Abstract
Freedom of movement of the hands is the most desired hope of stroke patients. However, stroke recovery is a long, long road for many patients. If artificial intelligence can assist human arm movement, the possibility of stroke patients returning to normal hand movement might be significantly increased. This study uses the artificial neuromolecular system (ANM system) developed in our laboratory as the core of motion control, in an attempt to learn to control the mechanical arm to produce actions similar to human rehabilitation training and the transition between different activities. This research adopts two methods. The first is hypothetical exploration, the so-called “artificial world” simulation method. The detailed approach uses the V-REP (Virtual Robot Experimentation Platform) to conduct different experimental runs to capture relevant data. Our policy is to establish an action database systematically to a certain extent. From these data, we use the ANM system with self-organization and learning capabilities to develop the relationship between these actions and establish the possibility of conversion between different activities. The second method of this study is to use the data from a hospital in Toronto, Canada. Our experimental results show that the ANM system can continuously learn for problem-solving. In addition, our three experimental results of adaptive learning, transfer learning, and cross-task learning further confirm that the ANM system can use previously learned systems to complete the delivered tasks through autonomous learning (instead of learning from scratch).
1. Introduction
Stroke has become an increasingly common disease in recent years. After stroke, patients tend to have so-called “abnormal coordinated movement problems” [1,2]. One of the typical problems is the difficulty isolating and activating specific muscle groups; the other is the simultaneous and unintended activation of multiple muscle groups. When patients face difficulties executing movements properly or fail to show improvement, intelligent robots, like clinical physical therapists, can assist when patients struggle to complete necessary movements independently [3]. In recent years, there have been considerable achievements in using machines to help stroke patients [4,5]. Electric [6,7] or pneumatic motors [8,9] mainly drive the design of general rehabilitation robots. Although the most effective approach remains undetermined, electrical muscle stimulation is the most commonly used method [10]. If we look at the needs of patients for body part rehabilitation, it generally goes in two directions: upper limb equipment and lower limb equipment. Most scholars will only emphasize research on using machines for upper or lower limb rehabilitation, but not both. However, some scholars [11] consider both upper and lower limb rehabilitation. The upper limbs play a more critical role in patients’ daily lives. People further subdivide rehabilitation into arm movements and hand operations. The former involves helping patients make appropriate movements with their entire hands. At the same time, the latter focuses on how to help patients coordinate their palms and fingers to make appropriate movements when touching objects.
As pointed out by Huang and Krakauer [12], current upper limb rehabilitation robots have two types: end-effector devices (such as MIT/IMT-Manus and GENTLE/s [13]) and exoskeleton systems (such as Armin [14], Pneu-WREX [15], RUPERT [16], and REHAROB [17].) The advantage of using a handheld end-effector is that it can be reconfigured for patients of different body types. End-point fixed systems are the most commonly used robots for stroke patient assistance. In 1998, Krebs et al. [18] introduced the MIT-MANUS, a planar-motion robotic arm that enables patients to perform hand movements with on-screen information. Cozens [19] developed a simple single-axis torque motor robotic system in 1999, demonstrating its efficacy in upper limb rehabilitation for patients with spasticity and muscle weakness. Hesse et al. [20] created the Bi-Manu-Track robot, which utilizes bimanual control to enhance forearm pronation/supination coordination and wrist flexion. These robotic designs primarily employ end-point contact with patients to facilitate various movements.
Brokaw et al. [21] designed a passive, lightweight, wearable device to assist patients in hand rehabilitation during daily activities. Yurkewich et al. [22] created a battery-powered, myoelectric, untethered robotic glove for sensing and helping the user’s fingers grasp or release objects. Park et al. [23] developed a robotic grasping rehabilitation device that integrated pressure sensors embedded in the handle with patient intention detection. Some studies [24] highlight how to design a home-computer-assisted arm rehabilitation (hCAAR) robotic device that stroke survivors with upper limb weakness can use independently at home. In addition, some scholars suggest exploring rehabilitation from the perspective of commercialization and the most advanced fields. In addition to using equipment, another group of students focused on discussing how to use Internet of Things (IoT) technology to record patients’ rehabilitation using equipment and the remote supervision of treatment by experts. In recent years, some scholars have devoted themselves to researching the most advanced intelligent rehabilitation robots and proposed modern and cutting-edge ideas [25,26,27]. Several scholars [28,29,30,31,32,33] provide systematic surveys of robotic systems for the rehabilitation and care of upper limb rehabilitation.
Previously, we implemented a humanoid robot controlled by a self-organizing model—an artificial neuromolecular (ANM) system—to assist upper limb rehabilitation [34,35]. This study has two significant differences from our previous study. First, we have continuously adopted the “artificial approach” and systematically implemented a complete survey of all possible combinations of movements of four possible actions: shrugging, swinging, up-lifting, and side-lifting. Moreover, we looked into the transfer learning among different movement combinations, simulating the alleviation of abnormal coordinated movement problems. The “artificial approach” allows us to perform various experiments with fewer constraints. The second difference is that we use the Toronto Rehab Stroke Pose (TRSP) dataset [36] collected by the Toronto Rehabilitation Institute (TRI-UHN), referred to as the “Toronto Rehabilitation dataset”, to analyze the relationship between the movements of stroke patients and regular participants. This analysis provides a basis for the ANM system to find correlations between movements, serving as a foundation for subsequent progressive rehabilitation learning. Using accurate rehabilitation data allows us to verify the ANM system’s ability to assist stroke patients in rehabilitation and achieve progressive rehabilitation learning assistance for stroke patients.
This study aims to use the artificial neuromolecular (ANM) system, a self-organizing model, to help stroke patients through the method of so-called progressive rehabilitation. The idea is to divide the gap between the movements of stroke patients and healthy people into several stages. In each improvement stage, the robot arm, controlled by the ANM system, performs a small improvement step. This allows the patient to experience the operation of the action. Assuming the patient feels comfortable, the robotic arm helps the patient experience their body’s reaction behavior and brain stimulation response. On the other hand, it means that relatively more movement changes can be performed (the so-called progressive rehabilitation). If the patient is experiencing discomfort, this indicates that the gap is too big for the patient (smaller gaps are needed for improvement.)
