Cases of Acute Flaccid Paralysis Associated with Coxsackievirus A2: Findings of a 20-Year Surveillance in the Russian Federation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Identification
2.2. Epidemiological and Clinical Data
2.3. Virological Investigation
2.4. Phylogenetic Studies
- (1)
- Collection date and place of sequences extracted from the Genbank database were retrieved from Genbank annotation. Sequences with missing metadata were omitted.
- (2)
- Too-short (250 nt) and too-long sequences (over 8000 nt) were removed from the dataset.
- (3)
- Both sequences downloaded from Genbank and 15 sequences of isolates collected in Russia were aligned using MAFFT v.7.304 [31].
- (4)
- Typing VP1 sequence was excised from an alignment automatically according to a reference sequence.
- (5)
- Sequences with multiple ambiguous nucleotides and obvious errors were omitted.
2.5. Ethical Statement
3. Results
3.1. Clinical and Epidemiological Characteristics of Cases
3.2. Virological Investigation
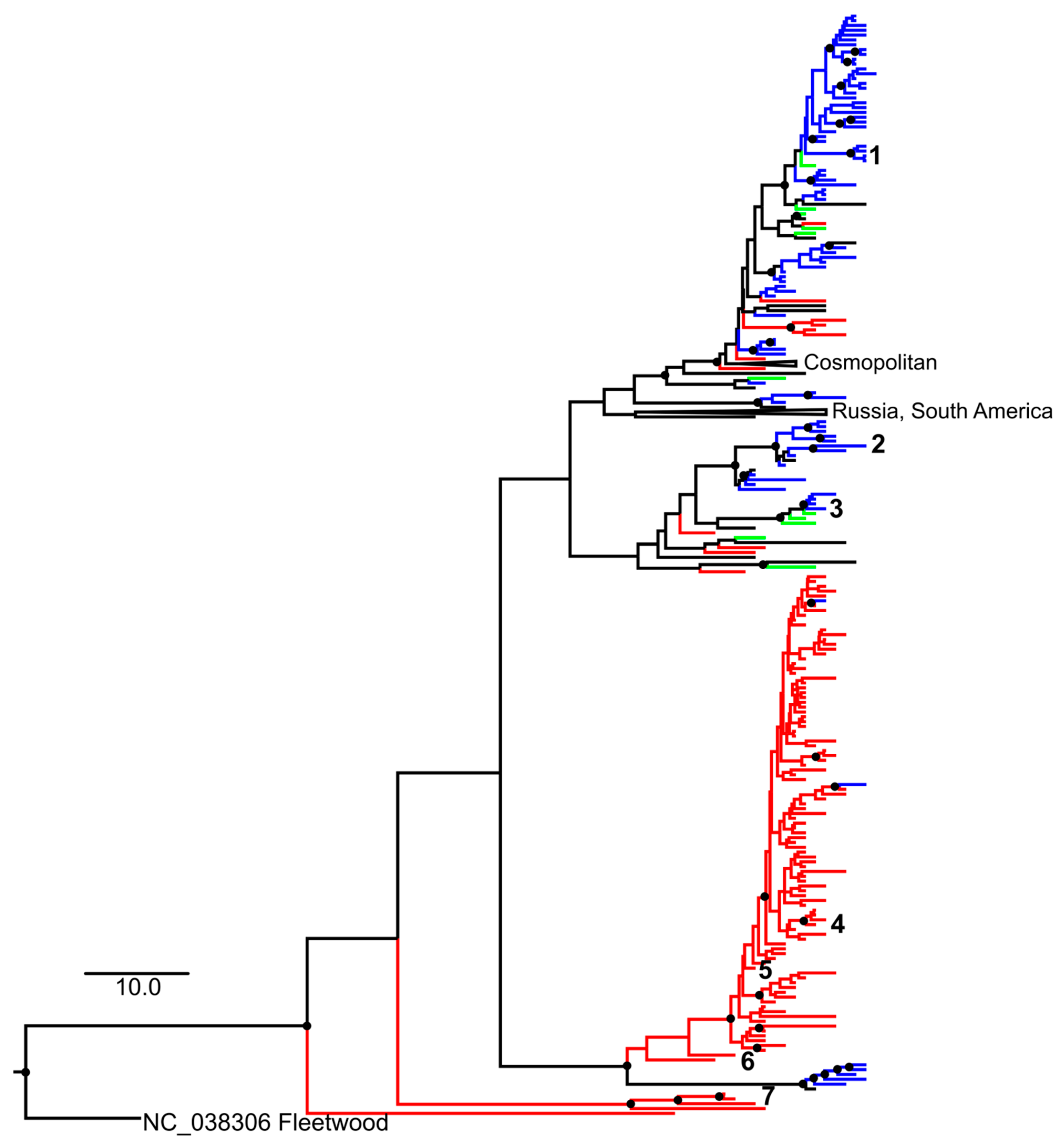
3.3. Phylogenetic Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Committee on Taxonomy of Viruses (ICTV). Available online: https://ictv.global/taxonomy/ (accessed on 27 October 2021).
