1. Introduction
The development of chemical technologies dates back to the 1950s and 60s. The first theoretical description of heat and mass transfer in the reactors was given within physicochemical hydrodynamics [
1,
2]. Further development of technologies based on these principles is described in [
3]. The traditional chemical production, as a rule, is carried out in the discontinuous stirred reactor (or batch reactor (BR)). In industry, discontinuous operations are well suited for rather complex reactions and allow one to drive the process by controlling the temperature [
4]. In contrast to the BR, the continuous operations traditionally were typical for large productions (for example, in the oil and gas industry) based on a more simple chemistry [
4]. Significant changes in this area occurred in the early 2000s when the new technologies penetrated an organic synthesis [
5,
6,
7]. Since the pharmaceutical production needs more flexibility in reconfiguring the synthesis system rather than large product volume, the process has been developing towards the design of increasingly miniature reactors or microreactors [
6,
7]. The advantages of this new reactor type, the continuous-flow microreactor (hereinafter, CFMR) against the BR are: high productivity due to the elimination of loading-unloading stage; the stable consumption of reagents and energy due to the small reactor zone; the replication of the production line to increase product yield, just to mention a few. Consequently, numerous studies have been published in recent years detailing the beneficial outcome of continuous-flow chemistry applied to single or indeed multi-step syntheses of target compounds on various reaction schemes and spatial scales [
8,
9,
10,
11].
To mix the reagents in the CFMR, two basic physical principles can be used: diffusion and/or convection. When using the diffusion as the main mixing mechanism, it is required to create higher gradients of diffusing components and use the channels of ever smaller cross section. Initially, the development of microreactor technology proceeded along this path [
6,
7]. However, with miniaturization of the connecting capillaries, the flow remains laminar, and the reaction zone is absent as unnecessary, since mixing occurs directly behind the junction of capillaries supplying fresh reagents. This approach has its drawbacks: pumping fluid through narrow channels requires the application of significant pressure at the ends of the installation, and the yield of the product decreases. Besides, there is a restriction on the type of reactions that can be implemented on such reactors, since the channel length determines the mixing time [
12]. Another approach involves the creation of a special reactor zone, where the convection is organized to mix the species (the advanced-flow microreactor, AFMR) [
8]. This leads to an increase in the product yield, and also allows to organize the process for reactions with smaller reaction rate [
12]. It should be noted that the development of the AFMR unit requires an individual approach to each reaction and large amount of preliminary theoretical and experimental work, the joint work of different specialists (both chemists and hydrodynamicists). At present, there are several examples of well-developed technologies based on the CFMR/AFMR for the production of pharmaceutical substances by the method of continuous-flow synthesis [
9,
10,
11,
13]. In the last few years, different types of convective micromixers for the AFMR unit have been proposed [
13,
14,
15]. Furthermore, various techniques have been developed for mixing typically laminar flows in the CFMR. Among them are passive methods, where the mixing effect is introduced by geometry [
16] or using bas-relief structures on the bottom of the channel [
17] and active methods using an external Braille pin actuator array [
18]. Further micromixer working principles are based on fluidic dielectrophoresis [
19] or centrifugal action [
20]. The literature on continuous–flow microreactors and micromixers is growing exponentially reflecting the interest of researchers in this field.
