Ribosomal Hibernation-Associated Factors in Escherichia coli
Abstract
:1. Introduction
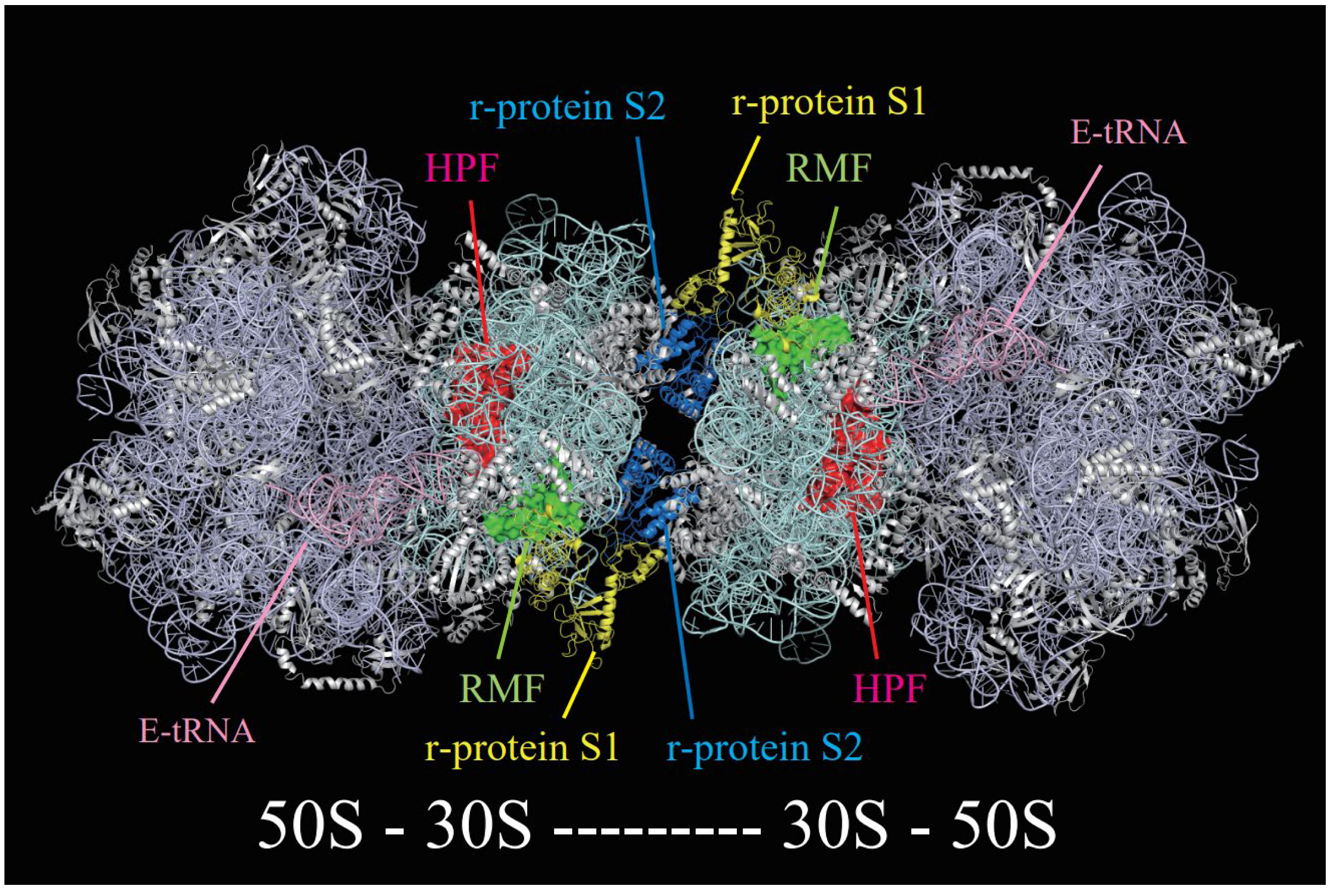
2. 100S Ribosome
3. Preparation of Ribosomal Hibernation
3.1. Synthesis of (p)ppGpp on the Ribosome by RelA
3.2. Changes in Ribosomes Because of Growth Phase Transition
4. Factors Affecting 100S Ribosome Formation
4.1. Ribosome Modulation Factor
4.2. RaiA and HPF
4.3. YqjD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolter, R.; Siegele, D.A.; Tormo, A. The stationary phase of the bacterial life cycle. Annu. Rev. Microbiol. 1993, 47, 855–874. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilmaerts, D.; Windels, E.M.; Verstraeten, N.; Michiels, J. General mechanisms leading to persister formation and awakening. Trends Genet. 2019, 35, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Verstraeten, N.; Knapen, W.; Fauvart, M.; Michiels, J. A Historical Perspective on Bacterial Persistence. Methods Mol. Biol. 2016, 1333, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Issakhanian, L.; Behzadi, P. Antimicrobial agents and urinary tract infections. Curr. Pharm. Des. 2019, 25, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, P.; García-Perdomo, H.A.; Karpiński, T.M.; Issakhanian, L. Metallo-ß-lactamases: A review. Mol. Biol. Rep. 2020, 47, 6281–6294. [Google Scholar] [CrossRef]
- Yoshida, H.; Wada, A. The 100S ribosome: Ribosomal hibernation induced by stress. WIREs RNA 2014, 5, 723–732. [Google Scholar] [CrossRef]
- Ueta, M.; Ohniwa, R.L.; Yoshida, H.; Maki, Y.; Wada, C.; Wada, A. Role of HPF (hibernation promoting factor) in translational activity in Escherichia coli. J. Biochem. 2008, 143, 425–433. [Google Scholar] [CrossRef]
- Ueta, M.; Wada, C.; Wada, A. Formation of 100S ribosomes in Staphylococcus aureus by the hibernation promoting factor homolog Sa HPF. Genes Cells 2010, 15, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Ueta, M.; Wada, C.; Daifuku, T.; Sako, Y.; Bessho, Y.; Kitamura, A.; Ohniwa, R.L.; Morikawa, K.; Yoshida, H.; Kato, T.; et al. Conservation of two distinct types of 100S ribosome in bacteria. Genes Cells 2013, 18, 554–574. [Google Scholar] [CrossRef]
- Wada, A.; Igarashi, K.; Yoshimura, S.; Aimoto, S.; Ishihama, A. Ribosome modulation factor: Stationary growth phase-specific inhibitor of ribosome functions from Escherichia coli. Biochem. Biophys. Res. Commun. 1995, 214, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Maki, Y.; Kato, H.; Fujisawa, H.; Izutsu, K.; Wada, C.; Wada, A. The ribosome modulation factor (RMF) binding site on the 100S ribosome of Escherichia coli. J. Biochem. 2002, 132, 983–989. [Google Scholar] [CrossRef]
- Sulthana, S.; Quesada, E.; Deutscher, M.P. RNase II regulates RNase PH and is essential for cell survival during starvation and stationary phase. RNA 2017, 23, 1456–1464. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, R.F.; Quendera, A.P.; Boavida, S.; Seixas, A.F.; Arraiano, C.M.; Andrade, J.M. Major 3′-5′ exoribonucleases in the metabolism of coding and non-coding RNA. Prog. Mol. Biol. Transl. Sci. 2018, 159, 101–155. [Google Scholar] [CrossRef]