The structure of this paper has six parts. The first part explains this study’s background, motivation, and purpose. The second part introduces the architecture, core processing components, and operating mechanism of the algorithm used in this study (the ANM system). The third part describes the research architecture, experimental equipment, datasets, input/output interface design of the ANM system, and the evaluation indicators used in the study. The fourth part introduces the respective experimental designs and results of both datasets. The fifth and sixth parts are the discussion and conclusion, respectively.
2. The ANM System
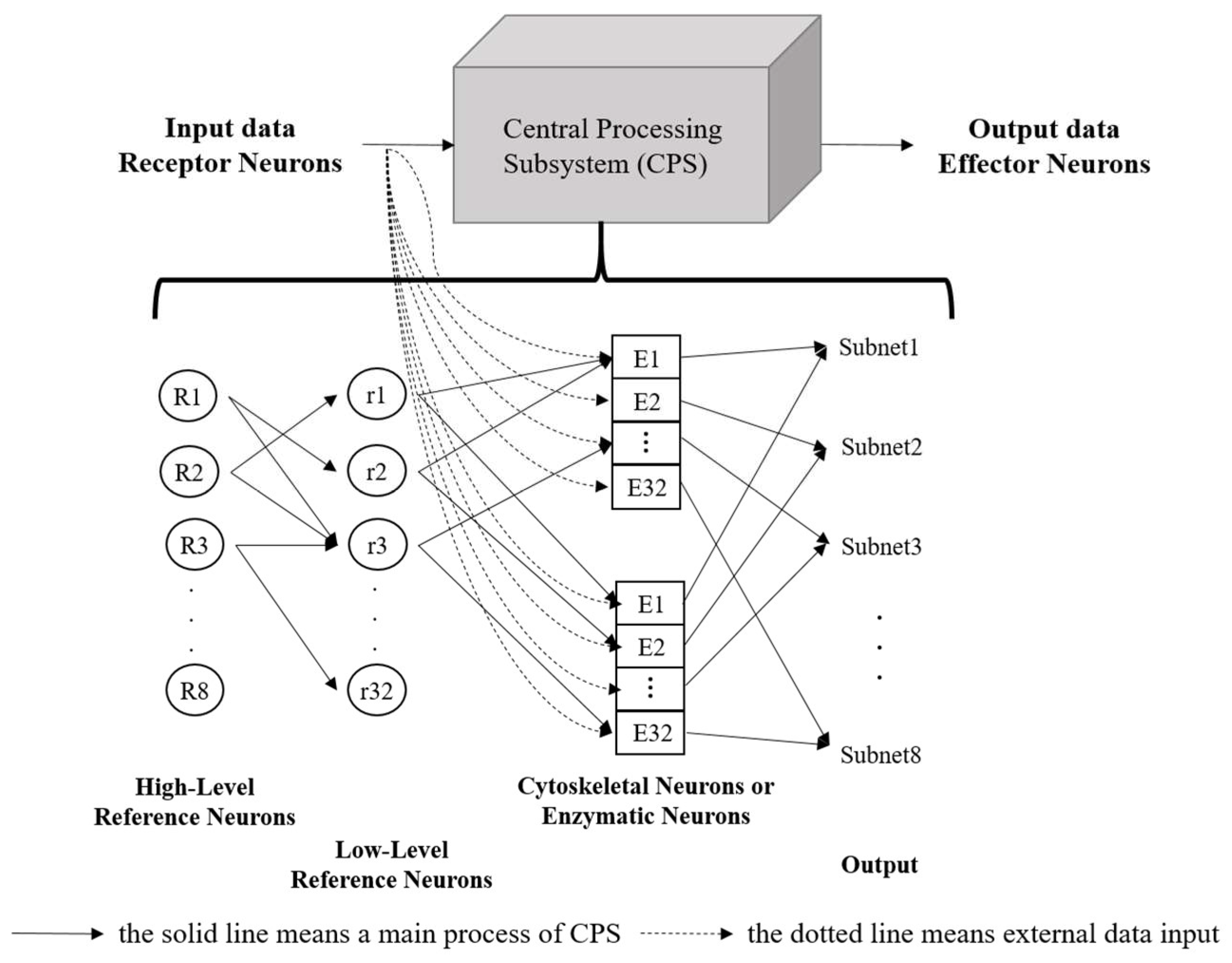
The core processing components of the ANM system include all control and information processing neurons, forming the Central Processing Subsystem (CPS). This system functions similarly to the brain’s processing mechanism and is viewed as a converter between input and output. The CPS mainly consists of a group of reference neurons and information-processing neurons (cytoskeletal neurons or enzymatic neurons), with the information-processing neurons functioning like a black box, as shown in Figure 1.
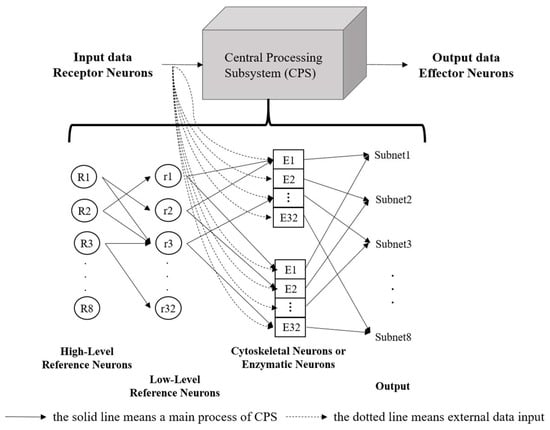
Figure 1.
The structure of the ANM system.
