J.P. Risks of Large-Scale Use of Systemic Insecticides to Ecosystem Functioning and Services. Environ. Sci. Pollut. Res. 2015, 22, 119–134. [Google Scholar] [CrossRef]
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.; Ferrer, A.; Peigné, J. Agroecological practices for sustainable agriculture. A review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef]
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; van Lanteren, J.C. The Status of Biological Control and Recommendations for Improving Uptake for the Future. Biol. Control 2018, 63, 155–167. [Google Scholar] [CrossRef]
- Wright, M.G.; Bennett, G.M. Evolution of Biological Control Agents Following Introduction to New Environments. Biol. Control 2017, 63, 105–116. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.; Lu, Y.; Morales, H.; Vazquez, L.L.; Legaspi, J.C.; Eliopoulos, P.A.; Hernandez, L.M. Current Status and Potential of Conservation Biological Control for Agriculture in the Developing World. Biol. Control 2013, 65, 152–167. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, I.; Verma, S. Microbe-based Inoculants: Role in Next Green Revolutio. In Environmental Concerns and Sustainable Development; Shukla, V., Kumar, N., Eds.; Springer Nature: Singapore, 2020; pp. 191–246. [Google Scholar] [CrossRef]
- Sulistyo, A.; Inayati, A. Mechanisms of Antixenosis, Antibiosis, and Tolerance of Fourteen Soybean Genotypes in Response to Whiteflies (Bemisia tabaci). Biodiversitas 2016, 17, 447–453. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Rossi, M.J.; Soares, C.R.; McConkey, B.J.; Glick, B.R. New Insights into 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase Phylogeny, Evolution and Ecological Significance. PLoS ONE 2014, 6, e99168. [Google Scholar] [CrossRef] [PubMed]
- Swarnalakshmi, K.; Senthilkumar, M.; Ramakrishnan, B. Endophytic Actinobacteria: Nitrogen Fixation, Phytohormone Production, and Antibiosis. In Plant Growth Promoting Actinobacteria; Subramaniam, G., Arumugam, S., Rajendran, V., Eds.; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Kaunat, H. Bidung Von Indolderivaten Durch Rhizospha Reenspezifisch Bakterien Und Aktinomyzeten. Zent. Bak. Abt. II 1969, 123, 501–515. [Google Scholar]
- Brown, M.E. Plant Growth Substances Produced by Micro Organisms of Soil and Rhizosphere. J. Appl. Bacteriol. 1972, 35, 443–451. [Google Scholar] [CrossRef]
- Wheeler, C.T.; Crozier, A.; Sandberg, G. The biosynthesis of Indole-3-Acetic Acid by Frankia. Plant Soil 1984, 78, 99–104. [Google Scholar] [CrossRef]
- Abd-Alla, M.H. Solubilization of rock phosphates by Rhizobium and Bradyrhizobium. Folia. Microbiol. 1994, 39, 53–56. [Google Scholar] [CrossRef]
- Mahadevan, B.; Crawford, D.L. Properties of the Chitinase of the Antifungal Biocontrol Agent Streptomyces Lydicus WYEC108. Enzym. Microb. Technol. 1997, 20, 489–493. [Google Scholar] [CrossRef]
- Tokala, R.K.; Strap, J.L.; Jung, C.M.; Crawford, D.L.; Salove, M.H.; Deobald, L.A.; Bailey, J.F.; Morra, M.J. Novel Plant-Microbe Rhizosphere Interaction Involving Streptomyces Lydicus WYEC108 and the pea plant (Pisums ativum). App. Environ. Microbiol. 2002, 68, 2161–2171. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Klimova, S.Y.; Cherdyntseva, T.A.; Netrusov, A.I. Microbial producers of Plant Growth-Stimulators and their Practical Use: A Review. App. Biochem. Microbiol. 2006, 42, 117–126. [Google Scholar] [CrossRef]
- El-Tarabily, K.A. Promotion of Tomato (Lycopersicon esculentum Mill.) Plant Growth by Rhizosphere Competent 1-Aminocyclopropane-1-Carboxylic Acid Deaminase-Producing Streptomyceteactino mycetes. Plant Soil 2008, 308, 161–174. [Google Scholar] [CrossRef]
- Khamna, S.; Yokota, A.; Peberdy, J.F.; Lumyong, S. Indole-3-Acetic Acid Production by Streptomyces Sp. Isolated from Some Thai Medicinal Plant Rhizosphere Soils. EurAsian J. BioSci. 2010, 4, 23–32. [Google Scholar] [CrossRef]
- Verma, V.C.; Singh, S.K.; Prakash, S. Bio-control and plant growth-promotion potential of siderophore producing endophytic Streptomyces from Azadirachtaindica A. Juss. J. Basic Microbiol. 2011, 51, 550–556. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; El-Sayed, E.S.A.; Rasmey, A.H.M. Indole-3-acetic acid (IAA) Production by Streptomyces Atrovirens Isolated from Rhizospheric Soil in Egypt. J. Biol. Earth Sci. 2013, 3, B182–B193. [Google Scholar]
- Lin, L.; Xu, X. Indole-3-acetic acid Production by Endophytic Streptomyces Sp. En-1 Isolated from Medicinal Plants. Curr. Microbiol. 2013, 67, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Kim, K.; Krishnamoorthy, R.; Sundaram, S.; Sa, T. Endophytic bacteria improve nodule function and plant nitrogen in soybean on co-inoculation with Bradyrhizobiumjaponicum MN110. Plant Growth Regul. 2014, 76, 327–332. [Google Scholar] [CrossRef]
- Sang-Mo, K.; Abdul-Latif, K.; Young-Hyun, Y.; Muhammad, K. Gibberellin production by newly isolated strain Leifsonia soli SE134 and its potential to promote plant growth. J. Microbiol. Biotechnol. 2014, 24, 106–112. [Google Scholar]
- Cacciari, I.; Grappelli, A.; Lippi, D.; Pietrosanti, W. Effect of Growth Rate on The Production of Phytohormone-Like Substances by an Arthrobacter Sp. in Chemostat Culture. J. Gen. Microbiol. 1980, 118, 549–552. [Google Scholar] [CrossRef][Green Version]
- Stevens, G.; Berry, A.M. Cytokinin secretion by Frankia sp. HFP ArI3 in defined medium. Plant Physiol. 1988, 87, 15–16. [Google Scholar] [CrossRef]
- Joshi, M.V.; Loria, R. Streptomyces Turgidiscabies Possesses a Functional Cytokinin Biosynthetic Pathway and Produces Leafy Galls. Mol. Plant Microbe. Interact. 2007, 20, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Pertry, I.; Vaclavikova, K.; Depuydt, S.; Galuszka, P.; Spichal, L.; Temmerman, W.; Vereecke, D. Identification of Rhodococcusfascianscytokinins and their modus Operandi to Reshape the Plant. PNAS 2009, 106, 929–934. [Google Scholar] [CrossRef]
- Katznelson, H.; Cole, S.E. Production of Gibberellin like Substances by Bacteria and Actinomycetes. Can. J. Microbiol. 1965, 11, 733–741. [Google Scholar] [CrossRef]
- Merckx, R.; Dijkra, A.; Hartog, A.D.; Veen, J.A.V. Production of Root-Derived Material and Associated Microbial Growth in Soil at Different Nutrient Levels. Biol. Fertil. Soil. 1987, 5, 126–132. [Google Scholar] [CrossRef]
- Jadhav, H.P.; Sayyed, R.Z. Hydrolytic Enzymes of Rhizospheric Microbes in Crop Protection. MOJ Cell. Sci. Rep. 2016, 3, 135–136. [Google Scholar]
- Mishra, J.; Tewari, S.; Singh, S.; Arora, N.K. Biopesticides: Where we Stand? Plant Microbes Symbiosis: Applied Facets. In Plant Microbes Symbiosis: Applied Facets; Springer: New Delhi, India, 2015; pp. 37–75. [Google Scholar]
- Mishra, N.; Khan, S.S.; Sundari, S.K. Native Isolate of Trichoderma: A Biocontrol Agent with Unique Stress Tolerance Properties. World J. Microbiol. Biotechnol. 2016, 32, 130. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial Biosurfactants Production, Applications and Future Potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef] [PubMed]
- López-Millán, A.F.; Ellis, D.R.; Grusak, M.A. Effect of Zinc and Manganese Supply on the Activities of Superoxide Dismutase and Carbonic Anhydrase in Medicago Truncatula Wild Type and Raz Mutant Plants. Plant Sci. 2005, 168, 1015–1022. [Google Scholar] [CrossRef]
- Pathak, D.; Lone, R.; Koul, K.K. Arbuscular Mycorrhizal Fungi (AMF) and Plant Growth Promoting Rhizobacteria (PGPR) Association in Potato (Solanum Tuberosum L.): A Brief Review. In Probiotics and Plant Health; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 401–420. [Google Scholar]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling Crop Diseases Using Induced Resistance: Challenges for the Future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induces Systemic Resistance by Beneficial Microbes. Ann. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Shockman, G.; Waksman, S.A. Rhodomycin-An Antibiotic Produced by A Red-Pigmented Mutant of Streptomyces Griseus. Antibiot Chem. 1951, 1, 68–75. [Google Scholar]