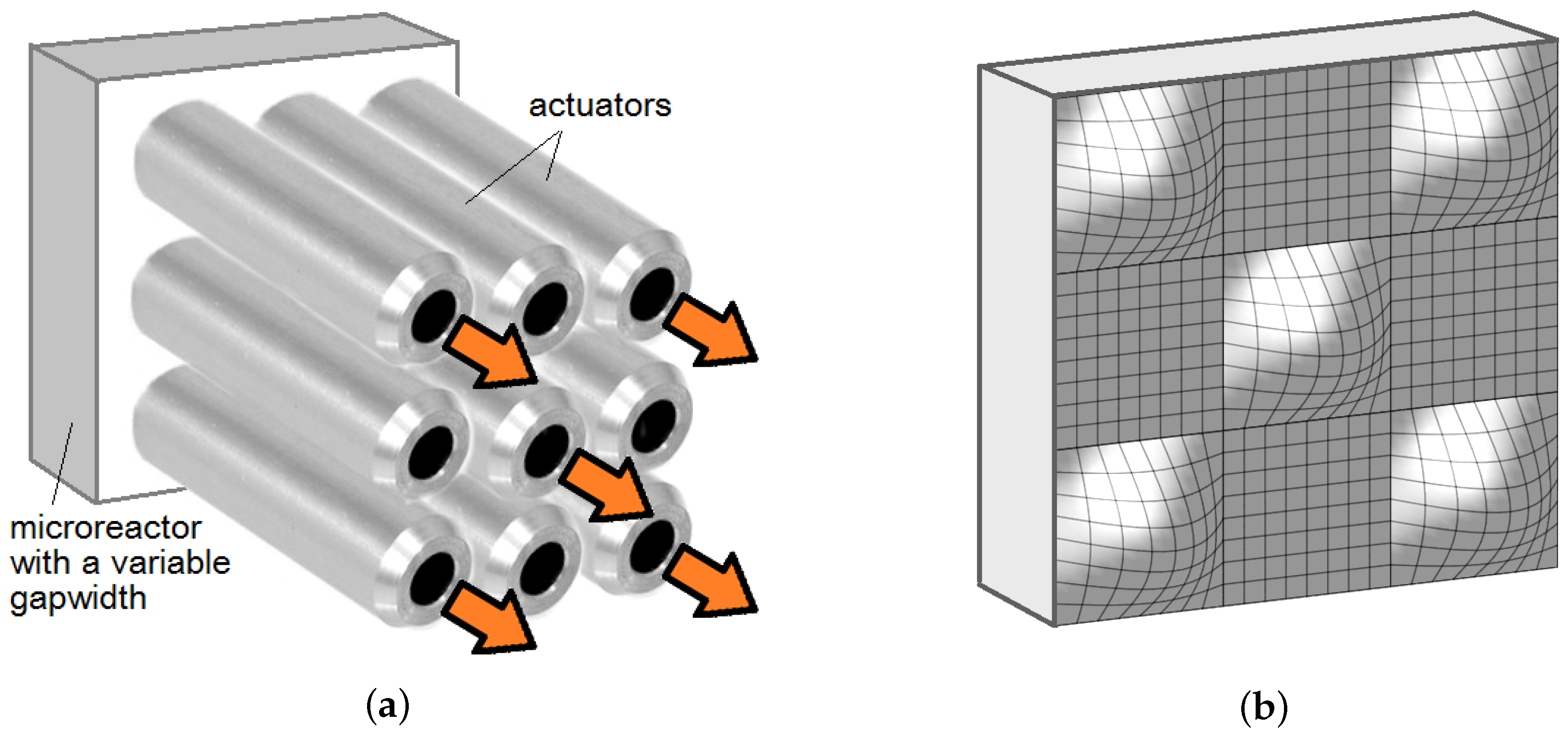
In this paper, we propose another way of controlling the mass transfer and mixing intensity in a microreactor. We suggest to use a quasi-plane Hele-Shaw (HS) cell with an adjustable gap width serving as the mixing zone in the AFMR unit (
Figure 1). In this case, the cell gap could vary both in space and in time due to a special system of actuators attached to the outer wall (or walls if control is carried out symmetrically from both sides) of the HS cell and having the ability to move slightly in the horizontal direction. Thus, the reactor zone would allow for more intensive convective motion. So far, the HS cell with tunable wide boundaries has been rarely considered in the literature since the HS cell was traditionally regarded as a simplified model of a quasi-two-dimensional fluid motion that arises between two parallel plates. So, from a theoretical point of view, the variation of the HS’s gap looked like an unnecessary complication of the problem. One the other hand, the precise manufacturing of plates with a slightly deformed and prescribed relief was technically not an easy task. However, at present, due to the appearance of new materials and the development of 3D-printing technology, the technical problems in manufacturing such HS cell are no longer relevant. There are just a few works dealing with non-planar HS cells. Zhao with colleagues studied both experimentally and theoretically the Saffman-Taylor flow in the HS cell with a uniform gradient of the gap [
21]. They found a significant effect of the cell wedging on the appearance of secondary instabilities. Viscous fingering in tapered HS cell was studied in [
22]. Non-stationary models have been considered in [
23,
24]. Works on reactive infiltration in a porous medium are also close to the topic considered in this paper, since the flow in a Hele-Shaw cell is similar in its properties to the flow in porous media [
25,
26,
27]. The reactive–infiltration instability is an important mechanism for pattern development in media with a heterogeneous permeability in geology.
