Abstract
Background: Sterols are the main components of fungal membranes. Inhibiting their biosynthesis is the mode of action of azole antifungal drugs that are widely used to treat fungal disease including aspergillosis. Azole resistance has emerged as a matter of concern but little is known about sterols biosynthesis in azole resistant Aspergillus fumigatus. Methods: We explored the sterol composition of 12 A. fumigatus isolates, including nine azole resistant isolates with TR34/L98H, TR46/Y121F/T289A or TR53 alterations in the cyp51A gene and its promoter conferring azole resistance. Modifications in sterol composition were also investigated after exposure to two azole drugs, itraconazole and voriconazole. Results: Overall, under basal conditions, sterol compositions were qualitatively equivalent, whatever the alterations in the target of azole drugs with ergosterol as the main sterol detected. Azole exposure reduced ergosterol composition and the qualitative composition of sterols was similar in both susceptible and resistant isolates. Interestingly TR53 strains behaved differently than other strains. Conclusions: Elucidating sterol composition in azole-susceptible and resistant isolates is of interest for a better understanding of the mechanism of action of these drugs and the mechanism of resistance of fungi.
1. Introduction
Invasive aspergillosis is a life-threatening infection in immunocompromised patients mainly due to Aspergillus fumigatus. Azole-drugs (itraconazole, voriconazole, posaconazole and isavuconazole) are the first-line therapy for prevention and treatment of Aspergillus infections [1,2]. They act by inhibiting the 14α-lanosterol demethylase, which has a key role in ergosterol biosynthesis [3]. Ergosterol is an important component of fungi, involved in membrane fluidity and cell integrity [4]. The burden of azole resistance in A. fumigatus has been pointed out since 2007 in Europe [5,6]. This worrisome problem has spread worldwide [7]. Mechanism of azole-resistance in A. fumigatus is mainly due to alteration in the cyp51a gene and/or its promotor, encoding for the 14α-demethylase. Two routes of azole-resistance are currently admitted: (i) the “clinical route” of azole resistance, in long-term treated patients with azole drugs and (ii) the “environmental route” of azole resistance, due to the wide use of azole fungicides in agriculture called demethylation inhibitors (DMI) which chemical structures are very close to clinical azole drugs [8]. Until now, the clinical route of azole resistance was associated with point mutations in the cyp51a gene while the environmental route of azole resistance led to complex alteration with tandem replication of a part of the promoter of the cyp51a gene, with or without point mutations in the cyp51a gene (TR34/L98H, TR46/Y121F/T289A, TR53…) [9].
As ergosterol biosynthesis is targeted by azole compounds, elucidating sterol composition in azole-susceptible and resistant A. fumigatus is of interest to understand the mechanism of action of these drugs and the mechanism of resistance of fungi. The ergosterol biosynthesis pathway has been described for some fungal pathogens such as Candida albicans [10], Cryptococcus neoformans [11], A. fumigatus [12] and more recently for Mucorales [13]. Concerning A. fumigatus, sterol studies mostly focused on azole-susceptible clinical isolates. So far, a single itraconazole-susceptible environmental strain has been studied [14]. In the few azole-resistant strains studied, resistance was induced by exposure to azole compounds [15] or by engineered mutations [12,16,17,18]; only one study included azole-resistant isolates from clinical origin [12]. Under basal condition, whatever the mutation in cyp51A, ergosterol remained the main sterol in azole-sensitive and azole-resistant strains and only minor changes in sterol profiles were observed [12,14]. Mutations in other genes encoding enzymes involved in ergosterol biosynthesis (erg3A, erg3B and hmg1) [16,17] or in the negative cofactor 2 complex (a transcriptional regulator) [18] modified the total ergosterol content and relative distribution of sterols. Some results of these studies are presented in Section 3.2.1. To our knowledge, the effect of azole drugs on ergosterol biosynthesis was studied only once, with voriconazole and isavuconazole [19].
The aims of this study were (i) to describe the sterol composition of azole resistant isolates harbouring environmental alterations (TR34/L98H, TR46/Y121F/T289A and TR53) and (ii) to explore how exposure to azole drugs, namely itraconazole and voriconazole, could affect their sterol composition in comparison with azole-susceptible isolates.
2. Materials and Methods
2.1. Isolates of A. fumigatus
Twelve isolates, gathered in four groups of three isolates, were explored during this study: azole-susceptible isolates (Group 1) and azole-resistant isolates, bearing TR34/L98H (Group 2) or TR46/Y121F/T289A (Group 3) or TR53 (Group 4) alterations. Itraconazole and voriconazole MICs were determined according to the EUCAST reference method [20]. When EUCAST MIC was >8 mg/L or >16 mg/L for itraconazole or voriconazole, respectively, the Etest® method (bioMerieux, Marcy l’Etoile) was additionally done to be closest to the MIC (Etest® strips concentration gradient up to 32 µg/mL). Characteristics of all isolates are given in Table 1.

Table 1.
Characteristics of isolates.