- Shih, H.D.; Liu, Y.C.; Hsu, F.L.; Mulabagal, V.; Dodda, R.; Huang, J.W. Fungichromin: A Substance from Streptomyces Padanus with Inhibitory Effects on Rhizoctoniasolani. J. Agric. Food Chem. 2003, 51, 95–99. [Google Scholar] [CrossRef]
- Ezra, D.; Castillo, U.F.; Strobel, G.A.; Hess, W.M.; Porter, H.; Jensen, J.B.; Condron, M.A.; Teplow, D.B.; Sears, J.; Maranta, M.; et al. Coronamycins, Peptide Antibiotics Produced by A Verticillate Streptomyces Sp. (MSU-2110) Endophytic On Monstera Sp. Microbiology 2004, 150, 785–793. [Google Scholar] [CrossRef]
- Weinstein, M.J.; Luedemann, G.M.; Oden, E.M.; Wagman, G.H. Everninomicin, A New Antibiotic Complex From Micromonosporacarbonacea. Antimicrob. Agents Chemother. 1964, 10, 24–32. [Google Scholar]
- Coronelli, C.; Pagani, H.; Bardone, M.R.; Lancini., G.C. Purpuromycin, a New Antibiotic Isolated from Actinoplanesianthinogenes N. sp. J. Antibiot. 1974, 27, 161–168. [Google Scholar] [CrossRef]
- Reimann, H.; Cooper, D.J.; Mallams, A.K.; Jaret, R.S.; Yehaskel, A.; Kugelman, M.; Vernay, H.F.; Schumacher, D. Structure of Sisomicin, a Novel Unsaturated Aminocyclitol Antibiotic from Micromonosporainyoensis. J. Org. Chem. 1974, 39, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Coronelli, C.; White, R.J.; Lancini, G.C.; Parenti, F. Lipiarmycin, a New Antibiotic from Actinoplanes. II. Isolation, Chemical, Biological and Biochemical Characterization. J. Antibiot. 1975, 28, 253–259. [Google Scholar] [CrossRef]
- Patel, M.; Horan, A.C.; Gullo, V.P.; Loebenberg, D.; Marquez, J.A.; Miller, G.H.; Waitz, J.A. Oxanthromicin, a Novel Antibiotic from Actinomadura. J. Antibiot. 1984, 37, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Maskey, R.P.; Li, F.C.; Qin, S.; Fiebig, H.H.; Laatsch, H. Chandrananimycins AC: Production of Novel Anticancer Antibiotics from a Marine Actinomadura Sp. Isolate M048 By Variation of Medium Composition and Growth Conditions. J. Antibiot. 2003, 56, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Parenti, F.; Beretta, G.; Berti, M.; Arioli, V. Teichomycins, New Antibiotics from Actinoplaneseichomyceticus Nov. Sp. I. Description of the Producer Strain, Fermentation Studies and Biological Properties. J. Antibiot. 1978, 1, 276–283. [Google Scholar] [CrossRef]
- Somma, S.; Gastaldo, L.; Corti, A. Teicoplanin, a New Antibiotic from Actinoplanesteichomyceticusnov.sp. Antimicrob. Agents Chemother. 1984, 26, 917–923. [Google Scholar] [CrossRef]
- Omura, S.; Imamura, N.; Oiwa, R.; Kuga, H.; Iwata, R.; Masuma, R.; Iwai, Y. Clostomicins, new antibiotics produced by Micromonosporaechinospora subsp. armeniaca Subsp. Nov.I Production, Isolation, and Physico-Chemical And Biological Properties. J Antibiot. 1986, 39, 1407–1412. [Google Scholar] [CrossRef]
- Boeck, V.D.; Fukuda, D.S.; Abbott, B.J.; Debono, M. Deacylation of Echinocandin B by Actinoplanesutahensis. J. Antibiot. 1989, 42, 382–388. [Google Scholar] [CrossRef]
- Kawamura, N.; Sawa, R.; Takahashi, Y.; Issiki, K.; Sawa, T.; Kinoshita, N.; Naganawa, H.; Hamada, M.; Takeuchi, T. Pyralomicins, New Antibiotics from Actinomadura Spiralis. J. Antibiot. 1995, 48, 435–437. [Google Scholar] [CrossRef][Green Version]
- Igarashi, Y.; Takagi, K.; Kajiura, T.; Furumai, T.; Oki, T. Glucosylquestiomycin, A Novel Antibiotic from Microbispora Sp. TP-A0184 Fermentation, Isolation, Structure Determination, Synthesis and Biological Activities. J. Antibiot. 1998, 51, 915–920. [Google Scholar] [CrossRef]
- Lam, Y.T.; Williams, D.L.; Sigmund, J.M.; Sanchez, M.; Genilloud, O.; Kong, Y.L.; Stevens-Miles, S.I.O.B.H.A.N.; Huang, L.; Garrity, G.M. Cochinmicins, Novel and Potent Cyclodepsipeptideendothelin Antagonists from a Microbispora sp. I. Production, Isolation, and Characterization. J. Antibiot. 1992, 45, 1709–1716. [Google Scholar] [CrossRef]
- He, H.; Ding, W.D.; Bernan, V.S.; Richardson, A.D.; Ireland, C.M.; Greenstein, M.; Ellestad, G.A.; Carter, G.T. Lomaiviticins A and B, Potent Antitumor Antibiotics from Micromonosporalomaivitiensis. J. Am. Chem. Soc. 2001, 123, 5362–5363. [Google Scholar] [CrossRef]
- Vertesy, L.; Ehlers, E.; Kogler, H.; Kurz, M.; Meiwes, J.; Seibert, G.; Vogel, M.; Hammann, P. Friulimicins: Novel Lipopeptide Antibiotics with Peptidoglycan Synthesis Inhibiting Activity from Actinoplanesfriuliensis Sp. Nov. II. Isolation and Structural Characterization. J. Antibiot. 2000, 53, 816–827. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P.D.N. Universal Primer Cocktails for Fish DNA Barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Li, W.; Leet, J.E.; Ax, H.A.; Gustavson, D.R.; Brown, D.M.; Turner, L.; Brown, K.; Clark, J.; Yang, H.; Fung-Tomc, J.; et al. Nocathiacins, New Thiazolyl Peptide Antibiotics From Nocardia Sp. I. Taxonomy, Fermentation and Biological Activities. J. Antibiot. 2003, 56, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.H.; Wang, Y.; Wang, Z.; Zhou, J.Q.; Jin, W.Z.; You, X.F.; Gao, H.; Zhao, L.X.; Si, S.Y.; Li, X. Chemomicin A: A New Angucyclinone Antibiotic Produced by Nocardiamediterranei subsp. Kanglensis 1747–64. J. Antibiot. 2007, 60, 211–215. [Google Scholar] [CrossRef]
- Engelhardt, K.; Degnes, K.F.; Kemmler, M.; Bredholt, H.; Fjaervik, E.; Klinkenberg, G.; Sletta, H.; Ellingsen, T.E.; Zotchev, S.B. Production of a New Thiopeptide Antibiotic, TP-1161, By A Marine Nocardiopsis Species. Appl. Environ. Microbiol. 2010, 76, 4969–4976. [Google Scholar] [CrossRef] [PubMed]
- El-Tarabily, K.A.; Nassar, A.H.; Hardy, G.E.S.J.; Sivasithamparam, K. Plant Growth-Promotion and Biological Control Of Pythiumaphanidermatum, A Pathogen of Cucumber, by Endophyticactin omycetes. J. Appl. Microbiol. 2009, 106, 13–26. [Google Scholar] [CrossRef]
- Loliam, B.; Morinaga, T.; Chaiyanan, S. Biocontrol of Pythiumaphanidermatumby the Cellulolytic Actinomycetes Streptomyces Rubrolavendulae S4. Sci. Asia 2013, 39, 584–590. [Google Scholar] [CrossRef]
- Ashokvardhan, T.; Rajithasri, A.B.; Prathyusha, P.; Satyaprasad, K. Actinomycetes from Capsicum annuum L. Rhizosphere Soil have the Bio control Potential Against Pathogenic Fungi. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 894–903. [Google Scholar]
- El-Tarabily, K.A. Anendophyticchitinase-Producing Isolate of Actinoplanesmissouriensis, with Potential for Biological Control of Root Rot of Lupine Caused by Plectosporium tabacinum. Aust. J. Bot. 2003, 51, 257–266. [Google Scholar] [CrossRef]
- Yandigeri, M.S.; Malviya, N.; Solanki, M.K.; Shrivastava, P.; Sivakumar, G. Chitinolytic Streptomyces vinaceusdrappus S5MW2 isolated from Chilika lake, India enhances plant growth and biocontrol efficacy through chitin supplementation against Rhizoctoniasolani. World J. Microbiol. Biotechnol. 2015, 31, 1217–1225. [Google Scholar] [CrossRef]
- Trejo-Estrada, S.R.; Sepulveda, I.; Crawford, D.L. In Vitro and in Vivo Antagonism of Streptomyces Violaceusniger YCED9 against Fungal Pathogens of Turfgrass. World J. Microbiol. Biotechnol. 1998, 14, 865–872. [Google Scholar] [CrossRef]
- Shekhar, N.; Bhattacharya, D.; Kumar, D.; Gupta, R.K. Biocontrol of wood-rotting fungi with Streptomyces violaceusniger XL2. Can. J. Microbiol. 2006, 52, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Arasu, M.V.; Esmail, G.A.; Al-Dhabi, N.A.; Ponmurugan, K. Managing Pests and Diseases of Grain Legumes with Secondary Metabolites from Actinomycetes. In Plant Growth Promoting Actinobacteria; Gopalakrishnan, S., Sathya, A., Vijayabharathi, R., Eds.; Springer: Singapore, 2016; pp. 83–98. [Google Scholar]
- USDA-ARS. Research Databases. 2008. Available online: http://www.ars.usda.gov/Services/docs.htm?docid=8908 (accessed on 12 July 2021).