In this paper, we consider the neutralization reaction as a model scheme. Second-order reactions coupled with flows have been attracting the attention of researchers because of their relatively simple but nonlinear kinetics [
28,
29,
30]. Previously, we have found that convection can arise in the presence of gravity due to the effect of concentration-dependent diffusion (CDD) of species [
31,
32,
33]. The CDD convection is used here to demonstrate how to control the mass transfer in the microreactor by manipulating its wall relief. The paper is organized as follows: in
Section 2 we derive the governing equation for the fluid flow in the HS cell with a variable gap width. In
Section 3 we formulate the general mathematical model for the mass transfer in the AFMR unit with the HS cell serving as a mixing zone. The results of the numerical simulations are presented in
Section 4. The last section summarizes the highlights of the paper.
2. Mathematical Formulation
We consider a thin cavity defined by
,
,
, where
, so that the HS approximation is applied (
Figure 2a). For the sake of clarity, suppose that the acceleration due to gravity is directed opposite to
z-axis. Assume further that the HS cell gap width can vary both in space and time
where
is an arbitrary dimensionless function symmetric with respect to the centre of the slot. Thus, the fluid flow in the slot can be approximately considered as quasi-two-dimensional even if the gap between the side walls is adjustable.
By assuming the fluid adhesion to the solid walls, the three-component vector field of the velocity
:
can be approximated as follows:
where
is the two-component velocity. The approximations (
2) should then be substituted into the Navier-Stokes equation and averaged across the slot
As a result, we obtain the motion equation written in the HS approximation
where
is the dynamic viscosity,
is the density of the solvent,
is the average medium density, which will be determined below. In addition to the correction factor
for a nonlinear term, the Equation (
4) differs from the standard Navier-Stokes equation by the term proportional to the velocity. This term may be interpreted as the average friction force due to the presence of the plates, and it is analogous to Darcy’s law for the porous medium. In our case, the friction term looks more complicated, since it includes the relief
of the HS wide walls. Thus, the manipulations with the gap width make it possible to strengthen or weaken locally the friction force acting on the fluid flow.
In this paper, we consider the chemoconvective structures of the characteristic size
l. Generally, this quantity is determined rather by the ratio of the reaction rate and the diffusion of the reactants than by the dimensions of the entire cavity, as it happens in the case of thermal convection, which arises due to external heating. To estimate the contribution of different terms, we write Equation (
4) in a dimensionless form using
l,
,
, and
as the units of measurement for the length, time, velocity, and pressure, respectively. Here
D is the tabular value of the diffusion coefficient of the fastest reagent. Then we get
The left-hand side of (
5) is multiplied by a dimensionless parameter
, where
stands for the Schmidt number. It is mainly used to quantify the relative timescales of viscous momentum transport and diffusive mass transport. In gases, the Schmidt number is of order of unity, while in the liquids, it is usually very large (about and even more than 1000). Thus, we can neglect the entire left side of the equation in (
5). If
, then we can neglect the process of the viscous diffusion of the fluid velocity. In the opposite case
, the fluid motion becomes so small-scale that the HS approximation (
5) is no longer applicable, and the standard Navier-Stokes equation must be used. If the resulting structures are slightly larger or, at least, of the same as the layer thickness, then Equation (
5) works well. In what follows, we assume that the condition
is fulfilled throughout the slot, and Equation (
5) can be reduced to the Darcy-like equation with a variable permeability of the layer
which is given in the dimensional form.
The continuity equation should also be averaged across the slot in the sense of (
3). It is convenient to write it in the form of the mass conservation law
Since
does not depend on
y, the amount of mass is determined only by the volume
V. By choosing
V in the form of the cylinder, the lateral surface of which is orthogonal to the slot, we can estimate it as
, where
stands for the area of cylinder’s cross-section. Then we obtain
Equation (
8) implies the effect of weak compressibility of the medium independently of the physical properties of the fluid. Generally, Equations (
1), (
6) and (
8) describe the fluid motion not only in a static situation, when the internal relief of walls is predetermined an apriori, but also in the case of external active control, when the relief is changed in time according to a certain law. The latter effect is taken into account by the first term in (
8). It is interesting to note that in the case of the static relief, the governing Equations (
6) and (
8) coincide with the equations for the filtration of a fluid through a porous medium with variable permeability. If the relief of the side walls does not change in time
, then a simpler formulation is possible. Indeed, instead of Equation (
8) we can write
In this case, no divergence condition is satisfied for the filtration rate
commonly used in porous media problems. Equation (
9) allows the introduction of the stream function
and the motion Equation (
6) then can be rewritten in the following form:
As a test reaction, we consider the neutralization of acid
A by base
B at the rate
k and resulting in the production of salt
SThe reaction enthalpy is about −57 kJ/mol. Although the reaction (
12) is exothermic, we have neglected the heat release in this work, since the effective removal of heat from the reactor zone can be organized with the help of highly heat-conducting wide walls of the HS cell. Let us assume the aqueous solutions of the acid and base be separated in space at the initial moment (acid at the top, base at the bottom). Since the solutions are aqueous, the system is miscible.