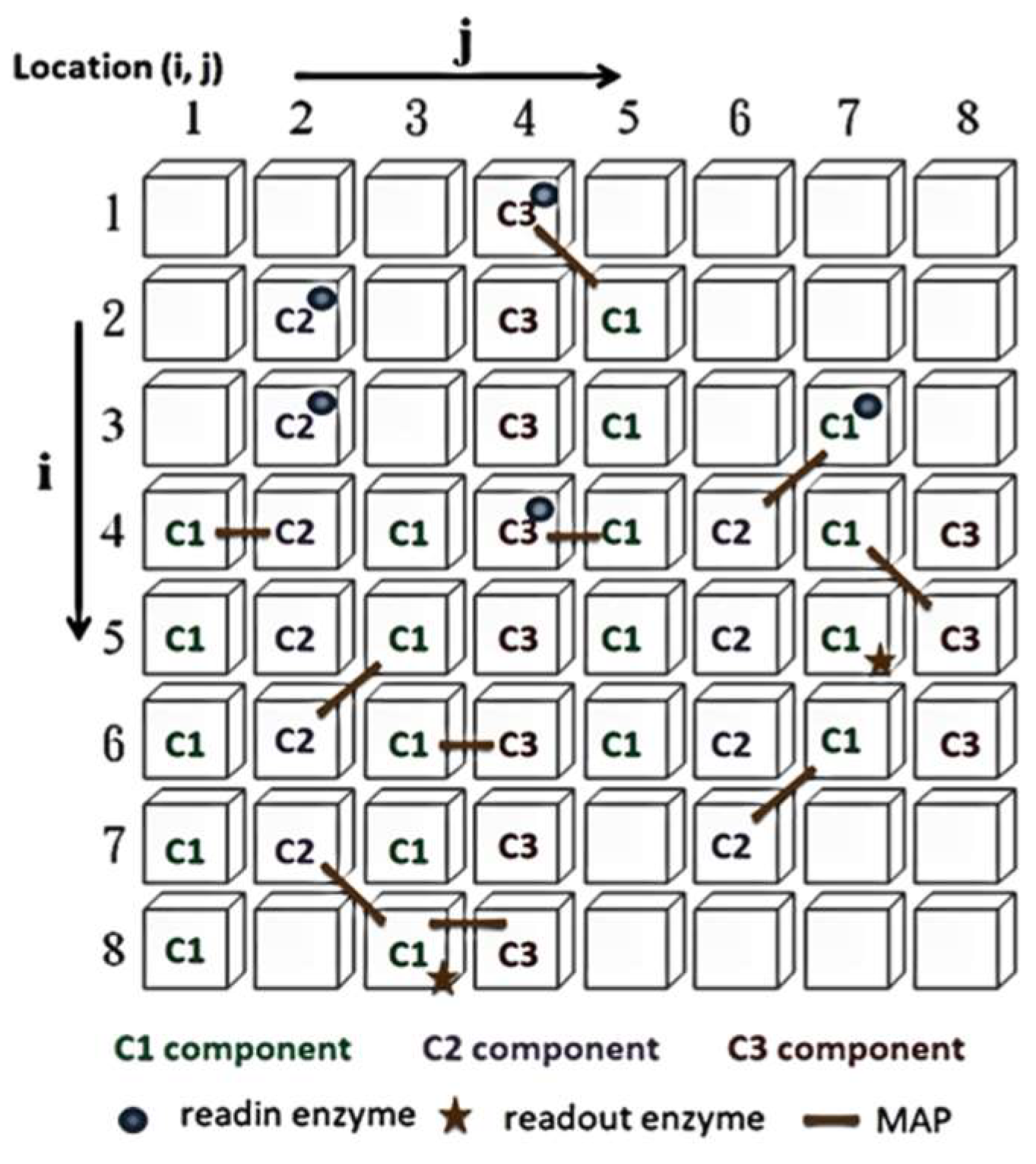
The control neurons in the ANM system architecture are further divided into high-level reference neurons and low-level reference neurons, which select and memorize the best combination of information-processing neurons to complete task requirements. The information-processing neurons form the central learning core of the ANM system, based on the assumption that information processing occurs on the neuron’s cytoskeleton. This study uses a two-dimensional cellular automaton (CA) to simulate the information processing on the cytoskeleton, as shown in Figure 2. The information-processing neuron comprises four components: essential constituent molecules, reading enzymes, readout enzymes, and microtubule-associated proteins (MAPs). The primary constituent molecules are further divided into three types: C1 has the highest energy but slowest speed; C2 has medium energy and speed; C3 has the lowest energy but fastest speed. These represent different transmission strengths and speeds. The neuron will trigger when the signal flows to the readout enzyme and the energy exceeds a certain threshold. A detailed explanation of information processing inside each neuron can be found in [14,15,16].
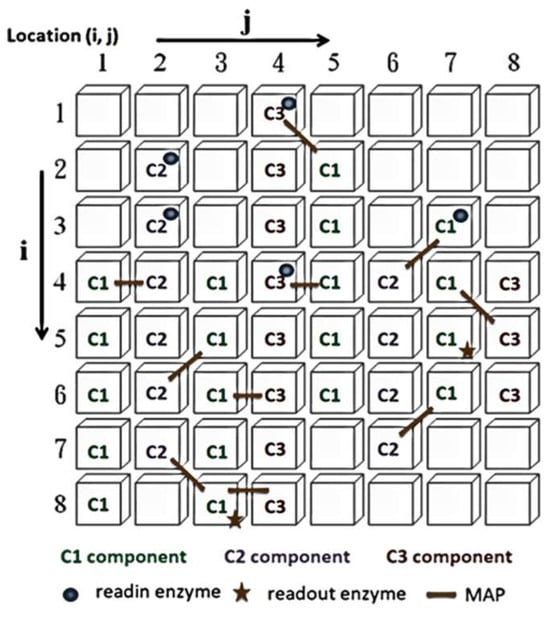
Figure 2.
Cytoskeleton elements.