- Franken, L.E.; Oostergetel, G.T.; Pijning, T.; Puri, P.; Arkhipova, V.; Boekema, E.J.; Poolman, B.; Guskov, A. A General mechanism of ribosome dimerization revealed by single-particle cryo-electron microscopy. Nat. Commun. 2017, 8, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckert, B.; Abdelshahid, M.; Schäfer, H.; Steinchen, W.; Arenz, S.; Berninghausen, O.; Beckmann, R.; Bange, G.; Turgay, K.; Wilson, D.N. Structure of the Bacillus Subtilis hibernating 100S ribosome reveals the basis for 70S dimerization. EMBO J. 2017, 36, 2061–2072. [Google Scholar] [CrossRef] [PubMed]
- Beckert, B.; Turk, M.; Czech, A.; Berninghausen, O.; Beckmann, R.; Ignatova, Z.; Plitzko, J.M.; Wilson, D.N. Structure of a hibernating 100S ribosome reveals an inactive conformation of the ribosomal protein S1. Nat. Microbiol. 2018, 3, 1115–1121. [Google Scholar] [CrossRef]
- Matzov, D.; Aibara, S.; Basu, A.; Zimmerman, E.; Bashan, A.; Yap, M.-N.F.; Amunts, A.; Yonath, A.E. The cryo-EM structure of hibernating 100S ribosome dimer from pathogenic Staphylococcus aureus. Nat. Commun. 2017, 8, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khusainov, I.; Vicens, Q.; Ayupov, R.; Usachev, K.; Myasnikov, A.; Simonetti, A.; Validov, S.; Kieffer, B.; Yusupova, G.; Yusupov, M.; et al. Structures and dynamics of hibernating ribosomes from Staphylococcus aureus mediated by intermolecular interactions of HPF. EMBO J. 2017, 36, 2073–2087. [Google Scholar] [CrossRef]
- Lake, J.A. Ribosome structure determined by electron microscopy of Escherichia coli small subunits, large subunits and monomeric ribosomes. J. Mol. Biol. 1976, 105, 131–159. [Google Scholar] [CrossRef]
- Lake, J.A. Ribosomal subunit orientations determined in the monomeric ribosome by single and by double-labeling immune electron microscopy. J. Mol. Biol. 1982, 161, 89–106. [Google Scholar] [CrossRef]
- Wada, A.; Yamazaki, Y.; Fujita, N.; Ishihama, A. Structure and probable genetic location of a “ribosome modulation factor” associated with 100S ribosomes in stationary-phase Escherichia coli cells. Proc. Natl. Aca. Sci. USA 1990, 87, 2657–2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prossliner, T.; Skovbo Winther, K.; Sørensen, M.A.; Gerdes, K. Ribosome hibernation. Annu. Rev. Genet. 2018, 52, 321–348. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yoshida, H.; Miyata, T.; Maki, Y.; Wada, A.; Namba, K. Structure of the 100S ribosome in the hibernation stage revealed by electron cryomicroscopy. Structure 2010, 18, 719–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz, J.O.; Brandt, F.; Matias, V.R.F.; Sennels, L.; Rappsilber, J.; Scheres, S.H.W.; Eibauer, M.; Hartl, F.U.; Baumeister, W. Structure of hibernating ribosomes studied by cryoelectron tomography in vitro and in situ. J. Cell Biol. 2010, 190, 613–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Maki, Y.; Furuike, S.; Sakai, A.; Ueta, M.; Wada, A. YqjD is an inner membrane protein associated with stationary-phase ribosomes in Escherichia coli. J. Bacteriol. 2012, 194, 4178–4183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garay-Arroyo, A.; Colmenero-Flores, J.M.; Garciarrubio, A.; Covarrubias, A.A. Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J. Biol. Chem. 2000, 275, 5668–5674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niven, G.W. Ribosome modulation factor protects Escherichia coli during heat stress, but this may not be dependent on ribosome dimerisation. Arch. Microbiol. 2004, 182, 60–66. [Google Scholar] [CrossRef]