2.2. Culture Conditions
A 4 × 107 spores/mL suspension in sterile water with Tween 20 (0.1%) was prepared from a fresh culture. Two hundred microliters were then inoculated to 25 mL of YPD (Yeast Peptone Dextrose) 20% glucose broth. Either antifungal agent (itraconazole and voriconazole, stock solution at 1.6 mg/mL in DMSO) or DMSO (basal condition) was then added to obtain 0.25 × MIC or 32 mg/L for strains with MIC >32 mg/L (Table 1). Cultures were incubated for 48 h at 37 °C under agitation (130 rpm). Each experiment was repeated three times. Absence of sterols in YPD broth was verified before running these experiments.
2.3. Total Sterol Extraction
Mycelia obtained from 48 h incubation were harvested by filtration on two layers of Whatman filter paper (n°1), washed with distilled water and weighted. The first step of sterol extraction was saponification, with the addition of 5 mL of fresh ethanolic potassium hydroxide solution, and incubation in water bath during 90 min at 90 °C. The non-saponifiable lipids (sterols) were extracted twice by adding 2 mL of hexane and manual mixing. Once the two layers were separated, the upper layer was harvested in a new tube. The organic layer was then washed twice by adding 1 mL of sterile water to the 4 mL of hexane. Organic phase was then dried by adding sodium sulfate. Final organic phase was transferred to a new collection tube after a 5 min centrifugation step at 720 g.
2.4. Sterol Derivatization
A volume of extract equivalent to 100 mg mycelia was transferred into a glass tube and hexane was evaporated by heating at 80 °C. Cholesterol was added as internal standard (2.5 µg). Sterols were derivatized with 100 μL of N-Méthyl-N-triméthyl-silyltrifluoroacetamide (TMS) (Sigma-Aldrich, Saint-Quentin-Fallavier, France) during 30 min at room temperature. The solvent was then evaporated at 80 °C under air flux. Dried sterols were solubilized with 200 μL dichloromethane and stored at −20 °C until analysis.
2.5. Sterol Content Analysis
Sterols as TMS derivatives were analyzed by Gas Chromatography-Mass Spectrometry (GC-MS) using an Agilent 7890A GC system, with a HP-5MS column (60 m × 0.25 mm, 0.25 μm, Agilent, Les Ulis, France) coupled with a mass detector (Agilent 5975C inert MSD—E.I. 70 eV). One microliter of each sample was injected in splitless mode at 250 °C. The carrier gas was helium at a flow rate of 1.2 mL/min. The oven was set at 150 °C for 0.5 min and then raised to 290 °C at 50 °C/min and from 290 °C to 305 °C at 2 °C/min for 7 min then 10 °C/min to 315 °C for 16 min. Sterols were identified via their electron ionization fragmentation pattern by comparison of the mass spectrum of each isolated sterol with previously published spectra (AMDIS data bank, [21,22,23]). Cholesterol was used as internal standard to calculate relative retention time. Sterol composition was expressed as a relative amount, i.e., percentage of the total amount of sterols detected.
For comparison between isolates and/or between conditions, only sterols with an amount of 1% or more were considered. Values are expressed as the mean of three independent experiments. Percentages lower than 1% have been obtained mathematically when this sterol has been detected only one or two times with a value higher than 1%. Relative amount and qualitative analysis of sterols were done using MSD ChemStation Data Analysis Application and AMDIS software.
2.6. Statistical Analysis
Univariate analyses of ergosterol content were performed with non-parametric two-tailed tests (Kruskal Wallis and Mann Whitney tests) with a type-I error fixed to 5%. Multivariate analyses were performed using ANOVA. Statistical analysis was performed using GraphPad Prism 7 and Stata 17 software.
3. Results and Discussion
3.1. Identification of Sterols
Thirteen sterols have been detected during this study and are listed in Table 2. Nomenclature of numbering sterols is presented in Figure 1. Eleven sterols have been identified and two are referred as “Unknown 1” and “Unknown 2” (PM 480 and PM 470 respectively). Ergosterol E (ergosta-5,7,22E-trien-3ß-ol) is the main sterol of the fungal membrane. Two ergosterol isomers (ergosta-5,7,22E-trien-3ß-ol and ergosta-5,7,22Z-trien-3ß-ol) were also identified but ergosterol Z has never been described in previous fungal sterol analysis. We hypothesized that isomerization could have occurred due to cell culture conditions and these two isomers’ quantifications have been pooled in all the analysis and considered as “ergosterol”.

Table 2.
List of the 13 sterols present with an amount higher than 1% of the total amount of sterols and identified during this study.
Figure 1.
Basic structure of a sterol with standard carbon numbering according to the IUPAC [30].
Overall, five sterols bearing a methyl in C14 position (14α-methyl sterols) have been identified: eburicol, obtusifoliol, obtusifolione, 14α-methylfecosterol and 14α-methylepisterol. Whereas eburicol is upstream in the biosynthesis pathway of ergosterol, the remaining four belong to an alternative pathway that ends into the synthesis of potentially toxic sterols (Figure 2). In our chromatographic conditions, because of the poor separation of obtusifoliol and obtusifolione when both present in a sample, we quantified them together.