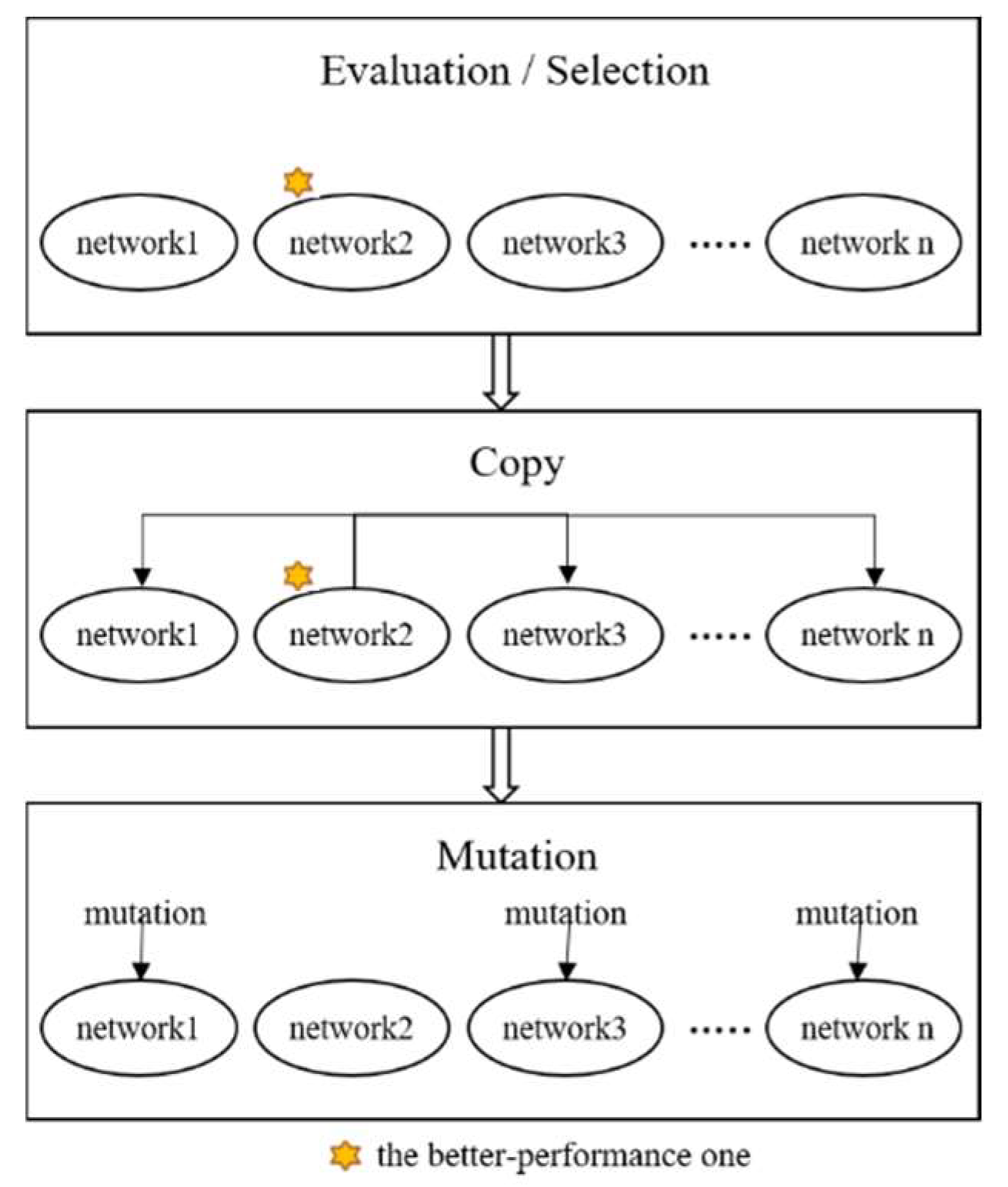
This evolutionary learning mechanism is divided into two stages: the first involves the control neuron layer, and the second consists of the information-processing neuron layer. The entire system training is achieved through cyclic training of these two stages. The learning steps for each stage can be summarized in three steps (Figure 3):
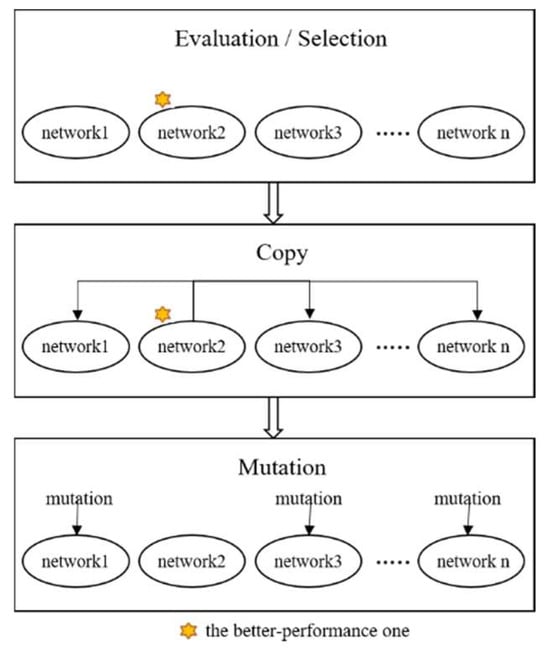
Figure 3.
Evolutionary learning of the ANM system.
- I.
- Evaluation: evaluate the performance of each sub-network according to experimental requirements and find the best sub-network.
- II.
- Copy: copy the variable settings of the best sub-network to other sub-networks.
- III.
- Mutation: randomly change the original settings of each sub-network.
3. Materials and Methods
This section will first introduce the model of this study. Next, we describe the devices and equipment used, including the robot simulation platform and the designed robot arm. Afterward, we describe the dataset used in this study and its linkage to the ANM system. Finally, we describe the evaluation criteria for learning performance.