- Song, S.; Wood, T.K. ppGpp ribosome dimerization model for bacterial persister formation and resuscitation. Biochem. Biophys. Res. Commun. 2020, 523, 281–286. [Google Scholar] [CrossRef]
- Aiso, T.; Yoshida, H.; Wada, A.; Ohki, R. Modulation of MRNA stability participates in stationary-phase-specific expression of ribosome modulation factor. J. Bacteriol. 2005, 187, 1951–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, A.; Shields, K.E.; Yap, M.-N.F. The hibernating 100S complex is a target of ribosome-recycling factor and elongation factor G in Staphylococcus aureus. J. Biol. Chem. 2020, 295, 6053–6063. [Google Scholar] [CrossRef] [Green Version]
- Moll, I.; Resch, A.; Bläsi, U. Discrimination of 5′-terminal start codons by translation initiation factor 3 is mediated by ribosomal protein S1. FEBS Lett. 1998, 436, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Flygaard, R.K.; Boegholm, N.; Yusupov, M.; Jenner, L.B. Cryo-EM structure of the hibernating Thermus thermophilus 100S ribosome reveals a protein-mediated dimerization mechanism. Nat. Commun. 2018, 9, 4179. [Google Scholar] [CrossRef] [PubMed]
- Izutsu, K.; Wada, A.; Wada, C. Expression of ribosome modulation factor (RMF) in Escherichia coli requires ppGpp. Genes Cells 2001, 6, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Potrykus, K.; Cashel, M. (p)ppGpp: Still magical? Annu. Rev. Microbiol. 2008, 62, 35–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patacq, C.; Chaudet, N.; Létisse, F. Crucial role of ppGpp in the resilience of Escherichia coli to growth disruption. mSphere 2020, 5, e01132-20. [Google Scholar] [CrossRef]
- Cachel, M.; Gentry, D.R.; Hernandez, V.J.; Vinella, D. Escherichia Coli and Salmonella: Cellular and Molecular Biology, 2nd ed.; Neidhardt, F.C., Ed.; ASM Press: Washington, DC, USA, 1996; ISBN 978-1-55581-084-9. [Google Scholar]
- Lemke, J.J.; Sanchez-Vazquez, P.; Burgos, H.L.; Hedberg, G.; Ross, W.; Gourse, R.L. Direct regulation of Escherichia coli ribosomal protein promoters by the transcription factors ppGpp and DksA. Proc. Natl. Acad. Sci. USA 2011, 108, 5712–5717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, G.C.; Tenson, T.; Hauryliuk, V. The RelA/SpoT homolog (RSH) superfamily: Distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS ONE 2011, 6, e23479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.; Kalman, M.; Ikehara, K.; Zemel, S.; Glaser, G.; Cashel, M. Residual guanosine 3′,5′-bispyrophosphate synthetic activity of RelA null mutants can be eliminated by SpoT null mutations. J. Biol. Chem. 1991, 266, 5980–5990. [Google Scholar] [CrossRef]