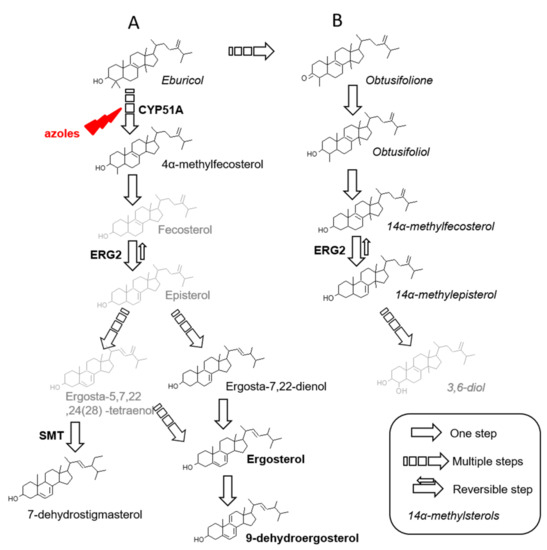
Figure 2.
Aspergillus fumigatus sterol biosynthesis pathway (A) Possible pathway for ergosterol biosynthesis (B) Alternative pathway, after CYP51 inhibition. In grey sterol intermediates not detected in this study. SMT: sterol methyltransferase.
In comparison to vertebrates, sterols from higher plants and fungi differ by the alkylation of the side chain at C24 position [31]. In fungi, most of C24 alkylated sterols are methylated (lanosterol, eburicol, obtusifoliol), but an ethylation is also possible as observed in higher plants, depending on the activity of S-adenosyl-L-methionine sterol C24 methyl transferases (SMTs). These enzymes have been studied in phytosterols biosynthesis and in Saccharomyces cerevisiae [32] but C24-ethylsterols and SMT are unexplored in A. fumigatus [4]. This sterol could be produced in two steps from 24-methylcholesta-5,7,24(28)-trien-3β-ol involving Erg6 (first step) then Erg4 and Erg5 (second step), as proposed by Bouvier-Navé for the formation of 4,4,14α-trimethyl-5α-stigmasta-8,22-dien-3β-ol from eburicol [31]. Here, we observed a C24 alkylated sterol, 7-dehydrostigmasterol (7-DHS: 24-éthylcholesta-5,7,22-trien-3β-ol) for all the isolates (Figure 2). In protozoans, such as T. cruzi and Leishmania spp. the major sterol components are ergosterol and its 24-alkylated and methylated derivatives i.e., 7-DHS [33]. Concerning the three last sterols identified, ergosta-7,22-dienol and 4α-methyl-fecosterol are common intermediates in A. fumigatus ergosterol biosynthesis pathway whereas 9-dehydroergosterol is the result of ergosterol degradation (Figure 2).
3.2. Qualitative Composition and Relative Amount of Sterols
Sterol membrane content determined for each strain in each experimental condition are presented in Table 3, Table 4, Table 5 and Table 6. Strains have been classified in four groups: itraconazole and voriconazole susceptible isolates (Group 1, Table 3), resistant isolates with TR34/L98H (Group 2, Table 4) or TR46/Y121F/T289A (Group 3, Table 5) or TR53 (Group 4, Table 6) alterations. Whatever the mutations in cyp51A gene, the alterations in its promoter and the growth conditions, ergosterol was the main sterol detected, accounting from 79.5% to 89.5% under basal conditions and from 61.8% to 81.1% and from 44.4% to 74.2% when exposed to itraconazole or voriconazole, respectively.

Table 3.
Relative composition of sterols of group 1, azole-susceptible strains, under basal conditions or after in vitro exposure to itraconazole (ITC) or voriconazole (VRC) (concentration = 0.25 × MIC). Values are expressed as the mean of three independent experiments, standard deviation is given in brackets.

Table 4.
Relative composition of sterols of group 2, strains with TR34/L98H alterations, under basal conditions or after in vitro exposure to itraconazole (ITC) (concentration = 32 mg/L) or voriconazole (VRC) (concentration = 0.25 × MIC). Values are expressed as the mean of three independent experiments, standard deviation is given in brackets.

Table 5.
Relative composition of sterols of group 3, strains with TR46/Y121F/298A alterations, under basal conditions or after in vitro exposure to itraconazole (ITC) (concentration = 0.25 × MIC) or voriconazole (VRC) (concentration = MIC). Values are expressed as the mean of three independent experiments, standard deviation is given in brackets.

Table 6.
Relative composition of sterols of group 4, strains with TR53 alterations, under basal conditions or after in vitro exposure to itraconazole (ITC) (concentration = 32 mg/L except AF124: concentration = 0.25 × MIC) or voriconazole (VRC) (concentration = 0.25 × MIC). Values are expressed as the mean of three independent experiments, standard deviation is given in brackets.