- Munns, R. Genes and Salt Tolerance: Bringing them together. Plant Physiol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Isayenkov, S.V. Physiological and Molecular Aspects of Salt Stress in Plants. Cytol. Genet. 2012, 46, 302–318. [Google Scholar] [CrossRef]
- Hernández, J.A.; Aguilar, A.B.; Portillo, B.; López-Gómez, E.; Beneyto, J.M.; García-Legaz, M.F. The Effect of Calcium on the Antioxidant Enzymes from Salt-Treated Loquat and Anger Plants. Funct. Plant Biol. 2003, 30, 1127–1137. [Google Scholar] [CrossRef]
- Mittova, V.; Guy, M.; Tal, M.; Volokita, M. Salinity Up-Regulates the Antioxidative System in Root Mitochondria and Peroxisomes of the Wild Salt-Tolerant Tomato Species Lycopersicon pennellii. J. Exp. Bot. 2004, 399, 1105–1113. [Google Scholar] [CrossRef]
- Taffouo, V.D.; Wamba, O.F.; Yombi, E.; Nono, G.V.; Akoe, A. Growth, Yield, Water Status and Ionic Distribution Response of Three Bambara Groundnut (Vigna subterranean (L.) verdc.) Landraces Grown Under Saline Conditions. Int. J. Bot. 2010, 6, 53–58. [Google Scholar] [CrossRef]
- Murillo-Amador, B.; Yamada, S.; Yamaguchi, T.; Rueda-Puente, E.; Ávila-Serrano, N.; García-Hernández, J.L.; López-Aguilar, R.; Troyo-Diéguez, E.; Nieto-Garibay, A. Salinity Toxicity Influence of Calcium Silicate on Growth Physiological Parameters and Mineral Nutrition in Two Legume Species under Salt Stress. J. Agron. Crop. Sci. 2007, 193, 413–421. [Google Scholar] [CrossRef]
- Incheira, P.; Quiroz, A. Microbial Volatiles as Plant Growth Inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef]
- Allard-Massicotte, R.; Tessier, L.; Lécuyer, F.; Lakshmanan, V.; Lucier, J.; Garneau, D.; Caudwell, L.; Vlamakis, H.; Bais, H.P.; Beauregard, P.B. Bacillus Subtilis Early Colonization of Arabidopsis Thaliana Roots Involves Multiple Chemotaxis Receptors. Microbe. Bio. 2016, 7, e01664-16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Hu, F.; Ma, L. Phosphate-solubilizing Bacteria from Safflower Rhizosphere and their Effect on Seedling Growth. Open. Life Sci. 2019, 14, 246–254. [Google Scholar] [CrossRef]
- Vaishnav, A.; Kumari, S.; Jain, S.; Verma, A.; Tuteja, N.; Choudhary, D.K. PGPR-Mediated Expression of Salt Tolerance Gene in Soybean through Volatiles under Sodium Nitroprusside. J. Basic Microbiol. 2016, 56, 1274–1288. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. A Review on Possible Elicitor Molecules of Cyanobacteria: Their Role in Improving Plant Growth and Providing Tolerance against Biotic or Abiotic Stress. J. App. Microbiol. 2014, 117, 1221–1244. [Google Scholar] [CrossRef] [PubMed]
- Kasotia, A.; Varma, A.; Choudhary, D.K. Pseudomonas-Mediated Mitigation of Salt Stress and Growth Promotion in Glycine Max. Agric. Res. 2015, 4, 31–41. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. Res. Fac. Rev. 2016, 5, 1554. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production Under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of Drought Stress on Photosynthesis and Photosynthetic Electron Transport Chain in Young Apple Tree Leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef] [PubMed]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Mishra, S.; Boldt, J.K. Effects of Drought on Nutrient Uptake and the Levels of Nutrient-Uptake Proteins in Roots of Drought-Sensitive and Tolerant Grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Mariotte, P.; Cresswell, T.; Johansen, M.P.; Harrison, J.J.; Keitel, C.; Dijkstra, F.A. Plant Uptake of Nitrogen and Phosphorus among Grassland Species Affected by Drought Along a Soil Available Phosphorus Gradient. Plant Soil 2020, 448, 121–132. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research Progress and Perspective on Drought Stress in Legumes: A Review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef]
- Zheng, M.; Tao, Y.; Hussain, S.; Jiang, Q.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Seed priming in Dry Direct-Seeded Rice: Consequences For Emergence, Seedling Growth and Associated Metabolic Events under Drought Stress. Plant Growth Regul. 2016, 78, 167–178. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, H.; Wu, J.; Xie, F. Effect of Drought Stress during Soybean R2–R6 Growth Stages on Sucrose Metabolism in Leaf and Seed. Int. J. Mol. Sci. 2020, 21, 618. [Google Scholar] [CrossRef]
- Thalmann, M.S. Starch as a Determinant of Plant Fitness under Abiotic Stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef]
- Majumdar, R.; Barchi, B.; Turlapati, S.A.; Gagne, M.; Minocha, R.; Long, S.; Minocha, S.C. Glutamate, Ornithine, Arginine, Proline, and Polyamine Metabolic Interactions: The Pathway is regulated at the Post-Transcriptional Level. Fronti Plant Sci. USA 2016, 7, 78. [Google Scholar] [CrossRef]
- Figueiredo, M.V.B.; Burity, H.A.; Martinez, C.R.; Chanway, C.P. Alleviation of Drought Stress in Common Bean (Phaseolus Vulgaris L.) by Coinoculation Withpaenibacillus Polymyxa and Rhizobium Tropici. Appl. Soil Ecol. 2008, 40, 182–188. [Google Scholar] [CrossRef]
- Wang, D.; Yang, S.; Tang, F.; Zhu, H. Symbiosis Specifi City in the Legume Rhizobial Mutualism. Cell Microbiol. 2012, 14, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, D.; Yapa, N. Arbuscular Mycorrhizal Fungi Inoculation Enhances Drought Stress Tolerance of Plants. Ground Water Sust. Dev. 2018, 7, 490–494. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought Stress in Plants: An Overview. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer Press: Berlin/Heidelberg, Germany, 2012; pp. 1–12. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic Stress, the Field Environment and Stress Combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, W. Climate change and livestock: Impacts, adaptation and mitigation. Climate Risk. Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Crafts-Brander, S.J.; Salvucci, M.E. Sensitivity to Photosynthesis in the C4 Plant Maize to Heat Stress. Plant Cell. 2002, 12, 54–68. [Google Scholar]
- Qu, M.; Chen, G.; Bunce, J.A.; Zhu, X.; Richard, C.S. Systematic biology analysis on photosynthetic carbon metabolism of maize leaf following sudden heat shock under elevated CO2. Sci. Rep. 2018, 8, 7849. [Google Scholar] [CrossRef]
- Nguyen, N.V. Global Climate Changes and Rice Food Security. IRC Rep. 2012, 24–31. Available online: http://www.fao.org/climatechange/1552603ecb62366f779d1ed45287e698a44d2e.pdf (accessed on 1 July 2021).
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat Tolerance in Plants: An Over View. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Koop, L.K.; Tadi, P. Physiology, Heat Loss (Convection, Evaporation, Radiation). In Stat Pearls; Stat Pearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541107/ (accessed on 1 July 2021).