- Shyp, V.; Tankov, S.; Ermakov, A.; Kudrin, P.; English, B.P.; Ehrenberg, M.; Tenson, T.; Elf, J.; Hauryliuk, V. Positive allosteric feedback regulation of the stringent response enzyme RelA by its product. EMBO Rep. 2012, 13, 835–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendrich, T.M.; Blaha, G.; Wilson, D.N.; Marahiel, M.A.; Nierhaus, K.H. Dissection of the mechanism for the stringent factor RelA. Mol. Cell 2002, 10, 779–788. [Google Scholar] [CrossRef]
- Arenz, S.; Abdelshahid, M.; Sohmen, D.; Payoe, R.; Starosta, A.L.; Berninghausen, O.; Hauryliuk, V.; Beckmann, R.; Wilson, D.N. The stringent factor RelA adopts an open conformation on the ribosome to stimulate ppGpp synthesis. Nucleic Acids Res. 2016, 44, 6471–6481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.; Fernández, I.S.; Gordiyenko, Y.; Ramakrishnan, V. Ribosome-dependent activation of stringent control. Nature 2016, 534, 277–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loveland, A.B.; Bah, E.; Madireddy, R.; Zhang, Y.; Brilot, A.F.; Grigorieff, N.; Korostelev, A.A. Ribosome•RelA structures reveal the mechanism of stringent response activation. eLife 2016, 5, e17029. [Google Scholar] [CrossRef] [PubMed]
- Karimi, R.; Pavlov, M.Y.; Buckingham, R.H.; Ehrenberg, M. Novel roles for classical factors at the interface between translation termination and initiation. Mol. Cell 1999, 3, 601–609. [Google Scholar] [CrossRef]
- Yoshida, H.; Ueta, M.; Maki, Y.; Sakai, A.; Wada, A. Activities of Escherichia coli ribosomes in IF3 and RMF change to prepare 100S ribosome formation on entering the stationary growth phase. Genes Cells 2009, 14, 271–280. [Google Scholar] [CrossRef]
- Qu, X.; Lancaster, L.; Noller, H.F.; Bustamante, C.; Tinoco, I. Ribosomal protein S1 unwinds double-stranded RNA in multiple steps. Proc. Natl. Acad. Sci. USA 2012, 109, 14458–14463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laughrea, M.; Tam, J. Interaction of ribosomal protein S1 and initiation factor IF3 with the 3’ major domain and the decoding site of the 30S subunit of Escherichia coli. Biochemistry 1991, 30, 11412–11420. [Google Scholar] [CrossRef]
- Shimada, T.; Yoshida, H.; Ishihama, A. Involvement of cyclic AMP receptor protein in regulation of the rmf gene encoding the ribosome modulation factor in Escherichia coli. J. Bacteriol. 2013, 195, 2212–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Shimada, T.; Ishihama, A. Coordinated hibernation of transcriptional and translational apparatus during growth transition of Escherichia coli to stationary phase. mSystems 2018, 3, e00057-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, A.; Steiner, S.; Zaehringer, F.; Casanova, A.; Hamburger, F.; Ritz, D.; Keck, W.; Ackermann, M.; Schirmer, T.; Jenal, U. Second messenger signalling governs Escherichia coli biofilm induction upon ribosomal stress. Mol. Microbiol. 2009, 72, 1500–1516. [Google Scholar] [CrossRef] [PubMed]