3.2.1. Under Basal Conditions
Under basal conditions besides ergosterol, 7-DHS (a C24-ethylsterol) was always detected, accounting from 10.5% to 20.0%, whatever the alteration involved. Sterols, other than ergosterol and 7-DHS, were inconstantly detected in only three isolates: obtusifoliol, obtusifolione and eburicol for AF1861 (susceptible strain), 4-methylfecosterol for AF23 (TR34/L98H strain) and 9-dehydroergosterol for AF1897 (TR34/L98H strain). This last sterol probably results from the degradation of ergosterol or 5,7-diene sterols [12]. Table 7 sums up the sterol composition of six wild type strains of A. fumigatus previously published in four articles between 2001 and 2019 [12,13,14,16,19]. Only sterols observed in our strains in basal conditions were detailed here i.e., ergosterol, ergosta-7,22-dienol, 7-DHS and eburicol. Differences in sterol content can be influenced by conditions of culture [34], by strains themselves and also by chromatographic conditions that have evolved along years. Ergosterol was always the main sterol with a variable amount (from 73.5 to 95%) and eburicol was also detected (from 0.3 to 2.72%). 7-DHS was only observed three times with an amount varying from 0.9 to 19.4% (in an environmental strain). Ergosta-7,22-dienol was only observed in CM237 strain by Alcazar-Fuoli et al. Interestingly 9-dehydroergosterol, 4-methylfecosterol, obtusifoliol and obtusifolione had never been reported. In Candida albicans, the balance of ergosterol and 14α-methylsterols is controlled by NSG2, a protein containing INSIG domain (insulin-induced genes) [35]. It would be interesting to search for putative orthologous genes to NSG2 in AF1861 isolate to explain the basal detection of obtusifoliol and obtusifolione.

Table 7.
Comparison of basal relative (%) composition of sterols of six WT strains of A. fumigatus published between 2001 and 2019.
3.2.2. Under Itraconazole or Voriconazole Exposure
When exposed to itraconazole or voriconazole, alongside the accumulation of eburicol, appearance of 14α-methylsterols (obtusifoliol, obtusifolione, 14α-methylfecosterol and 14α-methylepisterol) was observed for all strains, except for AF1861, where obtusifoliol and obtusifolione only increase as these sterols were detected in low amount in basal condition. These observations are linked to the mechanism of action of azole drugs (inhibitors of the 14α-demethylase), which lead to the accumulation of 14α-demethylase substrates and trigger deviation to a new biosynthesis pathway from eburicol and leading to synthesis of toxic sterol (3,6-diol) or at least its precursors [36] (Figure 2). Obtusifolione and obtusifoliol have been described in C. albicans after exposure to various azole drugs [37,38]. After ketoconazole (imidazole drug targeting 14α-demethylase) treatment, A. fumigatus accumulated obtusifoliol, 14α-methylfecosterol and 14α-methyl-ergosta-5,7,22,24(28)-tetraene-3βol [39]; obtusifoliol was also observed after triarimol (pyrimidines fungicide inhibiting 14α-demethylase) treatment [40]. In our study, we observed 14α-methylfecosterol only once but 14α-methylepisterol was constantly detected after voriconazole treatment and sometimes after itraconazole treatment. Conversion of fecosterol to episterol implies ERG2, a C8 isomerase [12]. This step is the unique reversible reaction in the biosynthesis of ergosterol but rather to the benefit of episterol synthesis [12], in ratio of 19:1 as shown in cholesterol biosynthesis pathway [41]. We hypothesized that both 14α-methylfecosterol and 14α-methylepisterol could be produced without the possibility of detection of 14α-methylfecosterol because of its shift toward the formation of 14α-methylepisterol (Figure 2). Ergosta-7,22-dienol, a precursor of ergosterol, was detected in four isolates (AF23 and AF1897 with TR34/L98H alteration, AF2226 with TR46/Y121F/T289A alteration and AF84 with TR53 alteration), when exposed to itraconazole. This intermediate between episterol and ergosterol was detected previously in basal conditions in A. fumigatus [12]. Interestingly, in Candida, ergosta-7,22-dienol is the ultimate sterol synthetized (instead of ergosterol) when erg3 is mutated but is never detected as intermediate of ergosterol biosynthesis [37]. Finally, after exposure to itraconazole or voriconazole, two sterols remained unidentified, but they were inconstantly present, and in a very low amount.
3.3. Statistical Comparison of Ergosterol Content
Under basal condition, ergosterol content was not significantly different comparing the three isolates in each group with the same kind of alteration (nonparametric Kruskal Wallis test). There was also no significant difference in ergosterol content between azole-susceptible isolates and azole resistant isolates bearing TR34/L98H or TR46/Y121F/T289A but it was significantly higher in azole resistant isolates bearing TR53 alterations than in azole-susceptible isolates (nonparametric Mann Whitney test, p = 0.006) (Figure 3).
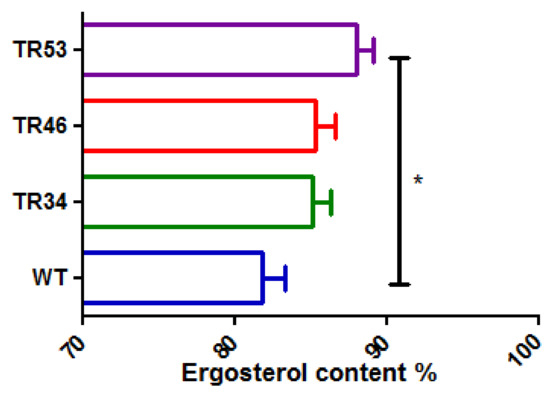
Figure 3.