- Chiang, K.-L.; Wei, S.-H.; Fan, H.-C.; Chou, Y.-K.; Yang, J.-Y. Outbreak of recombinant coxsackievirus A2 infection and polio-like paralysis of children, Taiwan, 2014. Pediatr. Neonatol. 2019, 60, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Gao, H.-H.; Zhang, O.; Liu, Y.-J.; Tao, R.; Cheng, Y.-P.; Shu, O.; Shang, S.-Q. Large outbreak of herpangina in children caused by enterovirus in summer of 2015 in Hangzhou, China. Sci. Rep. 2016, 6, 35388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Zhang, Y.; Yan, D.; Zhu, S.; Wang, D.; Ji, T.; Li, X.; Song, Y.; Gu, X.; Xu, W. Two genotypes of coxsackievirus A2 associated with hand, foot, and mouth disease circulating in China since 2008. PLoS ONE 2016, 11, e0169021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.; Fan, H.; Lu, P.-X.; Zhang, X.-F.; Ai, J.; Shi, C.; Huo, X.; Bao, C.-J.; Shan, J.; Jin, Y. Surveillance for severe hand, foot, and mouth disease from 2009 to 2015 in Jiangsu province: Epidemiology, etiology, and disease burden. BMC Infect. Dis. 2019, 19, 79. Available online: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-018-3659-7 (accessed on 29 December 2021). [CrossRef] [PubMed] [Green Version]
- Chen, S.P.; Huang, Y.C.; Li, W.C.; Chiu, C.H.; Huang, C.G.; Tsao, K.C.; Lin, T.Y. Comparison of clinical features between Coxsackievirus A2 and Enterovirus 71 during the enterovirus outbreak in Taiwan, 2008: A children’s hospital experience. Microbiol. Immunol. Infect. 2010, 43, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Tapparel, C.; Siegrist, F.; Petty, T.J.; Kaiser, L. Picornavirus and enterovirus diversity with associated human diseases. Infect. Genet. Evol. 2013, 14, 282–293. [Google Scholar] [CrossRef]
- Bendig, J.W.A.; O’Brien, P.S.; Muir, P.; Porter, H.J.; ECaul, E.O. Enterovirus sequences resembling Coxsackievirus A2 detected in stool and spleen from a girl with fatal myocarditis. J. Med. Virol. 2001, 64, 482–486. [Google Scholar] [CrossRef]
- Moleta, L.; Saloum, K.; Marque-Juillet, S.; Garbarg-Chenon, A.; Henquell, C.; Schuffenecker, I.; Peigue-Lafeuille, H.; Rozenberg, F.; Mirand, A. Enterovirus infections in hospitals of Ile de France region over 2013. J. Clin. Virol. 2016, 74, 37–42. [Google Scholar] [CrossRef]
- Ohara, N.; Kaneko, M.; Nishibori, T.; Sato, K.; Furukawa, T.; Koike, T.; Sone, H.; Kaneko, K.; Kamoi, K. Fulminant type 1 diabetes mellitus associated with Coxsackie virus type A2 infection: A case report and literature review. Intern. Med. 2016, 55, 643–646. [Google Scholar] [CrossRef] [Green Version]
- Marx, A.; Glass, J.D.; Sutter, R.W. Differential diagnosis of Acute Flaccid Paralysis and its role in poliomyelitis. Epidemiol. Rev. 2000, 22, 298–316. [Google Scholar] [CrossRef] [Green Version]
- Olivé, J.M.; Castillo, C.; Castro, R.G.; de Quadros, C.A. Epidemiologic study of Guillain-Barre syndrome in children <15 years of age in Latin America. J. Infect. Dis. 1997, 175 (Supple. 1), S160–S164. [Google Scholar] [CrossRef] [Green Version]
- World Health Assembly. Global Eradication of Poliomyelitis by the Year 2000; WHA Resolution No. WHA41.28; World Health Organization: Geneva, Switzerland, 1988; Available online: https://apps.who.int/iris/handle/10665/164531 (accessed on 29 December 2021).