- Pei, Z.M.; Ghassemian, M.; Kwak, C.M.; McCourt, P.; Schroeder, J.I. Role of Farnesyl Transferase in ABA Regulation of Guard Cell Anion Channels and Plant Water Loss. Science 1998, 282, 287–290. [Google Scholar] [CrossRef]
- De Zelicourt, A.; Al-Yousif, M.; Hirt, H. Rhizosphere Microbes as Essential Partners for Plant Stress Tolerance. Mol. Plant 2013, 6, 242–245. [Google Scholar] [CrossRef]
- Kao, C.H. Role of Glutathione in Abiotic Stress Tolerance of Rice Plants. J. Taiwan Agric. Res. 2015, 164, 167–176. [Google Scholar]
- Herawati, N.; Suzuki, S.; Hayashi, K.; Rivai, I.F.; Koyoma, H. Cadmium, Copper and Zinc Levels in Rice and Soil of Japan, Indonesia and China by Soil Type. Bull. Environ. Contam. Toxicol. 2000, 64, 33–39. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ali, S.; Hameed, A.; Bharwana, S.A.; Rizwan, M.; Ishaque, W.; Farid, M.; Mahmood, K.; Iqbal, Z. Cadmium Stress in Cotton Seedlings: Physiological, Photosynthesis and Oxidative Damages Alleviated by Glycine Betaine. South. African. J. Bot. 2016, 104, 61–68. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular Mechanisms for Heavy Metal Detoxification and Tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Ali, S.; Rizwan, M.; Saeed, R.; Tauqeer, H.M.; Sallah-Ud-Din, R.; Azam, A.; Raza, N. Microwave Irradiation and Citric Acid Assisted Seed Germination and Phytoextraction Of Nickel (Ni) By Brassica Napus L.; Morphophysiological And Biochemical Alterations Under Ni Stress. Environ Sci. Pollution Res. Int. 2017, 24, 21050–21064. [Google Scholar] [CrossRef]
- .Lin, A.; Zhang, X.; Zhu, Y.G. Arsenate-Induced Toxicity: Effects on Antioxidative Enzymes and DNA Damage In Vicia Faba. Environ. Toxicol. Chem. 2008, 27, 413–419. [Google Scholar] [CrossRef]
- Sarkar, B. Metal replacement in DNA-binding Zinc finger Proteins and its Relevance to Mutagenicity and Carcinogenicity through Free Radical Generation. Nutrition 1995, 11, 646–649. [Google Scholar]
- Ghasemi, F.; Heidari, R.; Jameii, R. Effects of Ni2+ Toxicity on Hill Reaction and Membrane Functionality in Maize. J. Stress. Physiol. Biochem. 2012, 8, 55–61. [Google Scholar]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F. Heavy Metal Stress and Some Mechanisms of Plant Defense Response. Sci. World J. 2015, 2015, 18. [Google Scholar] [CrossRef]
- Lombardi, L.; Sebastiani, L. Copper Toxicity in Prunus Cerasifera: Growth and Antioxidant Enzymes Responses of In Vitro Grown Plants. Plant Sci. 2005, 168, 797–802. [Google Scholar] [CrossRef]
- Ventrella, A.; Catucci, L.; Placido, T. Biomaterials Based on Photosynthetic Membranes as Potential Sensors for Herbicides. Biosens. Bioelectron. 2011, 26, 4747–4752. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Jia, L. Zinc-Induced Oxidative Damage, Antioxidant Enzyme Response and Proline Metabolism in Roots and Leaves of Wheat Plants. Ecotoxicol. Environ. Saf. 2013, 89, 150–157. [Google Scholar] [CrossRef]
- Ahmad, M.S.A.; Ashraf, M.; Tabassam, Q. Lead (Pb)-Induced Regulation of Growth, Photosynthesis, and Mineral Nutrition in Maize (Zea mays L.) Plants at Early Growth Stages. Biol. Trace. Elem. Res. 2011, 144, 1229–1239. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Abbas, F.; Bukhari, S.A.H.; Saeed, R.; Wu, L. Citric Acid Assisted Phyto-Extraction of Chromium By Sunflower; Morpho-Physiological And Biochemical Alterations In Plants. Ecotoxicol. Environ. Saf. 2017, 145, 90–102. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, S.; Asgher, M. Photosynthetic and Growth Responses of two Mustard Cultivars Differing in Phytocystatin Activity under Cadmium stress. Photosynthetica 2016, 54, 491–501. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Mangano, S.; Juarez, S.P.D.; Estevez, J.M. ROS Regulation of Polar Growth in Plant Cells. Plant Physiol. 2016, 171, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Bolwell, G.P. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shimizu, H.; Ito, S.; Yagasaki, Y.; Zou, C.; Zhou, G.; Zheng, Y. Effects of Elevated CO2, Warming and Precipitation Change on Plant Growth, Photosynthesis and Peroxidation in Dominant Species from North China Grassland. Planta 2014, 239, 421–435. [Google Scholar] [CrossRef]
- Pitzschke, A.; Hirt, H. Disentangling the Complexity of Mitogen-Activated Protein Kinases and Reactive Oxygen Species Signaling. Plant Physiol. 2009, 149, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Adrees, M. Cadmium Stress in Rice: Toxic Effects, Tolerance Mechanisms, and Management: A Critical Review. Environ. Sci. Poll. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid- A Potential Oxidant Scavenger and its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Anjum, N.A.; Ahmad, I.; Mohmood, I. Modulation of Glutathione and its Related Enzymes in Plants’ Responses to Toxic Metals and Metalloids –A Review. Environ. Exp. Bot. 2012, 75, 307–324. [Google Scholar] [CrossRef]
- Sofo, A.; Cicco, N.; Paraggio, M. Regulation of the ascorbate–glutathione cycle in plants under drought stress. In Ascorbate-glutathione pathway and stress tolerance in plants; Anjum, N.A., Umar, S., Chan, M.T., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 137–190. [Google Scholar]
- Srivastava, S.; Verma, P.C.; Chaudhry, V.; Singh, N.; Abhilash, P.C.; Kumar, K.V.; Sharma, N.; Singh, N. Influence of Inoculation of Arsenic-Resistant Staphylococcus arlettae on GRowth and Arsenic UPtake in Brassica juncea (L.) Czern.Var. R-46. J. Hazard Mater. 2013, 262, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Rajkumar, M.; Luo, Y.; Freitas, H. Phytoextraction of Heavy Metal Polluted Soils Using Sedum Plumbizincicola Inoculated with Metal Mobilizing Phyllobacterium Myrsinacearum RC6b. Chemosphere 2013, 93, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Adediran, G.A.; Ngwenya, B.T.; Mosselmans, J.F.W.; Heal, K.V. Bacteria–zinc Co localization Implicates Enhanced Synthesis of Cysteine-Richpeptides in Zinc Detoxification when Brassica Juncea is inoculated with Rhizobium leguminosarum. New Phytol. 2016, 209, 280–293. [Google Scholar] [CrossRef]
- Kiely, P.D.; Haynes, J.M.; Higgins, C.H.; Franks, A.; Mark, G.L.; Morrissey, J.P.; O’Gara, F. Exploiting New Systems-Based Strategies to Elucidate Plant-Bacterial Interactions in the Rhizosphere. Microb. Ecol. 2006, 51, 257–266. [Google Scholar] [CrossRef]
- Bevivino, A.; Dalmastri, C.; Tabacchioni, S.; Chiarini, L. Efficacy of Burkholderia Cepacia MCI 7 In Disease Suppression and Growth Promotion of Maize. Biol. Fertil. Soils 2000, 31, 225–231. [Google Scholar] [CrossRef]
- Harthmann, O.E.L.; Mógor, Á.F.; Wordell-Filho, J.A.; Luz, W.C.; Biasi, L.A. Tratamento De Sementes Com Rizobactérias Na Produção De Cebola. Cienc. Rural. 2009, 39, 2533–2538. [Google Scholar] [CrossRef]