Relative content of ergosterol in basal condition. WT: susceptible isolates, TR34: TR34/L98H, TR46: TR46/Y121F/T289A. * p = 0.006 (Mann Whitney test).
When exposed to itraconazole, the same observation was made: ergosterol content was not significantly different in each group of three isolates with the same kind of alteration (nonparametric Kruskal Wallis test), there is no significant difference in ergosterol content between azole-susceptible isolates (WT) and azole-resistant isolates bearing TR34/L98H or TR46/Y121F/T289A alterations but ergosterol content was lower in azole-resistant isolates bearing TR53 alteration than in azole-susceptible isolates (nonparametric Mann Whitney test, p = 0.0272). When exposed to voriconazole, ergosterol content was not significantly different in each group of three isolates with the same kind of alteration and ergosterol content was not different comparing each group of isolates with the same alteration to azole-susceptible isolates (nonparametric Kruskal Wallis test).
Then using ANOVA, an analysis of all ergosterol data together (whatever the alteration and the growth condition) was performed. In this model of analysis, ergosterol content was not significantly different for azole-susceptible, TR34/L98H and TR46/Y121F/T289A groups. The unique difference in ergosterol content concerned TR53 isolates (Group 4), when exposed to itraconazole. Overall ergosterol content reach 85.1% (IC 95 [82.8%; 87.4%]) whatever susceptibility or resistance to azoles and whatever growth conditions. This observation, together with the same sterol composition in basal condition suggested that neither TR34/L98H, nor TR46/Y121F/T289A nor TR53 alterations affected biosynthesis of ergosterol. The L98H modification in the 14α-steroldemethylase affect neither the biological activity of this protein nor the access of the natural ligands to the active site [42]; the duplication of 34 bases allows a better binding of cyp51A activator (SrbA) than repressor [43] leading to overexpression of cyp51A mRNA [42]. This knowledge about the mechanisms of resistance mediated by TR34/L98H alterations explains our findings on ergosterol content in basal condition. As for TR34, TR46 is associated with an overexpression of cyp51A mRNA while the Y121F mutation destabilizes the active site of the enzyme [44]. Basal sterols content in the 3 TR46/Y121F/T289A strains suggests that the enzyme activity is not affected by these alterations. TR53 alteration is also associated with a duplication of SRE1 and SRE2 (Sterol Regulatory Element) allowing a better binding of cyp51A activator (SrbA) than repressor [43]. In our growth conditions, itraconazole treatment decreased ergosterol content by 10.8% (IC 95 [7.3%; 14.3%]) for WT, TR34/L98H and TR46/Y121F/T289A isolates while ergosterol reduction was more important for TR53 isolates than for other groups (18.6%, IC 95 [13.5%; 23.8%]). Voriconazole treatment had the same impact for all isolates, with ergosterol decreasing by 20.7% (IC 95 [17.4%; 23.9%]).
3.4. Statistical Comparison of 14α-Methylsterols Content
Under exposure to itraconazole or voriconazole, 14α-methylsterols content was not significantly different in each group of three isolates with the same kind of alteration (nonparametric Kruskal Wallis test). Using ANOVA model, itraconazole exposure had the same effect on WT, TR34/L98H and TR46/Y121F/T289A isolates: 12.8% (IC 95 [9.4%; 16.1%]) of 14α-methylsterols were quantified. By comparison, TR53 isolates contained more 14α-methylsterols after itraconazole exposure, with 22.6% (IC 95 [16.8%; 28.4%]). Whatever the azole-susceptibility or resistance of strains, voriconazole had the same effect on 14α-methylsterols content (ANOVA). Overall, 14α-methylsterols content reach 31.4% (IC 95 [28.5%; 34.3%]) when exposed to voriconazole. Therefore, in our conditions, voriconazole appeared to be a stronger inhibitor of 14α-demethylase than itraconazole. Exposure to itraconazole or voriconazole at a concentration adapted to MIC of each isolate, can explain the same amount of 14α-methylsterols in azole-susceptible and azole-resistant strains with TR34/L98H or TR46/Y121F/T289A alterations. The TR53 genotype is not associated with Cyp51 mutations so that impairing the binding of azoles is not expected, which can explain a more important inhibition of ergosterol biosynthesis than for TR34/L98H or TR46/Y121F/T289A isolates. Interestingly, no 14-methylergosta-8,24-dien-3,6-diol was detected. It is the ultimate sterol after blockade of the 14α-demethylase and it is known to be toxic through the impairment of the membrane function of sterol due to the presence of the 6- hydroxy group [45,46]. We made the hypothesis that this absence of 14-methylergosta-8,24-dien-3,6-diol may be explained by the concentration of itraconazole or voriconazole added to fungal culture, which is not enough to end into the synthesis of this toxic sterol.