- Chouikha, A.; Rezig, D.; Driss, N.; Abdelkhalek, I.; Ben Yahia, A.; Touzi, H.; Meddeb, Z.; Ben Farhat, E.; Yahyaoui, M.; Triki, H. Circulation and molecular epidemiology of enteroviruses in paralyzed, immunodeficient and healthy individuals in Tunisia, a country with a polio-free status for decades. Viruses 2021, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Krzysztoszek, A. Molecular characterization of enteroviruses isolated from acute flaccid paralysis cases in Poland, 1999–2014. Pol. J. Microbiol. 2016, 65, 443–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrinelli, L.; Galli, C.; Primache, V.; Bubba, L.; Buttinelli, G.; Stefanelli, P.; Pariani, E.; Binda, S. Emerging non-polio enteroviruses recognized in the framework of the acute flaccid paralyses (AFP) surveillance system in Northern Italy, 2016–2018. Int. J. Infect. Dis. 2021, 106, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.M.A.; Apostol, L.N.; de Quiroz-Castro, M.; Jee, Y.; Roque, V.; Mapue, M.; Navarro, F.M.; Fe Tabada, C.; Tandoc, A. Non-polio enteroviruses among healthy children in the Philippines. BMC Public Health 2020, 20, 167. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Manual for the Virological Investigation of Polio, 4th ed.; WHO: Geneva, Switzerland, 2004; Available online: http://whqlibdoc.who.int/hq/2004/WHO_IVB_04.10.pdf (accessed on 27 October 2021).
- Suresh, S.; Rawlinson, W.D.; Andrews, P.I.; Stelzer-Braid, S. Global epidemiology of nonpolio enteroviruses causing severe neurological complications: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, e2082. [Google Scholar] [CrossRef]
- van der Sanden, S.M.G.; Koopmans, M.P.G.; van der Avoort, H.G.A.M. Detection of human enteroviruses and parechoviruses as part of the national enterovirus surveillance in the Netherlands, 1996–2011. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Khetsuriani, N.; LaMonte, A.; Oberste, M.S.; Pallansch, M. Neonatal enterovirus infections reported to the National Enterovirus Surveillance System in the United States, 1983–2003. Pediatr. Infect. Dis. J. 2006, 25, 889–893. [Google Scholar] [CrossRef] [Green Version]
- The National Committee for the Certification of Wild Poliovirus Eradication in Hong Kong (NCC). Fifteen years of acute flaccid paralysis surveillance in Hong Kong: Findings from 1997 to 2011. J. Paediatr. Child Health 2014, 50, 545–552. [Google Scholar] [CrossRef]
- Kim, H.; Kang, B.; Hwang, S.; Lee, S.W.; Cheon, D.-S.; Kim, K.; Jeong, Y.-S.; Hyeon, J.-Y. Clinical and enterovirus findings associated with acute flaccid paralysis in the Republic of Korea during the recent Decade. J. Med. Virol. 2014, 86, 1584–1589. [Google Scholar] [CrossRef]
- Sousa, I.P.; de Lourdes Aguiar Oliveira, M.; Burlandy, F.M.; Machado, R.S.; Oliveira, S.S.; Tavares, F.N.; Gomes-Neto, F.; da Costa, E.V.; da Silva, E.E. Molecular characterization and epidemiological aspects of non-polio enteroviruses isolated from acute flaccid paralysis in Brazil: A historical series (2005–2017). Emerg. Microbes Infect. 2020, 9, 2536–2546. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Regional Office for Europe. Available online: http://data.euro.who.int/cisid/default.aspx?TabID=539511 (accessed on 27 October 2021).
- The Ministry of Health of the Russian Federation; The State Committee for Sanitary and Epidemiological Supervision of the Russian Federation. On Approval of the Poliomyelitis Eradication Program on the Territory of the Russian Federation. Available online: https://base.garant.ru/4120180/ (accessed on 29 December 2021).