- Hungria, M.; Campo, R.J.; Souza, E.M.; Pedrosa, F.O. Inoculation with Selected Strains of Azospirillum Brasilense and A. Lipoferum Improves Yields of Maize and Wheat in Brazil. Plant Soil 2010, 331, 413–425. [Google Scholar] [CrossRef]
- Barraquio, W.L.; Segubre, E.M.; Gonzalez, M.A.S.; Verma, S.C.; James, E.K.; Ladha, J.K.; Tripathi, A.K. Diazotrophicenterobacteria: What is their Role in the Rhizosphere of Rice. In The Quest for Nitrogen Fixation in Rice; Ladha, J.K., Reddy, P.M., Eds.; International Rice Research Institute: Manila, Philippines, 2000; pp. 93–118. [Google Scholar]
- Compant, S.; Mitter, B.; Colli-Mull, J.G.; Gangl, H.; Sessitsch, A. Endophytes of Grapevine Fl owers, Berries, and Seeds: Identifi Cation of Cultivable Bacteria, Comparison with Other Plant Parts, and Visualization of Niches of Colonization. Microb. Ecol. 2011, 62, 188–197. [Google Scholar] [CrossRef]
- Szilagyi-Zecchin, V.J.; Ikeda, A.C.; Hungria, M.; Adamoski, D.; Kava-Cordeiro, V.; Glienke, C.; Galli-Terasawa, L.V. Identification and Characterization of Endophytic Bacteria from Corn (Zea mays L.) Roots with Biotechnological Potential in Agriculture. AMB. Exp. 2014, 4, 26. [Google Scholar] [CrossRef]
- Cruz, A.F.; Ishii, T.; Matsumoto, I.; Kadoya, K. Network Establishment of Vesicular Arbuscular Mycorrhizal Hyphae in the Rhizosphere between Trifoliate Orange and Some Plants. J. Jpn. Soc. Hortic. Sci. 2002, 71, 19–25. [Google Scholar] [CrossRef]
- Barea, J.M.; Pozo, M.J.; Azcon, R.; Azcon-Aguilar, C. Microbial Co-operation in the Rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef]
- Mishra, N.; Sundari, K.S. Native PGPM Consortium: A Beneficial Solution to Support Plant Growth in the Presence of Phytopathogens and Residual Organophosphate Pesticides. J. Bioproces Biotech. 2015, 5, 1–8. [Google Scholar]
- Rodriguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Dev. Plant Soil Sci. 2007, 102, 15–21. [Google Scholar]
- Simiyu, S.; Mumma, J.; Aseyo, E.; Cumming, O.; Czerniewska, A.; Baker, K.; Dreibelbis, R. Designing a Food Hygiene Intervention for Children 6–9 Months in an Informal Settlement in Kisumu, Kenya; Loughborough University: Loughborough, UK, 2018. [Google Scholar]
- Simiyu, S.; Czerniewska, A.; Aseyo, E.R.; Baker, K.K.; Cumming, O.; Mumma, J.A.O.; Dreibelbis, R. Designing a Food Hygiene Intervention in Low-Income, Peri-Urban Context of Kisumu, Kenya: Application of the Trials of Improved Practices Methodology. Am. J. Trop. Med. Hyg. 2020, 102, 1116. [Google Scholar] [CrossRef] [PubMed]
- Borriss, R. Use of Plant-Associated Bacillus Strains as Biofertilizers and Biocontrol Agents in Agriculture. In Bacteria in Agrobiology: Plant Growth Responses; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 41–76. [Google Scholar]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laine, P.; Goux, D.; Garnica, M.; Fuentes, M.; San Francisco, S.; Baigorri, R.; Cruz, F.; et al. Brassica Napus Growth Is Promoted By Ascophyllumnodosum (L.) Le Jol. Seaweed Extract: Microarray Analysis and Physiological Characterization of N, C, and S Metabolisms. J. Plant Growth Regul. 2013, 32, 31–52. [Google Scholar] [CrossRef]
- Lonhienne, T.; Mason, M.G.; Ragan, M.A.; Hugenholtz, P.; Paungfoo Lonhienne, S.S.C. Yeast as a Biofertilizer Alters Plant Growth and Morphology. Crop. Sci. 2014, 54, 785–790. [Google Scholar] [CrossRef]
- Bettoni, M.M.; Mógor, Á.F.; Pauletti, V.; Goicoechea, N. Growth and Metabolism of Onion Seedlings as Affected By The Application of Humic Substances, Mycorrhizal Inoculation and Elevated CO2. Sci. Hortic. 2014, 180, 227–235. [Google Scholar] [CrossRef]
- Civiero, C.; Armitage, J.J.; Goes, S.; Hammond, J.O. The Seismic Signature of Upper-Mantle Plumes: Application to the Northern East African Rift. Geochem. Geophy. Geosys. 2019, 20, 6106–6122. [Google Scholar] [CrossRef]
- Vinale, F. Biopesticides and Biofertilizers Based on Fungal Secondary Metabolites. J. Biofert. Biopest. 2014, 5, e119. [Google Scholar] [CrossRef]
- Kasiotis, K.M. Biopesticides Analysis: An Editorial. J. Biofertil. Biopestici. 2013, 4, e115. [Google Scholar] [CrossRef]
- Brasil, Ministério da Agricultura, Pecuária e Abastecimento. Lei de Fertilizantes, Corretivos, Inoculantes, Estimulantes ou Biofertilizantes. Decreton 4.954 de 14 de janeiro de 2004; Brasil, Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brazil, 2004. [Google Scholar]
- Brasil, Ministério da Agricultura, Pecuária e Abastecimento. Regulamento Técnico que estabelece as normas técnicas para os Sistemas Orgânicos de Produção. Instrução Normativa n° 64 de 18 de dezembro de 2008; Brasil, Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brazil, 2008. [Google Scholar]
- Kumar, A.; Bahadur, I.; Maurya, B.R.; Raghuwanshi, R.; Meena, V.S.; Singh, D.K.; Dixit, J. Does a Plant Growth-Promoting Rhizobacteria Enhance Agricultural Sustainability? J. Pure Appl. Microbiol. 2015, 9, 715–724. [Google Scholar]
- Ahmad, M.; Nadeem, S.M.; Naveed, M.; Zahir, Z.A. Potassium-Solubilizing Bacteria and their Application in Agriculture. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 293–313. [Google Scholar] [CrossRef]
- Meena, M.K.; Gupta, S.; Datta, S. Antifungal Potential of PGPR, Their Growth Promoting Activity on Seed Germination and Seedling Growth of Winter Wheat and Genetic Variabilities Among Bacterial Isolates. Int. J. Cur. Microbiol. Appl. Sci. 2016, 5, 235–243. [Google Scholar] [CrossRef]
- Jha, Y.; Subramanian, R.B. Regulation of Plant Physiology and Antioxidant Enzymes for Alleviating Salinity Stress by Potassium-Mobilizing Bacteria. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 149–162. [Google Scholar] [CrossRef]
- Rosselló-Mora, R.; Amann, R. The species concept for prokaryotes. FEMS Microbiol. Rev. 2001, 25, 39–67. [Google Scholar] [CrossRef]
- Vandamme, P.; Pot, B.; Gillis, M.; De Vos, P.; Kersters, K.; Swings, J. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol. Rev. 1996, 60, 407–438. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Cohan, F.M.; Lawrence, J.G.; Spratt, B.G.; Coenye, T.; Feil, E.J.; Stackebrandt, E.; Van de Peer, Y.; Vandamme, P.; Thompson, F.L.; et al. Opinion: Re-Evaluating Prokaryotic Species. Nat. Rev. Microbiol. 2005, 3, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.D.O.; Pires, A.J.V.; da Silva, F.F.; Veloso, C.M.; de Carvalho, G.G.P.; Cezario, A.S.; Santos, C.C. Effects of Feeding Cocoa Meal (Theobroma cacao L.) and Palm Kernel Cake (Elaeis guineensis, Jacq) on Milk Intake and Yield For Lactating Goats. Rev. Bras. Zootec. 2005, 34, 1786–1794. [Google Scholar] [CrossRef]