- El-Sharoud, W.M.; Niven, G.W. The activity of ribosome modulation factor during growth of Escherichia coli under acidic conditions. Arch. Microbiol. 2005, 184, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yamamoto, H.; Uchiumi, T.; Wada, A. RMF inactivates ribosomes by covering the peptidyl transferase centre and entrance of peptide exit tunnel. Genes Cells 2004, 9, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Polikanov, Y.S.; Blaha, G.M.; Steitz, T.A. How hibernation factors RMF, HPF, and YfiA turn off protein synthesis. Science 2012, 336, 915–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Nakayama, H.; Maki, Y.; Ueta, M.; Wada, C.; Wada, A. Functional sites of ribosome modulation factor (RMF) involved in the formation of 100S ribosome. Front. Mol. Biosci. 2021, 8, 661691. [Google Scholar] [CrossRef] [PubMed]
- Agafonov, D.E.; Kolb, V.A.; Nazimov, I.V.; Spirin, A.S. A protein residing at the subunit interface of the bacterial ribosome. Proc. Natl. Acad. Sci. USA 1999, 96, 12345–12349. [Google Scholar] [CrossRef] [Green Version]
- Agafonov, D.E.; Kolb, V.A.; Spirin, A.S. Ribosome-associated protein that inhibits translation at the aminoacyl-TRNA binding stage. EMBO Rep. 2001, 2, 399–402. [Google Scholar] [CrossRef] [Green Version]
- Agafonov, D.E.; Spirin, A.S. The ribosome-associated inhibitor A reduces translation errors. Biochem. Biophys. Res. Commun. 2004, 320, 354–358. [Google Scholar] [CrossRef]
- Maki, Y.; Yoshida, H.; Wada, A. Two proteins, YfiA and YhbH, associated with resting ribosomes in stationary phase Escherichia coli. Genes Cells 2000, 5, 965–974. [Google Scholar] [CrossRef]
- Ueta, M.; Yoshida, H.; Wada, C.; Baba, T.; Mori, H.; Wada, A. Ribosome binding proteins YhbH and YfiA have opposite functions during 100S formation in the stationary phase of Escherichia coli. Genes Cells 2005, 10, 1103–1112. [Google Scholar] [CrossRef]
- Vila-Sanjurjo, A.; Schuwirth, B.-S.; Hau, C.W.; Cate, J.H.D. Structural basis for the control of translation initiation during stress. Nat. Struct. Mol. Biol. 2004, 11, 1054–1059. [Google Scholar] [CrossRef]
- Sato, A.; Watanabe, T.; Maki, Y.; Ueta, M.; Yoshida, H.; Ito, Y.; Wada, A.; Mishima, M. Solution structure of the E. coli ribosome hibernation promoting factor HPF: Implications for the relationship between structure and function. Biochem. Biophys. Res. Commun. 2009, 389, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Sanchuki, H.B.S.; Gravina, F.; Rodrigues, T.E.; Gerhardt, E.C.M.; Pedrosa, F.O.; Souza, E.M.; Raittz, R.T.; Valdameri, G.; de Souza, G.A.; Huergo, L.F. Dynamics of the Escherichia coli Proteome in Response to Nitrogen Starvation and Entry into the Stationary Phase. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2017, 1865, 344–352. [Google Scholar] [CrossRef]
- DeLisa, M.P.; Wu, C.-F.; Wang, L.; Valdes, J.J.; Bentley, W.E. DNA microarray-based identification of genes controlled by autoinducer 2-stimulated quorum sensing in Escherichia coli. J. Bacteriol. 2001, 183, 5239–5247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmon, K.; Hung, S.; Mekjian, K.; Baldi, P.; Hatfield, G.W.; Gunsalus, R.P. Global gene expression profiling in Escherichia coli K12. The effects of oxygen availability and FNR. J. Biol. Chem. 2003, 278, 29837–29855. [Google Scholar] [CrossRef] [Green Version]