- Oberste, M.S.; Maher, K.; Kilpatrick, D.R.; Flemister, M.R.; Brown, B.A.; Pallansch, M.A. Typing of human enteroviruses by partial sequencing of VP1. J. Clin. Microbiol. 1999, 37, 1288–1293. [Google Scholar] [CrossRef] [Green Version]
- Federal Service for Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor). Program “Epidemiological Surveillance and Prevention of Enterovirus (Non-Polio) Infection for 2018–2022. Available online: https://fcgie.ru/download/koord_tsentr/%D0%AD%D0%92%D0%98%20%D0%9F%D0%A0%D0%9E%D0%93%D0%A0%D0%90%D0%9C%D0%9C%D0%90%2018-22.pdf (accessed on 29 December 2021).
- Nix, W.A.; Oberste, M.S.; Pallansch, M.A. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 2006, 44, 2698–2704. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://github.com/v-julia/GenAlignment (accessed on 29 December 2021).
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. 2 IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Hoang, D.P.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A.; Lam, T.T.; Carvalho, L.M.; Pybus, O.G. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen). Virus Evol. 2016, 2, vew007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vew016. [Google Scholar] [CrossRef] [Green Version]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2016, msw260. [Google Scholar] [CrossRef] [Green Version]
- Baele, G.; Lemey, P.; Bedford, T.; Rambaut, A.; Suchard, M.A.; Alekseyenko, A.V. Improving the Accuracy of Demographic and Molecular Clock Model Comparison While Accommodating Phylogenetic Uncertainty. Mol. Biol. Evol. 2012, 29, 2157–2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: http://tree.bio.ed.ac.uk/software/tracer (accessed on 29 December 2021).
- Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 29 December 2021).
- World Health Organization (WHO). Report of the Second Meeting of the Technical Consultation Group for Global Eradication of Poliomyelitis; WHO/EPI/GEN/98/04; WHO: Geneva, Switzerland, 1998; Available online: https://apps.who.int/iris/bitstream/handle/10665/63994/WHO_EPI_GEN_98.04.pdf?sequence=1&isAllowed=y (accessed on 29 December 2021).
- Leschinskaya, E.V.; Latysheva, I.N. The Clinic, Diagnosis and Treatment of Acute Poliomyelitis. In Guidelines. Moscow: M.P. Chumakov Institute of Poliomyelitis and Viral Encephalitis Them; M.P. Chumakov Russian Academy of Medical Sciences: Moscow, Russian, 1998. [Google Scholar]
- Yen, T.-Y.; Huang, Y.-P.; Hsu, Y.-L.; Chang, Y.-T.; Lin, H.-C.; Wu, H.-S.; Hwang, K.-P. A case of recombinant coxsackievirus A2 infection with neurological complications in Taiwan. J. Microbiol. Immunol. Infect. 2017, 50, 928–930. [Google Scholar] [CrossRef] [PubMed]
- Chumakov, M.; Voroshilova, M.; Shindarov, L.; Lavrova, I.; Gracheva, L.; Koroleva, G.; Vasilenko, S.; Brodvarova, I.; Nikolova, M.; Gyurova, S.; et al. Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch. Virol. 1979, 60, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Midgley, C.M.; Watson, J.T.; Nix, W.A.; Curns, A.T.; Rogers, S.L.; Brown, B.A.; Conover, C.; Dominguez, S.R.; Feikin, D.R.; The EV-D68 Working Group; et al. Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): A descriptive epidemiological investigation. Lancet Respir. Med. 2015, 3, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Clusters of acute respiratory illness associated with human enterovirus 68—Asia, Europe, and United States, 2008–2010. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 1301–1304. [Google Scholar]
- Poelman, R.; Schuenecker, I.; Van Leer-Buter, C.; Josset, L.; Niesters, H.G.M.; Lina, B. European surveillance for enterovirus D68 during the emerging North-American outbreak in 2014. J. Clin. Virol. 2015, 71, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Acute Flaccid Myelitis (AFM) 2020 Case Definition. Available online: https://wwwn.cdc.gov/nndss/conditions/acute-flaccid-myelitis/case-definition/2020/ (accessed on 27 October 2021).