- Martens, M.; Delaere, M.; CoopmanDe Vos, R.P.; Gillis, M.; Willems, A. Multilocus Sequence Analysis of Ensifer and Related Taxa. Int. J. SystEvol. Microbiol. 2007, 57, 489–503. [Google Scholar] [CrossRef]
- Naser, S.; Thompson, F.L.; Hoste, B.; Gevers, D.; Vandemeulebroecke, K.; Cleenwerck, I.; Thompson, C.C.; Vancanneyt, M.; Swings, J. Phylogeny and Identification of Enterococci Using Atpa Gene Sequence Analysis. J. Clin. Microbiol. 2005, 43, 2224–2230. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Barcellos, F.G.; Thompson, F.L.; Hungria, M. Multilocus sequence analysis of Brazilian Rhizobium Microsymbionts of Common Bean (Phaseolus vulgaris L.) Reveals Unexpected Taxonomic Diversity. Res. Microbiol. 2009, 160, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.L.; Gevers, D.; Thompson, C.C.; Dawyndt, P.; Naser, S.; Hoste, B.; Munn, C.B.; Swings, J. Phylogeny and molecular identification of vibrios on the basis of multilocus sequence analysis. App. Environ. Microbiol. 2005, 71, 5107–5115. [Google Scholar] [CrossRef]
- Laranjo, M.; Young, J.P.W.; Oliveira, S. Multilocus Sequence Analysis Reveals Multiple Symbiovars within Mesorhizobium Species. Syst. Appl. Microbiol. 2012, 35, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Dall’Agnol, R.F.; Ribeiro, R.A.; Ormeno-Orrillo, E.; Rogel, M.A.; Delamuta, J.R.M.; Andrade, D.S.; Martínez-Romero, E.; Hungria, M. Rhizobium Freirei sp. nov., a Symbiont of Phaseolus Vulgaris that is Very Effective At Fi Xing Nitrogen. Int. J. Syst. Evol. Microbiol. 2013, 63, 4167–4173. [Google Scholar] [CrossRef] [PubMed]
- Amutha, R.; Karunakaran, S.; Dhanasekaran, S.; Hemalatha, K.; Monika, R.; Shanmugapriya, P.; Sornalatha, T. Isolation and Mass Production of Biofertilizer (Azotobacter and phosphobacter). Int. J. Lat. Res. Sci. Tech. 2014, 3, 79–81. [Google Scholar]
- Palai, J.B.; Malik, G.C.; Maitra, S.; Banerjee, M. Role of Rhizobium on Growth and Development of Groundnut: A Review. Int. J. Agric. Environ. Biotechnol. 2021, 14, 63–73. [Google Scholar] [CrossRef]
- Baset Mia, M.A.; Shamsuddin, Z.H. Rhizobium as a Crop Enhancer and Biofertilizer for Increased Cereal Production. Afr. J. Biotechnol. 2010, 9, 6001–6009. [Google Scholar]
- Parewa, H.P.; Yadav, J.; Rakshit, A.; Meena, V.S.; Karthikeyan, N. Plant Growth Promoting Rhizobacteriaenhance Growth and Nutrient Uptake of Crops. Agric. Sustain. Dev. 2014, 2, 101–116. [Google Scholar]
- Prakash, S.; Verma, J.P. Global Perspective of Potash for Fertilizer Production. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 327–331. [Google Scholar] [CrossRef]
- Dominguez-Nunez, J.A.; Benito, B.; Berrocal-Lobo, M.; Albanesi, A. Mycorrhizal Fungi: Role in the Solubilization of Potassium. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 77–98. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Meena, V.D.; Basak, B.B.; Meena, R.S. Potassium Uptake by Crops as Well As Microorganisms. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 267–280. [Google Scholar] [CrossRef]
- Jaiswal, D.K.; Verma, J.P.; Prakash, S.; Meena, V.S.; Meena, R.S. Potassium as an Important Plant Nutrient In Sustainable Agriculture, A State of the Art. Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 21–29. [Google Scholar] [CrossRef]
- Rossman, A.Y.; Palm, M.E. Why are Phytophthora and other Oomycota not true fungi? Outlooks Pest Manag. 2007, 17, 217–219. [Google Scholar] [CrossRef]
- Kang, S.; Mansfi-eld Park, M.A.; Geiser, D.M.; Ivors, K.L.; Coffey, M.D.; Grünwald, N.J.; Martin, F.N.; Lévesque, C.A.; Blair, J.E. The Promise and Pitfalls of Sequence– Based Identifi Cation of Plant–Pathogenic Fungi and Oomycetes. Phytopathology 2010, 100, 732–737. [Google Scholar] [CrossRef]
- Brun, S.; Madrid, H.B.; Gerrits-Van-Den-Ende, B.; Andersen, B.; Marinach–Patrice, C.; Mazier, D.; De Hoog, G.S. Multilocus Phylogeny and MALDI–TOF Analysis of the Plant Pathogenic Species Alternaria Dauci and Relatives. Fungal Biol. 2013, 117, 32–40. [Google Scholar] [CrossRef]
- Slippers, B.; Boissin, E.; Phillips, A.J.L.; Groenewald, J.Z.; Wingfi-eld, M.J.; Postma, A.; Burgess, T.; Crous, P.W. Phylogenetic Lineages in the Botryosphaeriales: A Systematic and Evolutionary Framework. Stud. Mycol. 2013, 76, 31–49. [Google Scholar] [CrossRef]
- Sharma, R.; Polkade, A.V.; Shouche, Y.S. Species Concept in Microbial Taxonomy and Systematics. Curr. Sci. 2015, 108, 1804–1814. [Google Scholar]
- Shivas, R.G.; Beasley, D.R.; McTaggart, A.R. Online Identifi Cation Guides for Australian Smut Fungi (Ustilagino mycotina) and Rust Fungi (Pucciniales). IMA Fungus 2014, 5, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Malfanova, N. Endophytic Bacteria with Plant Growth Promoting Properties and Biocontrol Abilities. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 2013; p. 166. [Google Scholar]
- Raven, J.A.; Beardall, J.; Flynn, K.J.; Maberly, S.C. Phagotrophy in the Origins of Photosynthesis in Eukaryotes and as Complementary Mode of Nutrition in Phototrophs: Relation to Darwin’s Insectivorous Plants. J. Exp. Bot. 2009, 60, 3975–3987. [Google Scholar] [CrossRef]
- Seghers, D.; Wittebolle, L.; Top, E.M.; Verstraete, W.; Siciliano, S.D. Impact of agricultural Practices on the Zea mays L. Endophytic Community. App. Environ. Microbiol. 2004, 70, 1475–1482. [Google Scholar] [CrossRef]
- Podolich, O.; Ardanov, P.; Zaets, I.; Maria Pirttilä, A.; Kozyrovska, N. Reviving of the Endophytic Bacterial Community as a Putative Mechanism of Plant Resistance. Plant Soil. 2014, 388, 367–377. [Google Scholar] [CrossRef]
- Tan, H.M.; Cao, L.X.; He, Z.F.; Su, G.J.; Lin, B.; Zhou, S.N. Isolation of Endophytic Actinobacteria from Different Cultivars of Tomato and their Activities Against Ralstonia solanacearum in vitro. World J. Microbiol. Biotechnol. 2006, 22, 1275–1280. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Martinez-Romero, E. Bacterial Endophytes and their Interactions with Hosts. Mol. Plant. Microb. Interact. 2006, 19, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of Bacterial Endophytes and Their Proposed Role in Plant Growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef]
- Miyamoto, T.; Kawahara, M.; Minamisawa, K. Novel Endophytic Nitrogen-Fixing Clostridia from the Grass Miscanthus Sinensis as Revealed by Terminal Restriction Fragment Length Polymorphism Analysis. Appl. Environ. Microbiol. 2004, 70, 6580–6586. [Google Scholar] [CrossRef] [PubMed]
- Benhizia, Y.; Benhizia, H.; Benguedouar, A.; Muresu, R.; Giacomini, A.; Squartini, A. Gamma Proteobacteria can Nodulate Legumes of the Genus Hedysarum. Syst. Appl. Microbiol. 2004, 27, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; Hardoim, C.C.; Van Overbeek, L.S.; Van Elsas, J.D. Dynamics of Seed-Borne Rice Endophytes on Early Plant Growth Stages. PLoS ONE 2012, 7, e30438. [Google Scholar] [CrossRef] [PubMed]