In the last years, this simple, irreversible chemical reaction occurring in liquid-liquid miscible systems was extensively studied, to mention but a few [
28,
29,
31,
32,
33]. The Rayleigh-Taylor plumes and fingers are commonly observed under gravity when the heavier
A overlies the lighter
B [
28]. Another mechanism breaking the equilibrium was found to be double diffusion instability and diffusive-layer convection [
29]. Recently, we have reported a new type of instability, the CDD convection [
31,
32,
33]. It belongs to the family of double-diffusion phenomena and arises when the diffusion coefficients of species depend on their concentrations. We have shown that the CDD effect can result in the development of a perfectly regular convective pattern [
31]. The effect was found primarily for the pair HNO
/NaOH, but then it was demonstrated also for other systems, for example, HNO
/KOH, HCl/NaOH [
32,
33].
The Boussinesq approximation for the convection problems assumes that the density
changes due to heat and/or reagent concentrations are taken into account only in terms depending on gravity (for example, in the last term in Equation (
6)). In all other terms, density variations are neglected. Thus, we express the medium density
through the concentrations of reagents dissolved in water
Here, stand for the set of solutal expansion coefficients, respectively.
We scale the problem by using
,
,
and
as the units of measurement for the length, time, velocity and concentration, respectively. Here
is the tabular value of the diffusion coefficient of acid in water at the temperature of 25
C and ultra-low concentration.
stands for the initial concentration of acid. Then we obtain the set of equations for species coupled to the Navier-Stokes equation, written in the dimensionless form:
In this paper, we use the concentration-dependent diffusion model developed for the pair HNO
/NaOH, which works well up to 3 mol/L [
31]
The dimensionless parameters which appearing in Equations (
14)–(
17) are the Damköhler number
, and the set of concentration Rayleigh numbers
(
). The parameter
is estimated to be about
. The Rayleigh numbers can be estimated from experimental data as
[
33].
3. Microreactor Configuration
Let us assume that the acid solution is continuously supplied through the upper boundary, and the base solution is fed through the lower boundary (
Figure 2b)
where
Q is the dimensionless feed rate of the reagent solutions through the incoming channels. This is another important parameter of the system.
Let us assume further that the product removal from the reaction zone occurs through the side tubes (
Figure 2b). The boundary conditions for the concentrations at the outlets can be specified assuming that the flow in output channels is sufficiently strong to neglect the diffusion transport [
34]
To be specific, let us define the wall profile by the following function:
where
stands for the Heaviside step function. Equation (
21) ensures that gap variation occurs only within the band
, and the amplitude
uniquely determines the deviation of the walls from the planar shape.
Figure 2a gives the example of non-planar HS cell for
. For sidewalls, we specify
In order to characterize the nonlinear dynamics resulting from the coupling between chemical reactions and hydrodynamic flows, various types of the integral measurements have been performed during the numerical simulations.
The important measure is given by the reaction rate
computed in terms of the area of the reaction zone, i.e., the number of points where the concentration of salt
is larger than an arbitrary threshold
. In other words, we compute a time-dependent quantity
where
if
(
is set to be
) and zero otherwise. The integral (
23) is normalized by the area of the HS unit. It is normally expected that the rate of reaction should increase because of the fluid convection.