4. Conclusions
In this study, we described for the first-time relative sterol composition of azole-resistant Aspergillus isolates with complex alterations in the cyp51A gene and/or its promotor, which are linked to environmental exposition to azole fungicides. This study highlights that TR34/L98H and TR46/Y121F/T289A alterations had no impact on relative composition of sterols in basal conditions when compared with susceptible isolates. Moreover, in our conditions, after itraconazole and voriconazole exposure the qualitative composition of sterols was similar in susceptible and resistant isolates. Interestingly in basal condition relative ergosterol content was higher for TR53 strains and it was significantly lower than for other alterations after itraconazole and voriconazole treatment. It would be interesting to look for other mechanisms of resistance in these isolates. Moreover, in this study we did not explore point substitution in 14α-lanosterol demethylase (including M220 and G54); it could be of interest to compare the effects of point mutation with the effects of complex alterations studied here.
Author Contributions
Conceptualization, R.-A.L., F.P., P.L.P. and I.O.-G.; methodology, R.-A.L., M.A. and I.O.-G.; formal analysis, R.-A.L., M.A., J.-B.H. and I.O.-G.; resources, C.A.-M.; writing—original draft preparation, R.-A.L. and I.O.-G.; writing—review and editing, R.-A.L., M.A., J.-B.H., C.A.-M., F.P., F.M., P.L.P., I.O.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We gratefully acknowledge Aurélie Couzinet-Mossion for scientific discussion and Vony Rabesaotra for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.E.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef] [Green Version]
- Patterson, T.F.; Thompson, G.R.; Denning, D.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Executive Summary: Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Arastehfar, A.; Lass-Flörl, C.; Garcia-Rubio, R.; Daneshnia, F.F.; Ilkit, M.; Boekhout, T.; Gabaldon, T.; Perlin, D.S. The Quiet and Underappreciated Rise of Drug-Resistant Invasive Fungal Pathogens. J. Fungi 2020, 6, 138. [Google Scholar] [CrossRef]
- Alcazar-Fuoli, L.; Mellado, E. Ergosterol biosynthesis in Aspergillus fumigatus: Its relevance as an antifungal target and role in antifungal drug resistance. Front. Microbiol. 2013, 3, 439. [Google Scholar] [CrossRef] [Green Version]
- Verweij, P.E.; Mellado, E.; Melchers, W. Multiple-Triazole–Resistant Aspergillosis. New Engl. J. Med. 2007, 356, 1481–1483. [Google Scholar] [CrossRef] [Green Version]
- Mellado, E.; Garcia-Effron, G.; Alcázar-Fuoli, L.; Melchers, W.J.G.; Verweij, P.; Cuenca-Estrella, M.; Rodríguez-Tudela, J.L. A New Aspergillus fumigatus Resistance Mechanism Conferring In Vitro Cross-Resistance to Azole Antifungals Involves a Combination of cyp51A Alterations. Antimicrob. Agents Chemother. 2007, 51, 1897–1904. [Google Scholar] [CrossRef] [Green Version]
- Lestrade, P.P.A.; Meis, J.F.; Melchers, W.J.G.; Verweij, P.E. Triazole resistance in Aspergillus fumigatus: Recent insights and challenges for patient management. Clin. Microbiol. Infect. 2019, 25, 799–806. [Google Scholar] [CrossRef]
- Nywening, A.V.; Rybak, J.M.; Rogers, P.D.; Fortwendel, J.R. Mechanisms of triazole resistance in Aspergillus fumigatus. Environ. Microbiol. 2020, 22, 4934–4952. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; Cuenca-Estrella, M.; Mellado, E. Triazole Resistance in Aspergillus Species: An Emerging Problem. Drugs 2017, 77, 599–613. [Google Scholar] [CrossRef]
- Martel, C.M.; Parker, J.E.; Bader, O.; Weig, M.; Gross, U.; Warrilow, A.G.S.; Kelly, D.E.; Kelly, S.L. A Clinical Isolate of Candida albicans with Mutations in ERG11 (Encoding Sterol 14α-Demethylase) and ERG5 (Encoding C22 Desaturase) Is Cross Resistant to Azoles and Amphotericin B. Antimicrob. Agents Chemother. 2010, 54, 3578–3583. [Google Scholar] [CrossRef] [Green Version]
- Ghannoum, M.A.; Spellberg, B.J.; Ibrahim, A.S.; Ritchie, J.A.; Currie, B.; Spitzer, E.D.; Edwards, J.E.; Casadevall, A. Sterol composition of Cryptococcus neoformans in the presence and absence of fluconazole. Antimicrob. Agents Chemother. 1994, 38, 2029–2033. [Google Scholar] [CrossRef] [Green Version]