3.1. Research Model
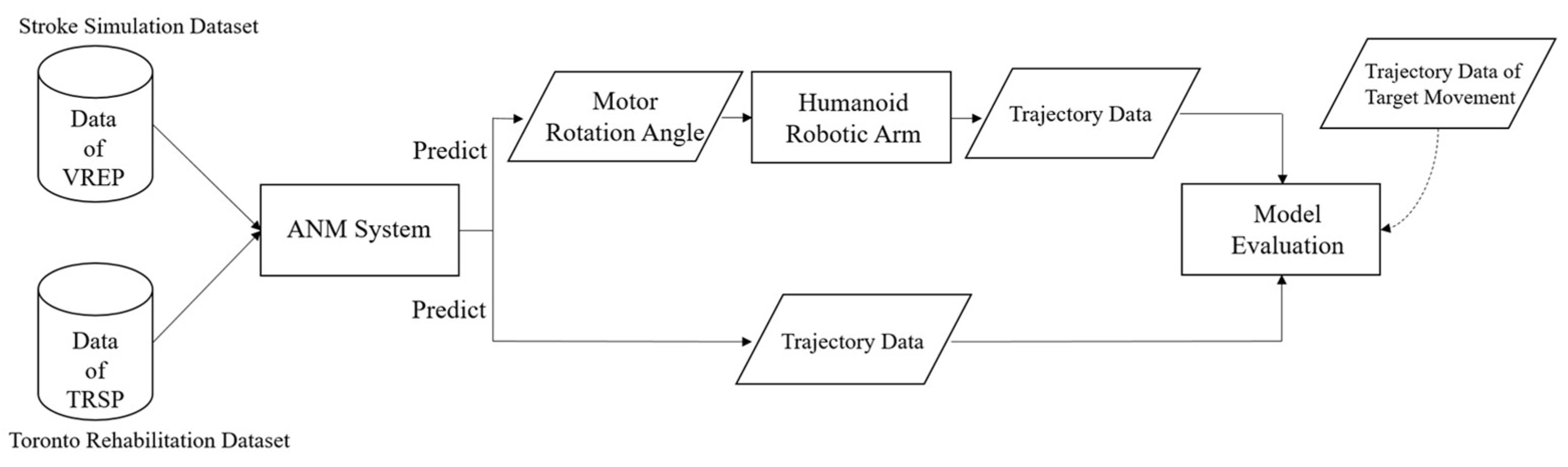
To validate the ANM system’s ability to leverage its adaptive learning capabilities in assisting stroke patients with rehabilitation tasks, this research utilizes two data sources: the first data source, termed the “Artificial World dataset”, combines the concept of an “artificial world” with a seven-degrees-of-freedom humanoid robotic arm. This virtual framework generates various motion datasets, including stroke rehabilitation movements. The second source is the publicly available Toronto Rehabilitation dataset (TRSP) from the Toronto Rehabilitation Institute, comprising data from 10 healthy people and nine stroke patients.
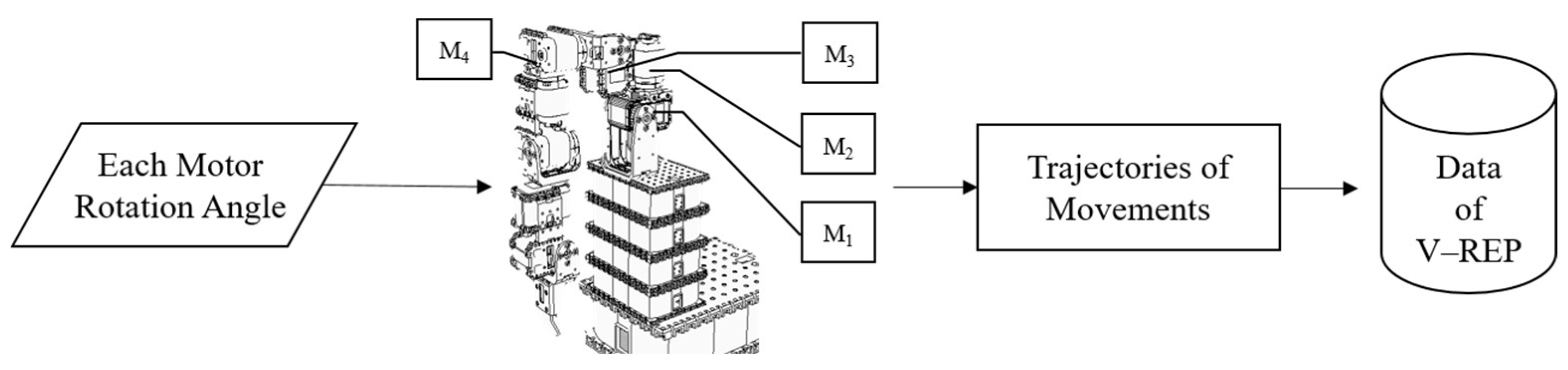
Both datasets are input into the ANM system to learn the relationships between different movements. The input for the Artificial World dataset is the motion trajectory simulated by the humanoid robotic arm. In contrast, the output (learned motion) is the rotation angle of each motor when the robotic arm acts. This allows the ANM system to learn from rough movements to more precise, muscle-group-specific actions. For the Toronto Rehabilitation dataset, input and output are motion trajectory data. The learning results from the ANM system are then compared with the target motion trajectories for similarity, using dynamic time warping (DTW) as the performance indicator. Since the learning output for the Artificial World dataset is the motor rotation angles of the robotic arm, these angle values need to be input back into the humanoid robotic arm to generate corresponding motion trajectories. The complete research model is illustrated in Figure 4.
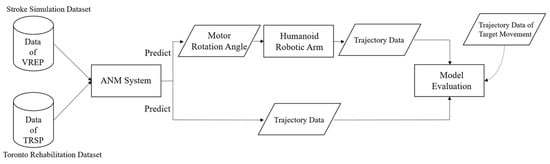
Figure 4.
Research model.