- Durfee, T.; Hansen, A.-M.; Zhi, H.; Blattner, F.R.; Jin, D.J. Transcription profiling of the stringent response in Escherichia coli. J. Bacteriol. 2008, 190, 1084–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferdosh, S.; Banerjee, S.; Pathak, B.K.; Sengupta, J.; Barat, C. Hibernating ribosomes exhibit chaperoning activity but can resist unfolded protein-mediated subunit dissociation. FEBS J. 2021, 288, 1305–1324. [Google Scholar] [CrossRef]
- Theng, S.; Williamson, K.S.; Franklin, M.J. Role of hibernation promoting factor in ribosomal protein stability during Pseudomonas aeruginosa dormancy. Int. J. Mol. Sci. 2020, 21, 9494. [Google Scholar] [CrossRef] [PubMed]
- Feaga, H.A.; Kopylov, M.; Kim, J.K.; Jovanovic, M.; Dworkin, J. Ribosome dimerization protects the small subunit. J. Bacteriol. 2020, 202, e00009-20. [Google Scholar] [CrossRef] [PubMed]
- Feaga, H.A.; Dworkin, J. Transcription regulates ribosome hibernation. Mol. Microbiol. 2021, 116, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Prossliner, T.; Gerdes, K.; Sørensen, M.A.; Winther, K.S. Hibernation factors directly block ribonucleases from entering the ribosome in response to starvation. Nucleic Acids Res. 2021, 49, 2226–2239. [Google Scholar] [CrossRef]
- Lipońska, A.; Yap, M.-N.F. Hibernation-promoting factor sequesters Staphylococcus aureus ribosomes to antagonize RNase R-mediated nucleolytic degradation. mBio 2021, 12, e00334-21. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Q.; Wood, T.K. The R1 conjugative plasmid increases Escherichia coli biofilm formation through an envelope stress response. Appl. Environ. Microbiol. 2008, 74, 2690–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Name | Mr | Gene Position | Main Function Related to 100S Ribosomes |
|---|---|---|---|
| Factors directly involved in 100S ribosome formation | |||
| RMF | 6.5 k | 1016137–1016304 | Dimerizing ribosomes |
| HPF | 10.8 k | 3346028–3346315 | Stabilizing 100S ribosomes |
| Major factors indirectly involved in 100S ribosome formation | |||
| RaiA | 12.8 k | 2735810–2736151 | Inhibiting ribosome dimerization |
| RelA | 83.9 k | 2910073–2912307 | Synthesis of (p)ppGpp |
| YqjD | 11.1 k | 3248031–3248336 | Localizing ribosomes to membrane |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maki, Y.; Yoshida, H. Ribosomal Hibernation-Associated Factors in Escherichia coli. Microorganisms 2022, 10, 33. https://doi.org/10.3390/microorganisms10010033
Maki Y, Yoshida H. Ribosomal Hibernation-Associated Factors in Escherichia coli. Microorganisms. 2022; 10(1):33. https://doi.org/10.3390/microorganisms10010033
Chicago/Turabian StyleMaki, Yasushi, and Hideji Yoshida. 2022. "Ribosomal Hibernation-Associated Factors in Escherichia coli" Microorganisms 10, no. 1: 33. https://doi.org/10.3390/microorganisms10010033
APA StyleMaki, Y., & Yoshida, H. (2022). Ribosomal Hibernation-Associated Factors in Escherichia coli. Microorganisms, 10(1), 33. https://doi.org/10.3390/microorganisms10010033