- Alcazar-Fuoli, L.; Mellado, E.; Garcia-Effron, G.; Lopez, J.F.; Grimalt, J.O.; Cuenca-Estrella, J.M.; Rodriguez-Tudela, J.L. Ergosterol biosynthesis pathway in Aspergillus fumigatus. Steroids 2008, 73, 339–347. [Google Scholar] [CrossRef]
- Müller, C.; Neugebauer, T.; Zill, P.; Lass-Flörl, C.; Bracher, F.; Binder, U. Sterol Composition of Clinically Relevant Mucorales and Changes Resulting from Posaconazole Treatment. Mol. 2018, 23, 1218. [Google Scholar] [CrossRef] [Green Version]
- Dannaoui, E.; Persat, F.; Borel, E.; Piens, M.A.; Picot, S. Sterol composition of itraconazole-resistant and itraconazole-susceptible isolates of Aspergillus fumigatus. Can. J. Microbiol. 2001, 47, 706–710. [Google Scholar] [CrossRef]
- Hagiwara, D.; Arai, T.; Takahashi, H.; Kusuya, Y.; Watanabe, A.; Kamei, K. Non-cyp51A Azole-Resistant Aspergillus fumigatus Isolates with Mutation in HMG-CoA Reductase. Emerg. Infect. Dis. 2018, 24, 1889–1897. [Google Scholar] [CrossRef] [Green Version]
- Alcazar-Fuoli, L.; Mellado, E.; Garcia-Effron, G.; Buitrago, M.J.; López, J.; Grimalt, J.; Cuenca-Estrella, J.M.; Rodriguez-Tudela, J.L. Aspergillus fumigatus C-5 Sterol Desaturases Erg3A and Erg3B: Role in Sterol Biosynthesis and Antifungal Drug Susceptibility. Antimicrob. Agents Chemother. 2006, 50, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Rybak, J.M.; Ge, W.; Wiederhold, N.P.; Parker, J.E.; Kelly, S.L.; Rogers, P.D.; Fortwendel, J.R. Mutations in hmg1, Challenging the Paradigm of Clinical Triazole Resistance in Aspergillus fumigatus. mBio 2019, 10, e00437-19. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, T.; Van Rhijn, N.; Fraczek, M.; Gsaller, F.; Davies, E.; Carr, P.; Gago, S.; Fortune-Grant, R.; Rahman, S.; Gilsenan, J.M.; et al. The negative cofactor 2 complex is a key regulator of drug resistance in Aspergillus fumigatus. Nat. Commun. 2020, 11, 427. [Google Scholar] [CrossRef] [Green Version]
- Warrilow, A.; Parker, J.; Price, C.L.; Rolley, N.J.; Nes, W.D.; Kelly, D.E.; Kelly, S.L. Isavuconazole and voriconazole inhibition of sterol 14α-demethylases (CYP51) from Aspergillus fumigatus and Homo sapiens. Int. J. Antimicrob. Agents 2019, 54, 449–455. [Google Scholar] [CrossRef]
- Arendrup, M.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J. The Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing. EUCAST Definitive Document E.Def 9.3.2: Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Forming Moulds. Available online: https://www.eucast.org/astoffungi/methodsinantifungalsusceptibilitytesting/ast_of_moulds/ (accessed on 10 November 2021).
- Müller, C.; Junker, J.; Bracher, F.; Giera, M. A gas chromatography–mass spectrometry-based whole-cell screening assay for target identification in distal cholesterol biosynthesis. Nat. Protoc. 2019, 14, 2546–2570. [Google Scholar] [CrossRef]
- The Lipid Web. Available online: https://www.lipidmaps.org/resources/lipidweb/index.php?page=index.html (accessed on 1 November 2021).
- Akihisa, T.; Goad, J. Analyse des Sterols; Blackie Academic and Professional: London, UK, 1997; ISBN 0751402303. [Google Scholar]
- Müller, C.; Binder, U.; Bracher, F.; Giera, M. Antifungal drug testing by combining minimal inhibitory concentration testing with target identification by gas chromatography–mass spectrometry. Nat. Protoc. 2017, 12, 947–963. [Google Scholar] [CrossRef]
- Müller, C.; Staudacher, V.; Krauss, J.; Giera, M.; Bracher, F. A convenient cellular assay for the identification of the molecular target of ergosterol biosynthesis inhibitors and quantification of their effects on total ergosterol biosynthesis. Steroids 2013, 78, 483–493. [Google Scholar] [CrossRef]
- Brooks, C.J.W.; Horning, E.C.; Young, J.S. Characterization of sterols by gas chromatography-mass spectrometry of the trimethylsilyl ethers. Lipids 1968, 3, 391–402. [Google Scholar] [CrossRef]
- Weete, J.D.; Gandhi, S.R. Sterols of the Phylum Zygomycota: Phylogenetic Implications. Lipids 1997, 32, 1309–1316. [Google Scholar] [CrossRef]
- Quail, M.A.; Arnold, A.; Moore, D.J.; Goosey, M.W.; Kelley, S.L. Ketoconazole-mediated growth inhibition in Botrytis cinerea and Saccharomyces cerevisiae. Phytochemistry 1993, 32, 273–280. [Google Scholar] [CrossRef]