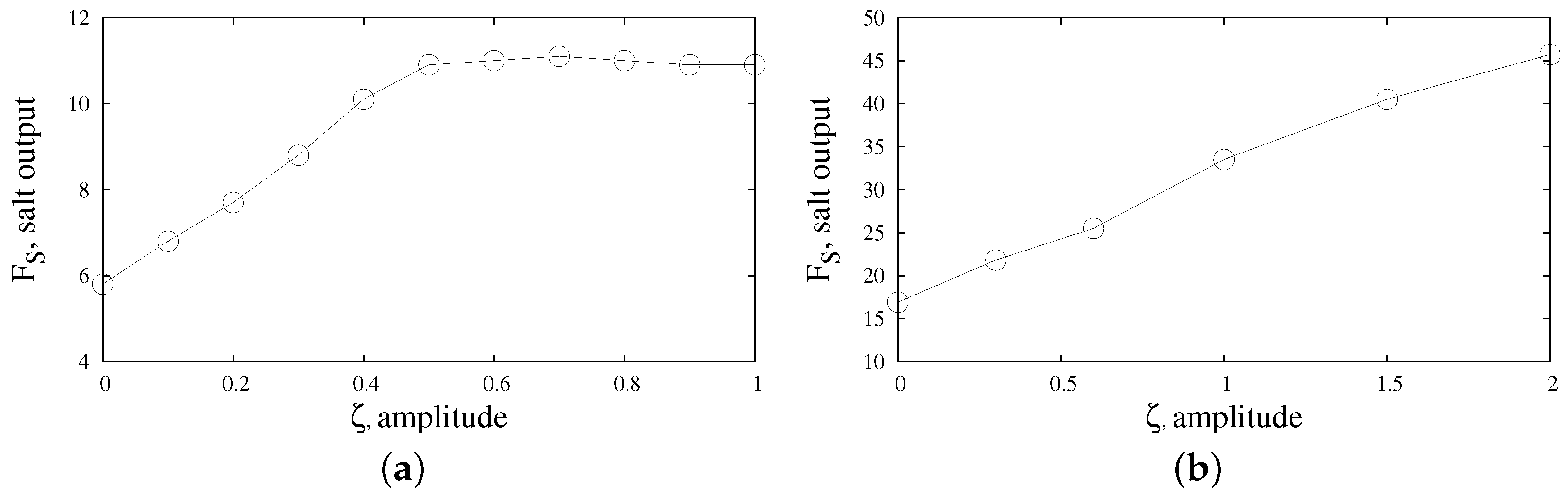
The main control parameter in the problem is the total yield of the reaction product through the output channels. We can estimate it by calculating the instantaneous flux of solvent that removes salt
S from the reactor zone through the right and left channels
Since the process can be time-periodic or even non-stationary, it makes sense to average the fluid flow (
24) over time
where the averaging procedure should be carried out over the entire time
of the observation so that the quantity
becomes the total average yield of the reaction product through the output channels.
The formulated non-stationary boundary value problem (
14)–(
22) for the variables
,
A,
B, and
S was solved numerically by a finite-difference method. The partial differential equations were approximated by standard finite-difference expressions using one-sided forward differences for time derivatives and central differences for spatial derivatives. The main calculations were carried out on uniform grids with the coordinate step of
. For example, for the reaction zone
, a square grid of
nodes was used. As it was shown in [
32], such a grid guarantees a sufficiently high resolution for detecting CDD convection.
5. Discussion and Conclusions
The theory and practice of the continuous-flow microreactors, which use hydrodynamic or convective motions of fluid for mixing reagents, are being actively developed for chemical technologies of pharmaceutical production. The production of the pharmaceutical substances requires flexibility in reconfiguring the production line and the continuity of its operation. Besides, the pharmaceutical production usually consists of a large number of intermediate stages, continuously flowing one into another, which is difficult to achieve in the framework of a traditional batch reactor. Being developed quite recently, the continuous-flow microreactors have already demonstrated their efficiency compared to their counterparts.
In this paper, we theoretically consider the influence of the shape of the microreactor walls on the mass transfer processes inside it. To be more precise, we consider the Hele-Shaw cell, which is externally controlled by the system of actuators enabling the variation of the width of the cell gap in both time and space. Our paper shows that such manipulations open up broad possibilities for controlling the processes inside the reactor. Such control can be organized in various ways. For example, the control can be organized passively by pre-prototyping the shape of the reactor walls to maintain certain flow patterns. In this case, the shape of the wall profile can be computed and tested. For instance, the intensity of an undesirable flow can be significantly reduced by setting a narrower gap between the sidewalls of the reactor, i.e., by reducing the local permeability of the medium. In the opposite case of the flow, that has a useful effect in the microreactor, it can be supported by expanding locally the gap in the area where this flow is present. To apply active control, the walls of the microreactor must be deformable. Then one has to establish a real-time feedback between the actuators and sensors signaling the mass transfer processes inside the reactor. If the product yield is taken as a control parameter, then the controller could be programmed so that it would stimulate useful convective structure in real time.
In conclusion, in this paper, we have studied the effect of the wall shape changes on mass transfer in the CFMR reactor. This method to control the microreactor has not yet been discussed in the literature, since, until the invention of 3D-printing, the fabrication of microcells with slightly non-planar walls was not a trivial task. To implement it, the walls of the microreactor must be made of a deformable, chemically inert and resistant material. If this condition is fulfilled, then it is possible to design a device that would change the local permeability of the quasi-planar microreactor in real-time. We have shown that manipulations of the wall shape open up broad possibilities for controlling the processes in the reactor.