- Manter, D.K.; Delgado, J.A.; Holm, D.G.; Stong, R.A. Pyrosequencing Reveals a Highly Diverse and Cultivar Specific Bacterial Endophyte Community in Potato Roots. Microb. Ecol. 2010, 60, 157–166. [Google Scholar] [CrossRef]
- Bulgarelli, A.; Rott, M.; Schlaeppi, K.; Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing Structure and Assembly Cues for Arabidopsis Root-Inhabiting Bacterial Microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Sessitsch, A.; Hardoim, P.; Döring, J.; Weilharter, A.; Krause, A.; Woyke, T.; Mitter, B.; Hauberg-Lotte, L.; Friedrich, F.; Rahalkar, M.; et al. Functional Characteristics of an Endophyte Community Colonizing Rice Roots as Revealed by Metagenomic Analysis. Mol Plant Microb. Interact. 2012, 25, 28–36. [Google Scholar] [CrossRef]
- Bodenhausen, N.; Horton, M.W.; Bergelson, J. Bacterial Communities Associated with the Leaves and the Roots of Arabidopsis thaliana. PLoS ONE 2013, 8, e56329. [Google Scholar] [CrossRef]
- Gond, S.K.; Verma, V.C.; Mishra, A.; Kumar, A.; Kharwar, R.N. Role of Fungal Endophytes in Plant Protection. In Management of Fungal Plant Pathogens; Arya, A., Perello, A.E., Eds.; CAB: London, UK, 2010; pp. 183–197. [Google Scholar]
- Brem, A.D. Leuchtmann Epichloë Grass Endophytes Increase Herbivore Resistance in the Woodland Grass Brachypodium sylvaticum. Oecologia 2001, 126, 522–530. [Google Scholar] [CrossRef]
- Li, H.; Wei, D.; Shen, M.; Zhou, Z. Endophytes and Their Role in Phytoremediation. Fungal Divers. 2012, 54, 11–18. [Google Scholar] [CrossRef]
- Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. Thermo Tolerance Conferred to Plant Host and Fungal Endophyte During Mutualistic Symbiosis. Science 2002, 298, 1581. [Google Scholar] [CrossRef] [PubMed]
- Kharwar, R.N.; Verma, S.K.; Mishra, A.; Gond, S.K.; Sharma, V.K.; Afreen, T.; Kumar, A. Assessment of Diversity, Distribution and Antibacterial Activity of Endophytic Fungi Isolated from a Medicinal Plant Adenocalymma Alliaceum Miers. Symbiosis 2011, 55, 39–46. [Google Scholar] [CrossRef]
- Selvakumar, G.; Kim, K.; Hu, S.; Sa, T. Effect of Salinity on Plants and the Role of Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting rhizobacteria in Alleviation of Salt Stress. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Ahmad, P., Wani, M.R., Eds.; Springer: New York, NY, USA, 2014; pp. 115–144. [Google Scholar]
- Hu, C.; Qi, Y. Long-term Effective Microorganisms Application Promote Growth and Increase Yields and Nutrition of Wheat in China. Euro. J. Agron. 2013, 46, 63–67. [Google Scholar] [CrossRef]
- Muttalib, S.A.A.; Ismail, S.N.S.; Praveena, S.M. Application of Effective Microorganism (EM) in Food Waste Composting: A Review. Asia Pacific Environ. Occup. Health J. 2016, 2, 37–47. [Google Scholar]
- Isa, D.M.; Abdullah, S.; Noor, N.M.; Ismail, H.B. The Natural Way for Water Quality Improvement Using Effective Microorganism. Int. J. Environ. Engin. 2021, 11, 169–182. [Google Scholar] [CrossRef]
- Tsai, S.Q.; Joung, J.K. Defining and Improving the Genomewide Specificities of CRISPR–Cas9 Nucleases. Nat. Rev. Gene. 2016, 17, 300. [Google Scholar] [CrossRef]
- Dale, P.J.; Clarke, B.; Fontes, E.M.G. Potential for the Environmental Impact of Transgenic Crops. Nat. Biotechnol. 2002, 20, 567. [Google Scholar] [CrossRef] [PubMed]
- Thal, B.; Braun, H.P.; Eubel, H. Proteomic Analysis Dissects the Impact of Nodulation and Biological Nitrogen Fixation on Vicia faba Root Nodule Physiology. Plant. Mol. Biol. 2018, 97, 233–251. [Google Scholar] [CrossRef]
- Azhar, A.; Deris, S.; Napis, S.; Sinnott, R.O. A Hybrid of Ant Colony Optimization and F Lux Variability Analysis to Improve the Production of L-Phenylalanine and Biohydrogen. Int. J. Adv. Soft. Comp. App. 2016, 8, 161–180. [Google Scholar]
- Sanghera, G.S.; Wani, S.H.; Hussainm, W.; Singhm, N.B. Engineering Cold Stress Tolerance in Crop Plants. Curr. Genomic. 2011, 12, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Markovich, N.A.; Kononova, G.L. Lytic Enzymes of Trichoderma and their Role in Protecting Plants from Fungal Diseases. Prikl. Biokhim. Mikrobiol. 2003, 39, 389–400. [Google Scholar] [PubMed]
- Gajera, H.P.; Bambharolia, R.P.; Patel, S.V.; Khatrani, T.J.; Goalkiya, B.A. Antagonism Of Trichoderma Spp. Against Macrophominaphaseolina: Evaluation of Coiling and Cell Wall Degrading Enzymatic Activities. Plant Pathol. Microb. 2012, 3, 1000149. [Google Scholar]
- Swiontek-Brzezinska, M.; Jankiewicz, U.; Burkowska, A.; Walczak, M. Chitinolytic Microorganisms and Their Possible Application in Environmental Protection. Curr. Microbiol. 2014, 68, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Schnider-Keel, U.; Seematter, A.; Maurhofer, M.; Blumer, C.; Duffy, B.; Gigot-Bonnefoy, C.; Reimmann, C.; Notz, R.; Défago, G.; Haas, D.; et al. Autoinduction of 2,4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescens CHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J. Bacteriol. 2000, 182, 1215–1225. [Google Scholar] [CrossRef]
- Souza, J.T.; Raaijmakers, J.M. Polymorphisms within the prnD and pltC genes from Pyrrolnitrin and Pyoluteorin-Producing Pseudomonas and Burkholderia spp. FEMS Microbiol. Ecol. 2003, 43, 21–34. [Google Scholar] [CrossRef]
- .Kurth, C.; Kage, H.; Nett, M. Siderophores as Molecular Tools in Medical and Environmental Applications. Org. Biomol. Chem. 2016, 14, 8212–8227. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in Environmental Research: Roles and Applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- .Kim, J.G.; Park, B.K.; Kim, S.U.; Choi, D.; Nahm, B.H.; Moon, J.S.; Reader, J.S.; Farrand, S.K.; Hwang, I. Bases of Biocontrol, Sequence Predicts Synthesis And Mode Of Action Of Agrocin 84, the Trojan Horse Antibiotic That Controls Crown Gall. Proc. Natl. Acad. Sci. USA 2006, 103, 8846–8851. [Google Scholar] [CrossRef]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial Activities of Bacteriocins: Application in Foods and Pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [PubMed]
- Zhong, J.; Chen, D.; Zhu, H.J.; Gao, B.D.; Zhou, Q. Hypovirulence of Sclerotiumrolfsii Caused by Associated RNA Mycovirus. Front. Microbiol. 2016, 7, 1798. [Google Scholar] [CrossRef] [PubMed]