- Shirane, N.; Murabayashi, A.; Masuko, M.; Uomori, A.; Yoshimura, Y.; Seo, S.; Uchida, K.; Takeda, K. Effect on ergosterol biosynthesis of a fungicide, SSF-109, in Botrytis cinerea. Phytochemistry 1990, 29, 2513–2520. [Google Scholar] [CrossRef]
- Moss, G.P. Nomenclature of steroids (Recommendations 1989). Pure Appl. Chem. 1989, 61, 1783–1822. [Google Scholar] [CrossRef]
- Bouvier-Nave, P.; Husselstein, T.; Benveniste, P. Two families of sterol methyltransferases are involved in the first and the second methylation steps of plant sterol biosynthesis. JBIC J. Biol. Inorg. Chem. 1998, 256, 88–96. [Google Scholar] [CrossRef]
- Nes, W. Sterol methyl transferase: Enzymology and inhibition. Biochim. et Biophys. Acta BBA Bioenerg. 2000, 1529, 63–88. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Waterman, M.R. Sterol 14alpha-demethylase (CYP51) as a therapeutic target for human trypanosomiasis and leishmaniasis. Curr. Top. Med. Chem. 2011, 11, 2060–2071. [Google Scholar] [CrossRef]
- Nes, W.; Xu, S.; Haddon, W.F. Evidence for similarities and differences in the biosynthesis of fungal sterols. Steroids 1989, 53, 533–558. [Google Scholar] [CrossRef]
- Lv, Q.-Z.; Qin, Y.-L.; Yan, L.; Wang, L.; Zhang, C.; Jiang, Y.-Y. NSG2 (ORF19.273) Encoding Protein Controls Sensitivity of Candida albicans to Azoles through Regulating the Synthesis of C14-Methylated Sterols. Front. Microbiol. 2018, 9, 218. [Google Scholar] [CrossRef]
- Georgopapadakou, N.H.; Walsh, T.J. Antifungal agents: Chemotherapeutic targets and immunologic strategies. Antimicrob. Agents Chemother. 1996, 40, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Martel, C.M.; Parker, J.E.; Bader, O.; Weig, M.; Gross, U.; Warrilow, A.G.S.; Rolley, N.; Kelly, D.E.; Kelly, S.L. Identification and Characterization of Four Azole-Resistant erg3 Mutants of Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 4527–4533. [Google Scholar] [CrossRef] [Green Version]
- Marichal, P.; Gorrens, J.; Laurijssens, L.; Vermuyten, K.; Van Hove, C.; Le Jeune, L.; Verhasselt, P.; Sanglard, D.; Borgers, M.; Ramaekers, F.C.S.; et al. Accumulation of 3-Ketosteroids Induced by Itraconazole in Azole-Resistant Clinical Candida albicans Isolates. Antimicrob. Agents Chemother. 1999, 43, 2663–2670. [Google Scholar] [CrossRef] [Green Version]
- Venkateswarlu, K.; Kelly, S.L. Biochemical characterisation of ketoconazole inhibitory action on Aspergillus fumigatus. FEMS Immunol. Med Microbiol. 1996, 16, 11–20. [Google Scholar] [CrossRef]
- Sherald, J.L.; Sisler, H.D. Antifungal mode of action of triforine. Pestic. Biochem. Physiol. 1975, 5, 477–488. [Google Scholar] [CrossRef]
- Nes, W.D. Biosynthesis of Cholesterol and Other Sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar] [CrossRef]
- Snelders, E.; Karawajczyk, A.; Verhoeven, R.J.; Venselaar, H.; Schaftenaar, G.; Verweij, P.E.; Melchers, W.J. The structure–function relationship of the Aspergillus fumigatus cyp51A L98H conversion by site-directed mutagenesis: The mechanism of L98H azole resistance. Fungal Genet. Biol. 2011, 48, 1062–1070. [Google Scholar] [CrossRef]
- Gsaller, F.; Hortschansky, P.; Furukawa, T.; Carr, P.D.; Rash, B.; Capilla, J.; Muller, C.; Bracher, F.; Bowyer, P.; Haas, H.; et al. Sterol Biosynthesis and Azole Tolerance Is Governed by the Opposing Actions of SrbA and the CCAAT Binding Complex. PLoS Pathog. 2016, 12, e1005775. [Google Scholar] [CrossRef]
- Snelders, E.; Camps, S.M.; Karawajczyk, A.; Rijs, A.J.; Zoll, J.; Verweij, P.; Melchers, W.J. Genotype–phenotype complexity of the TR46/Y121F/T289A cyp51A azole resistance mechanism in Aspergillus fumigatus. Fungal Genet. Biol. 2015, 82, 129–135. [Google Scholar] [CrossRef]
- Watson, P.; Rose, M.; Ellis, S.; England, H.; Kelly, S. Defective sterol C5-6 desaturation and azole resistance: A new hypothesis for the mode of action of azole antifungals. Biochem. Biophys. Res. Commun. 1989, 164, 1170–1175. [Google Scholar] [CrossRef]
- Kelly, S.L.; Lamb, D.C.; Kelly, D.E.; Manning, N.J.; Loeffler, J.; Hebart, H.; Schumacher, U.; Einsele, H. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol Δ5,6 -desaturation. FEBS Lett. 1997, 400, 80–82. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).