- Kramer, R.; Sabatier, M.; Wirth, T.; Pichon, M.; Lina, B.; Schuffenecker, I.; Josset, L. Molecular diversity and biennial circulation of enterovirus D68: A systematic screening study in Lyon, France, 2010 to 2016. Eurosurveillance 2018, 23, 1700711. [Google Scholar] [CrossRef] [Green Version]
- Dickson, C.; Ho Mi Fane, B.; Squires, S.G. Acute flaccid myelitis in Canada, 2018 to 2019. Can. Commun. Dis. Rep. 2020, 46, 349–353. [Google Scholar] [CrossRef]
- Simonen-Tikka, M.L.; Hiekka, A.K.; Klemola, P.; Poussa, T.; Ludvigsson, J.; Korpela, R.; Vaarala, O.; Merja Roivainen, M. Early human entero- virus infec-tions in healthy Swedish children participating in the PRODIA pilot study. J. Med. Virol. 2012, 84, 923–930. [Google Scholar] [CrossRef]
- Witso, E.; Palacios, G.; Cinek, O.; Stene, L.C.; Grinde, B.; Janowitz, D.; Lipkin, W.I.; Rønningen, K.S. High prevalence of human entero- virus a infec-tions in natural circulation of human enteroviruses. J. Clin. Microbiol. 2006, 44, 4095–4100. [Google Scholar] [CrossRef] [Green Version]
- Solomon, T.; Lewthwaite, P.; Perera, D.; Cardosa, M.J.; McMinn, P.; Ooi, M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010, 10, 778–790. [Google Scholar] [CrossRef]
- Gaunt, E.; Harvala, H.; Österback, R.; Sreenu, V.B.; Thomson, E.; Waris, M.; Simmonds, P. Genetic characterization of human coxsackievirus A6 variants associated with atypical hand, foot and mouth disease: A potential role of recombination in emergence and pathogenicity. J. Gen. Virol. 2015, 96, 1067–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, O.E.; Yarmolskaya, M.S.; Eremeeva, T.P.; Babkina, G.M.; Baykova, O.Y.; Akhmadishina, L.V.; Krasota, A.Y.; Kozlovskaya, L.I.; Lukashev, A.N. Environmental Surveillance for Poliovirus and Other Enteroviruses: Long-Term Experience in Moscow, Russian Federation, 2004–2017. Viruses 2019, 11, 424. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Manual for Virological Investigation of Poliomyelitis; WHO/EPI/GEN/97.1; WHO: Geneva, Switzerland, 1997; Available online: https://apps.who.int/iris/bitstream/handle/10665/62186/WHO_EPI_CDS_POLIO_90.1.pdf?sequence=1&isAllowed=y (accessed on 27 October 2021).
- Ivanova, O.E.; Yurashko, O.V.; Eremeeva, T.P.; Baikova, O.Y.; Morozova, N.S.; Lukashev, A.N. Adenovirus isolation rates in acute flaccid paralysis patients. J. Med. Virol. 2012, 84, 75–80. [Google Scholar] [CrossRef]
- de Azevedo, J.P.R.; Nascimento, L.R.; Cortinovis, M.C.; Oliveira, S.S.; da Costa, E.V.; da Silva, E.E. Characterization of species B adenoviruses isolated from fecal specimens taken from poliomyelitis-suspected cases. J. Clin. Virol. 2004, 31, 248–252. [Google Scholar] [CrossRef]
- Yousefi, M.; Nejati, A.; Zahraei, S.M.; Mahmoudi, S.; Parhizgari, N.; Farsani, S.M.J.; Mahmoodi, M.; Nategh, R.; Shahmahmoodi, S. Enteroviruses and Adenoviruses in stool specimens of paralytic children- can they be the cause of paralysis? Iran. J. Microbiol. 2018, 10, 194–201. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc6087694/ (accessed on 29 December 2021). [PubMed]
- Haddad-Boubaker, S.; Joffret, M.-L.; Pérot, P.; Bessaud, M.; Meddeb, Z.; Touzi, H.; Delpeyroux, F.; Triki, H.; Eloit, M. Metagenomic analysis identifies human adenovirus 31 in children with acute flaccid paralysis in Tunisia. Arch. Virol. 2019, 164, 747–755. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Polio Eradication Strategy 2022−2026: Executive Summary; 2021(WHO/Polio/21.04); Licence CC BY-NC-SA 3.0 IGO. World Health Organization: Geneva, Switzerland. Available online: https://polioeradication.org/wp-content/uploads/2021/10/9789240031937-eng.pdf (accessed on 27 October 2021).