3.2. Equipment and Devices
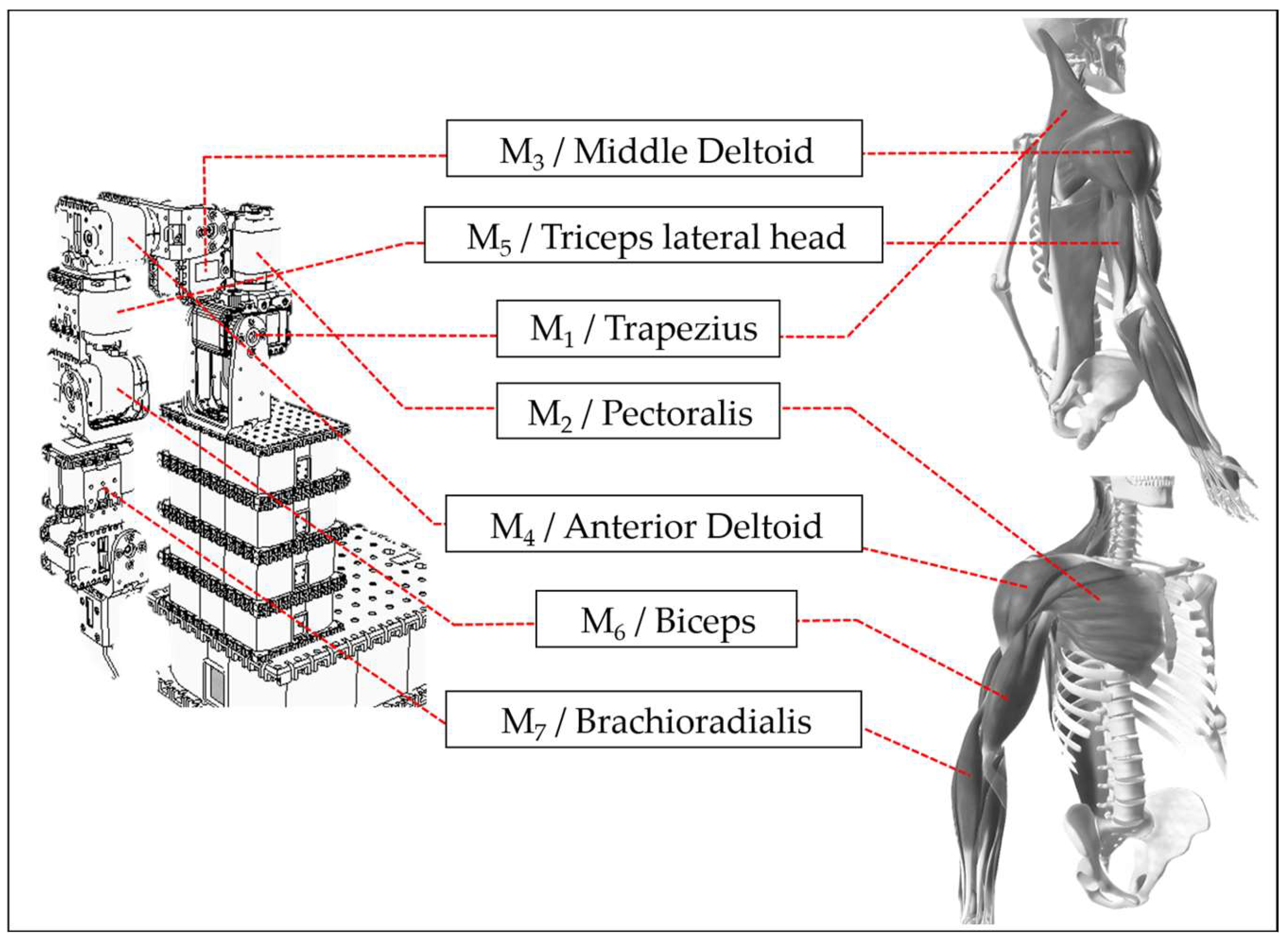
To achieve the ultimate goal of assisting stroke patient rehabilitation, this study uses V-REP simulation software (V-REP_PRO_EDU v3.1.2, now renamed CoppeliaSim) to design a humanoid robotic arm with seven degrees of freedom, each controlled by a motor. Each motor corresponds to human muscle joints. The study selects one primary muscle for movement from the muscle groups involved in each joint’s operation. It assembles the humanoid robotic arm in the V-REP simulation software based on the motor’s rotation direction. This creates a correspondence between human joints, muscle groups, and the robotic arm’s motors, allowing the ANM system to learn the relationship between motion trajectories and motor rotation angles. Motor 1 corresponds to the trapezius muscle, motor 2 to the pectoralis major, motor 3 to the middle deltoid, and motor 4 to the anterior deltoid. A detailed comparison between the humanoid robotic arm and human muscle joints [37] is shown in Figure 5. In designing arm movements, this study only considers actions that can be roughly imitated by the humanoid robotic arm, excluding human arm movements that cannot be simulated due to motor design and assembly limitations. The current experiment is primarily based on movements achievable by motors 1 to 4.
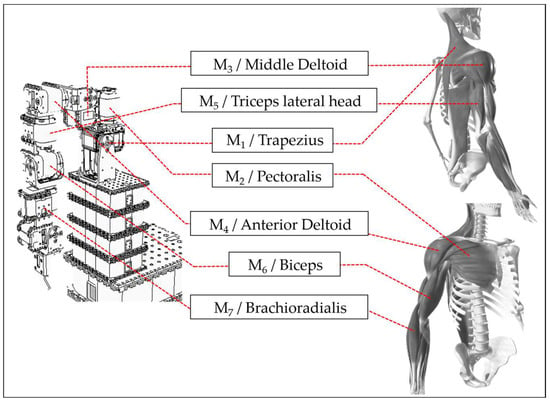
Figure 5.
Comparison of muscle joints between robotic arm and human arm.