- Hoegger, P.J.; Heiniger, U.; Holdenrieder, O.; Rigling, D. Differential Transfer and Dissemination of Hypovirus and Nuclear and Mitochondrial Genomes of a Hypovirus-Infected Cryphonectria 3 Biological Control Agents: Diversity, Ecological Significance Parasitica Strain after Introduction into a Natural Population. Appl. Environ. Microbiol. 2003, 69, 3767–3771. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Yao, Z.; Zhou, Y.; Shang, J.; Lin, H.; Nuss, D.L.; Chen, B. Deletion of the cpku80 Gene In The Chestnut Blight Fungus, Cryphonectria Parasitica, Enhances Gene Disruption Efficiency. Curr. Genet. 2008, 53, 59–66. [Google Scholar] [CrossRef]
- Bardin, M.; Ajouz, S.; Comby, M.; Lopez-Ferber, M.; Graillot, B.; Siegwart, M.; Nicot, P.C. Is the Efficacy of Biological Control against Plant Diseases Likely to be More Durable than That of Chemical Pesticides? Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Brunner, K.; Zeilinger, S.; Ciliento, R.; Woo, S.L.; Lorito, M.; Kubicek, C.P.; Mach., R.L. Improvement of the Fungal Biocontrol Agent Trichoderma atroviride to Enhance Both Antagonism and Induction of Plant Systemic Disease Resistance. Appl. Environ. Microbiol. 2005, 71, 3959–3965. [Google Scholar] [CrossRef]
- Bubici, G.; Kaushal, M.; Prigigallo, M.I.; Gómez-Lama Cabanás, C.; Mercado-Blanco, J. Biological Control Agents Against Fusarium Wilt of Banana. Front. Microbiol. 2019, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Higa, T.; Parr, J.F. Beneficial and Effective Microorganisms for a Sustainable Agriculture and Environment; International Nature Farming Research Center: Atami, Japan, 1994; Volume 1. [Google Scholar]
- Bhattacharyya, P.N.; Goswami, M.P.; Bhattacharyya, L.H. Perspective of beneficial microbes in agriculture under changing climatic scenario: A review. J. Phytol. 2016, 8, 26–41. [Google Scholar] [CrossRef]
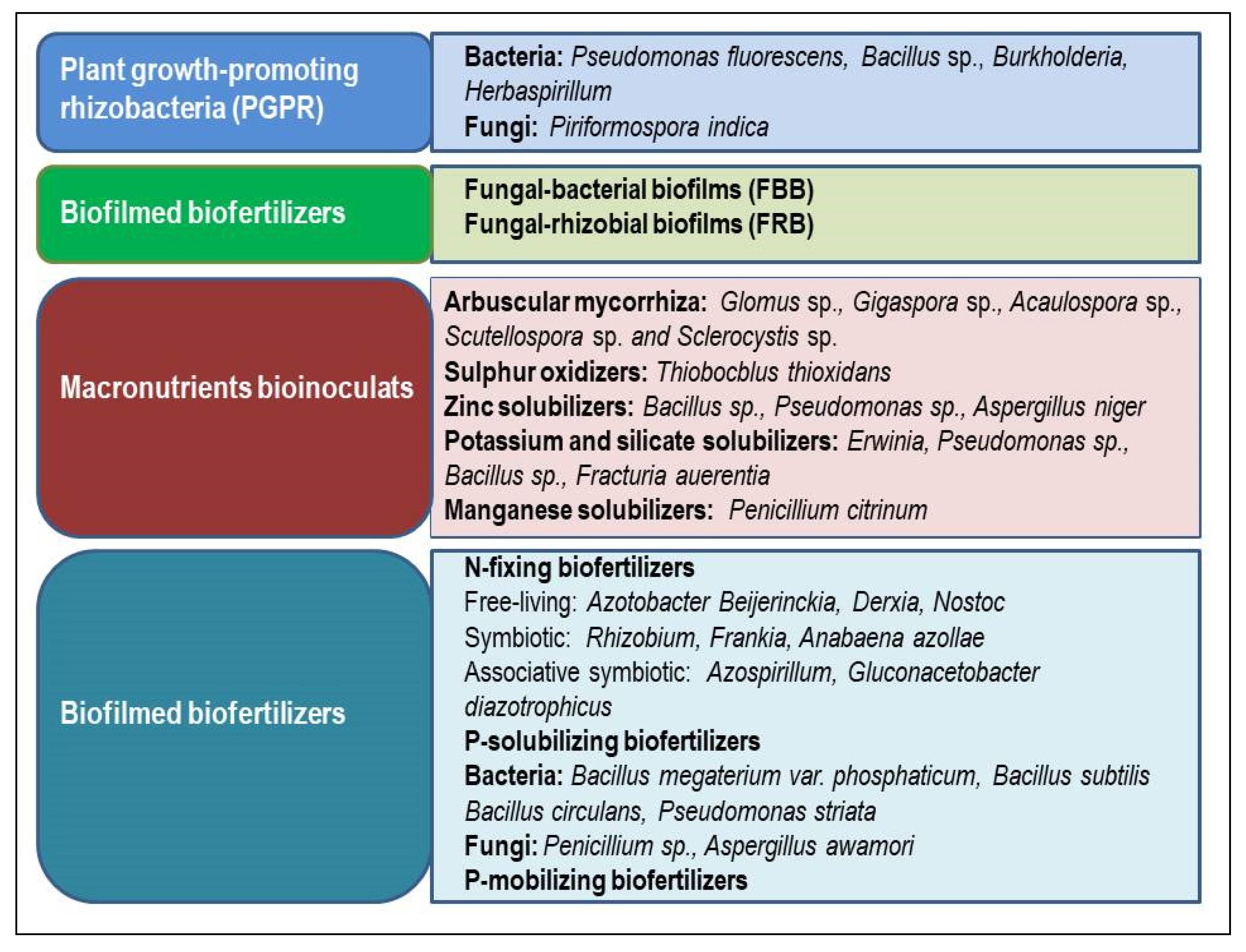
| Microorganisms | Crop Performance over Control (Untreated) | Associated Crop | References |
|---|---|---|---|
| Glomus faciculatum Bacillus megaterium | 30% increase in yield | Banana | [74] |
| Bacillus firlmus Enterobacter spp. Burkholderia spp. Pseudomonas sp. Paenibacillus kribbensis | 12% enhancement in yield, increase in the tillers number and yield | Paddy | [74] |
| Phosphobacterium variety SBS 1 | Increase in yield up to 70% | Sword bean | [74] |
| Azotobacter chroococcum | Increase yield up to 74% | Wheat | [75] |
| Rhizobium lupini | Higher Nitrogen shoot content, enhanced nutrient uptake, increased root and shoot weight | Alfalfa | [76] |
| Pseudomonas cepacia R85, Pseudomonas fluorescens R22 | Increased plant height and yield | Wheat | [77] |
| R. phaseoli | Increase in root nodule formation | Clover | [78] |
| Frateuria aurantia isolate KSBD-58 | Growth parameters, head diameter, test weight, seed yield and potassium content | Sunflower | [79] |
| Co-inoculation of Rhizobium meliloti, Paenibacillus polymyxa and Bacillus megaterium | Increase in dry matter, nodule and dry root weight | Bean | [80] |
| Bradyrhizobium japonicum | Enhanced root nodule formation and yield | Soybean | [81] |
| Azospirillum brasilense, Bacillus, and Pseudomonas fluorescens | Shoot yield, P accumulation in cane, reduced P fertilization by 75% | Sugarcane | [82] |
| Rhizobium leguminosarum sv. viciae | Enhanced nodulation and growth | Pea | [83] |
| Azospirillum brasilense | Increased leaf chlorophyll index, stem girth, grain yield | Maize | [84] |
| Azospirillum brasilense | Seed inoculation increased growth and grain yield (26.7%), nutrient uptake | Wheat | [84,85] |
| Azospirillum sp. | Increase of growth and yield | Finger millet | [23] |
| Azospirillum brasilense Ab-V5 | Increased growth, yield and nitrogen use efficiency (NUE) | Maize | [86] |
| Acinetobacter sp. RC04 and Sinorhizobium sp. RC02 | Seed germination, seedling growth | Safflower | [86] |
| Co-inoculation of BradyrhizobiumAzospirillum, Bacillus and Pseudomonas | Increased nodule number (11.40%) and biomass of nodule (6.47%), root (12.84%), and shoot (6.53%) | Soybean | [87] |
| Phytohormone/ACC Deaminase | PGP Bacteria | References |
|---|---|---|
| ACC deaminase | Arthrobacter, Streptomyces spp., Leifsonia soli sp. nov, Microbacteriumazadirachtae sp. nov., Rhodococcus sp. R04, Micrococcus spp., | [185,186] |
| Auxin/IAA | Actinomyces sp., Bradyrhizobium, Bacillus megaterium, Frankia sp., Micrococcus, Methylobacterium oryzae, Nocardia sp., Rhizobium, Streptomyces spp., S. atrovirens, S. griseoviridis K61, S. lydicus WYEC108, S. olivaceoviridis, S. rimosus, S. rochei, S.viridis | [187,188,189,190,191,192,193,194,195,196,197,198,199] |
| Cytokinins | Arthrobacter, Frankia sp., Leifsonia soli, Rhodococcusfascians, Pseudomonas, Streptomyces turgidiscabies | [186,200] |
| Gibberellin | Actinomyces sp., Bacillus, Arthrobacter, Micrococcus, Nocardia sp., Streptomyces sp. | [187,188,193,201,202,203,204,205,206] |
| Diseases | Pathogen | Antagonistic Strain | References |
|---|---|---|---|
| Crown rot | P. aphanidermatum | M. chalcea | [237] |
| Damping-off | P. aphanidermatum, P. aphanidermatum, F. oxysporum | A. campanulatus, S. rubrolavendulae S4, Streptomyces sp. | [237,238,239] |
| Lupin root rot | P. tabacinum, F. oxysporum, R. solani | A. missouriensis, S. halstedii AJ-7, S. vinaceusdrappus | [240,241] |
| Root rot of lupine | P. cinnamomi | M. carbonacea | [240] |
| Root rot of turfgrass | P. infestans | S. violaceusniger strain YCED-9 | [242] |
| Wood rot | P. chrysosporium, P. placenta, C. versicolor, G. trabeum | S. violaceusniger XL-2 | [243,244] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maitra, S.; Brestic, M.; Bhadra, P.; Shankar, T.; Praharaj, S.; Palai, J.B.; Shah, M.M.R.; Barek, V.; Ondrisik, P.; Skalický, M.; et al. Bioinoculants—Natural Biological Resources for Sustainable Plant Production. Microorganisms 2022, 10, 51. https://doi.org/10.3390/microorganisms10010051
Maitra S, Brestic M, Bhadra P, Shankar T, Praharaj S, Palai JB, Shah MMR, Barek V, Ondrisik P, Skalický M, et al. Bioinoculants—Natural Biological Resources for Sustainable Plant Production. Microorganisms. 2022; 10(1):51. https://doi.org/10.3390/microorganisms10010051
Chicago/Turabian StyleMaitra, Sagar, Marian Brestic, Preetha Bhadra, Tanmoy Shankar, Subhashisa Praharaj, Jnana Bharati Palai, M. Mostafizur Rahman Shah, Viliam Barek, Peter Ondrisik, Milan Skalický, and et al. 2022. "Bioinoculants—Natural Biological Resources for Sustainable Plant Production" Microorganisms 10, no. 1: 51. https://doi.org/10.3390/microorganisms10010051
APA StyleMaitra, S., Brestic, M., Bhadra, P., Shankar, T., Praharaj, S., Palai, J. B., Shah, M. M. R., Barek, V., Ondrisik, P., Skalický, M., & Hossain, A. (2022). Bioinoculants—Natural Biological Resources for Sustainable Plant Production. Microorganisms, 10(1), 51. https://doi.org/10.3390/microorganisms10010051