| Characteristic | Patients | ||||||
|---|---|---|---|---|---|---|---|
| H.A. | K.G. | D.A. | N.T. | K.O. | |||
| Place of residence | Bryansk | Saratov | St. Petersburg | Vladimir region | Nizhny Novgorod region | ||
| Gender | F | M | M | F | M | ||
| Age, years | 1 | 2 | 4 | 3 | 3 | ||
| Number of doses and the type of polio vaccine | 2 OPV | 5 OPV | 4 IPV | 3 IPV, 2 OPV | 2 IPV, 3 OPV | ||
| Date of the paralysis onset | 5 October 2008 | 9 September 2008 | 23 September 2015 | 10 September 2019 | 23 September 2019 | ||
| Time from the last vaccination to the disease onset, months | 13 | 5 | 24 | 8 | 2 | ||
| Time from the disease onset to the full manifestation of paralysis, days | 10 | 4 | 1 | 1 | 2 | ||
| Body temperature at the disease onset | normal | 39.0 °C | elevated, value unknown | 39.0 °C | 37.8 °C | ||
| Localization of paralysis | left leg, right leg | left leg > right leg | left leg | left hand > right hand | quadriparesis (lower limbs > upper limbs) | ||
| Proximal/distal | proximal | proximal > dystal | both | proximal > distal | both | ||
| Other neurological signs | encopresis, enuresis | ||||||
| Virus source and type isolated | feces, CVA2 | feces, CVA2 | feces, CVA2 | feces, CVA2 oropharyngeal swab, CVA2 | feces, CVA2 | ||
| CSF study results | Day since disease onset | unknown | 09.12.19 | 27 September 2019 | 4 October 2019 | ||
| Cytosis, cells/mm3 | no data | 5 | no data | 3 | 61 | 12 | |
| Protein, g/L | 0.99 | 0.84 | 0.14 | 0.57 | |||
| Glucose, mmol/L | no data | no data | 1.81 | 2.0 | |||
| Lymphocytes, % | 80 | 84 | |||||
| Neutrophils, % | 20 | 16 | |||||
| Health condition before AFP | healthy | infectious mononucleosis one month prior to paresis | healthy | convalescence of bilateral focal pneumonia | aplasia of the left kidney, minor anomaly of heart development. | ||
| Residual paralysis after 60 days from the onset | no | no | yes | yes | yes | ||
| Clinical diagnosis at discharge * | Paraparesis of the lower limbs associated with CVA2. Organic lesion of central nervous system. | Polyradiculo-neuritis associated with non-polio enterovirus | Monoparesis of left lower limb | Enterovirus infection, cervical myelitis, upper flaccid paraparesis | Acute meningomyelitis | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, O.E.; Shakaryan, A.K.; Morozova, N.S.; Vakulenko, Y.A.; Eremeeva, T.P.; Kozlovskaya, L.I.; Baykova, O.Y.; Shustova, E.Y.; Mikhailova, Y.M.; Romanenkova, N.I.; et al. Cases of Acute Flaccid Paralysis Associated with Coxsackievirus A2: Findings of a 20-Year Surveillance in the Russian Federation. Microorganisms 2022, 10, 112. https://doi.org/10.3390/microorganisms10010112
Ivanova OE, Shakaryan AK, Morozova NS, Vakulenko YA, Eremeeva TP, Kozlovskaya LI, Baykova OY, Shustova EY, Mikhailova YM, Romanenkova NI, et al. Cases of Acute Flaccid Paralysis Associated with Coxsackievirus A2: Findings of a 20-Year Surveillance in the Russian Federation. Microorganisms. 2022; 10(1):112. https://doi.org/10.3390/microorganisms10010112
Chicago/Turabian StyleIvanova, Olga E., Armen K. Shakaryan, Nadezhda S. Morozova, Yulia A. Vakulenko, Tatyana P. Eremeeva, Liubov I. Kozlovskaya, Olga Y. Baykova, Elena Y. Shustova, Yulia M. Mikhailova, Natalia I. Romanenkova, and et al. 2022. "Cases of Acute Flaccid Paralysis Associated with Coxsackievirus A2: Findings of a 20-Year Surveillance in the Russian Federation" Microorganisms 10, no. 1: 112. https://doi.org/10.3390/microorganisms10010112
APA StyleIvanova, O. E., Shakaryan, A. K., Morozova, N. S., Vakulenko, Y. A., Eremeeva, T. P., Kozlovskaya, L. I., Baykova, O. Y., Shustova, E. Y., Mikhailova, Y. M., Romanenkova, N. I., Rozaeva, N. R., Dzhaparidze, N. I., Novikova, N. A., Zverev, V. V., Golitsyna, L. N., & Lukashev, A. N. (2022). Cases of Acute Flaccid Paralysis Associated with Coxsackievirus A2: Findings of a 20-Year Surveillance in the Russian Federation. Microorganisms, 10(1), 112. https://doi.org/10.3390/microorganisms10010112