The virtual robotic arm designed in this research experiment has seven actuators, four of which are mainly responsible for arm movement, and the remaining three are responsible for hand operation. Generally, arm movements are gross, while hand operations are delicate. For stroke patients, arm movement may be more urgent than hand manipulation. This is because, in daily life, everyone must be able to move their hands to the appropriate position before further performing hand operations. Therefore, when arm movement is not improved, emphasis on hand manipulation will not significantly help stroke patients with daily activities. Thus, this research experiment uses only four of the seven actuators of the mobile virtual robotic arm in the current research phase. When the system matures considerably, this study will add hand operation research.
3.3. Dataset
3.3.1. Artificial World Dataset
The Artificial World dataset is derived from a seven-degrees-of-freedom humanoid robotic arm constructed in the V-REP simulation software, combined with the concept of an artificial world. This approach simulates human arm movements rather than replicating actual human arm motions. Consequently, it is not bound by the inherent limitations of human arm movement, allowing the artificial world method to provide a relatively larger dataset for executing various arm movements. The data collection process begins by gathering arm motion trajectories that simulate the single movements of a healthy person. Subsequently, more complex arm movement trajectories are generated by combining multiple movements simultaneously. Finally, the Artificial World dataset combines these various arm movement combinations. The data collection workflow is illustrated in Figure 6.
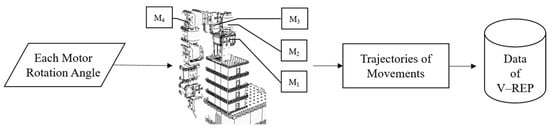
Figure 6.
Artificial World dataset data collection flowchart.
For people with healthy hands, the brain can freely control both hands to perform different types of movements. Therefore, this study designs four movements that healthy people can execute freely: shrugging, swinging, side-lifting, and up-lifting. The V-REP software can simulate these four movements by inputting preset angles for the humanoid robotic arm’s motors. During the execution of each movement, the spatial coordinate position of the robotic arm’s end-point (corresponding to human fingertips) is recorded every 300 milliseconds. These end-point spatial coordinates are then connected to form a complete movement trajectory for the robotic arm. Executing a single movement is relatively easy for people with healthy hands. Combining two or three movements may present some difficulty but is still manageable. However, simultaneously executing all four movements poses a considerable challenge.
3.3.2. Toronto Rehabilitation Dataset
The TRSP dataset [38,39], collected by the Toronto Rehabilitation Institute (TRI-UHN), analyzes the relationship between stroke patients’ movements and regular participants. This dataset also supports research in developing and evaluating algorithms for monitoring upper body posture and movement post-stroke.
Experimental Tools
The study used Microsoft Kinect (K4W) to track subjects’ arm movements, employing image-based markerless tracking technology. We note that K4W s manufactured by Microsoft, which is based in Redmond, Washington, USA. K4W was chosen over marker-based motion capture systems like MoCap, which can cause discomfort to subjects, are costly, and are unsuitable for clinical environments. K4W extracts human posture using depth and video sequences captured by infrared and color cameras. A K4W v2 SDK 2.0 application was developed for detecting, tracking, and recording movements. Additionally, arm movements were executed using a handheld end-effector with two degrees of freedom, capable of assisting and resisting shoulder and elbow rehabilitation movements. The experimental tools are shown in Figure 7a,b.

Figure 7.
(a) Microsoft Kinect (k4w) v2. (b) A handheld end-effector with two degrees of freedom.
Data Collection
The study recruited ten stroke patients with varying degrees of upper limb impairment from a Toronto rehabilitation hospital (TRI-UHN). Recruitment was based on therapist recommendations and the hospital’s central recruitment process, using assessment standards such as the Fugl-Meyer Assessment of the Upper Extremity (FMAUE) and Chedoke–McMaster Stroke Assessment (CMSA). Due to software issues, data from one participant were not correctly recorded, leaving the dataset with nine stroke patients. Ten healthy participants were also recruited, totaling 19 subjects. The data collection process followed these steps:
- I.
- Using Microsoft Kinect (K4W) with K4W v2 SDK 2.0, 3D positions (x, y, and z coordinates) and orientations of 10 upper body parts and joints relative to the depth sensor were tracked and recorded every 30 milliseconds.
- II.
- Subjects performed a series of short scripted movements, each repeated five times at a comfortable speed and range of motion.
- III.
- Each movement was performed first with the left arm, then the right, for consistency. Participants could rest between repetitions if needed.
- IV.
- Researchers counted each repetition and signaled participants when to start.
- V.
- Stroke patients were asked to perform two types of scripted movements with both upper limbs using a two-degrees-of-freedom haptic robot (handheld end-effector): reach-forward–backward (Fwr_Bck) and reach-side-to-side (Sd2Sd_Bck). Please see Table 1.
 Table 1. Stroke patients performed motions similar to simulated movements performed by healthy participants.
Table 1. Stroke patients performed motions similar to simulated movements performed by healthy participants. - VI.
- To avoid prolonged experimental sessions for stroke patients, healthy participants completed the same scripted movements and then, under expert guidance, performed additional sets simulating everyday post-stroke compensatory movements, including lean-forward (LnFwr), trunk rotation (TrRot), and shoulder elevation (ShElev). Please see Table 1.
3.4. Input/Output Interface (Linkage to the ANM System)
This study trains the ANM system using the Stroke Simulation and Toronto Rehabilitation datasets. The input for both datasets consists of trajectory data. For the Artificial World dataset, 50 data points are input into the ANM system for learning.
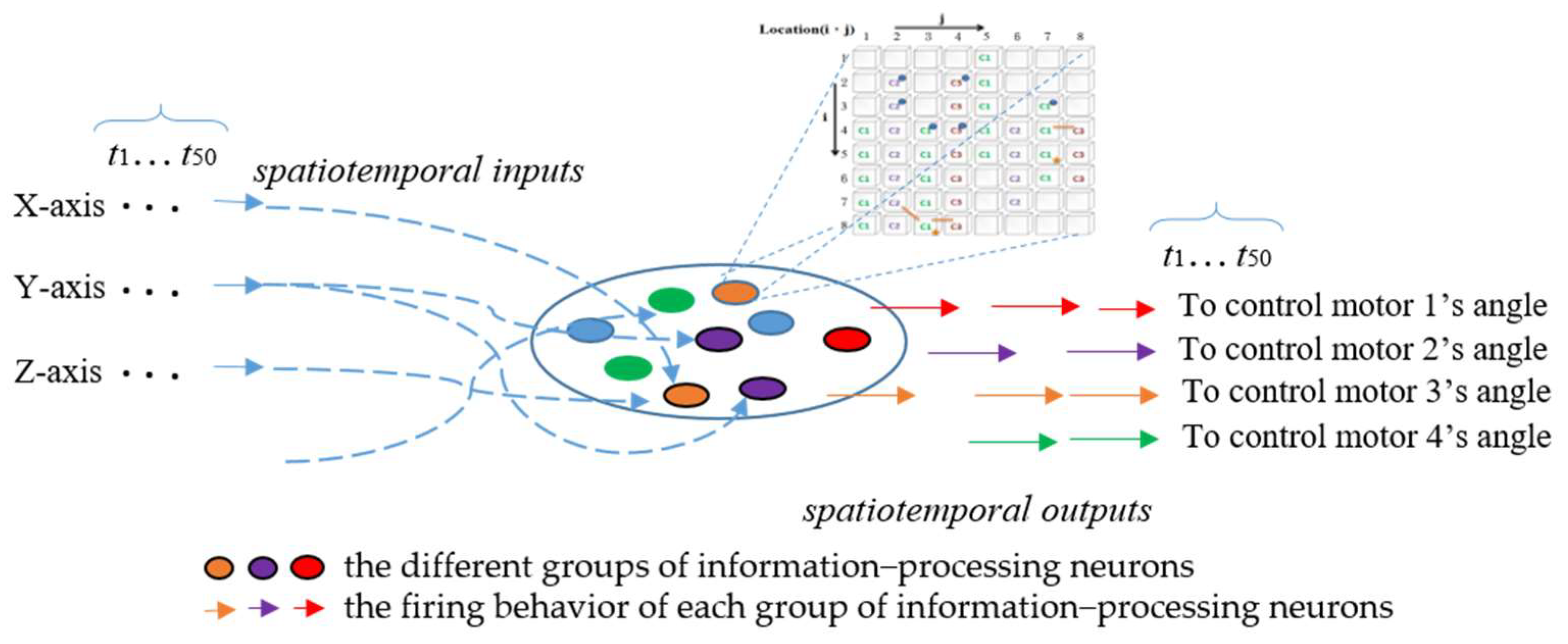
The ANM system activates internal neurons through input data and returns corresponding arm movements. In the ANM system for the Artificial World dataset, all information-processing neurons of the ANM system are equally divided into four groups according to the four motors of the humanoid robotic arm. The firing behavior of each group of information-processing neurons represents the manipulation control of the humanoid robotic arm at specific motor rotation angles, as shown in Figure 8. In the current implementation, we use the time difference (Δt) between two adjacent firing neurons of the same group to describe the degree of actuation for that motor’s rotation angle. This relationship between time difference and degree of actuation follows an S-shaped waveform, as shown in Equation (1), where the magnitude of actuation gradually decreases over time. The four output motor angles correspond to joints that achieve shrugging, swinging, side-lifting, and up-lifting. Longer firing times result in larger motor rotation angles and vice versa. The ANM system outputs angles by converting the time difference between two firings of the corresponding information-processing neuron group through a Sigmoid function, then normalizing the results to −100 to 150 degrees to meet the study’s requirements for motor rotation angles.
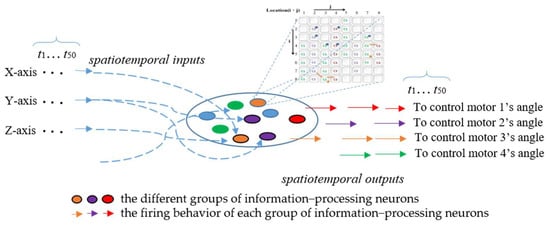
Figure 8.
Comparison of learning results at different stages of the ANM system.
For the Toronto Rehabilitation dataset, 30 data points are input into the system. In the ANM system of this dataset, all information-processing neurons are evenly divided into three groups based on the target motion trajectory (three-dimensional coordinates). The firing behavior of each group of information-processing neurons for a specific axis represents the manipulation of the robotic arm. The calculation of the degree of actuation in a particular axis is shown in Equation (2).
3.5. Dynamic Time Warping
This study first outputs the learned motor rotation angle data from the model to evaluate the learning performance on the Artificial World dataset. These angle data are then input into a V-REP humanoid robotic arm to generate the learned motion trajectory data. The difference between this learned trajectory and the target motion trajectory is then calculated. The same calculation is performed for the Toronto Rehabilitation dataset, comparing the two trajectories. Although both trajectory sequences are temporally consistent, the research aims to assist stroke patients in improving their condition to achieve arm movements closer to those of healthy people. Therefore, the comparison focuses on the similarity of motion trajectories rather than point-to-point correspondence. To this end, dynamic time warping (DTW) is used to evaluate the distance between the model’s output and target motion trajectory. Using Euclidean distance for point-to-point calculation might erroneously classify similar trajectory data as two entirely different motions.
This study employs FastDTW, proposed by Salvador and Chan, as the computational method for dynamic time warping. The trajectory data of the learned and target motions are separated into their X, Y, and Z axes. FastDTW distance is calculated individually for each axis and then summed to obtain the three-dimensional movement trajectory error between the two movements, as shown in Equation (3). The DTW distance between the two sequences is implemented using the “fastest” Python package [40].
where and represent the target trajectory generated by the humanoid robotic arm from the V-REP system and the trajectory generated by the ANM system, respectively. i = x-axis, y-axis, and z-axis.
4. Experiments
This study uses two datasets: Artificial World and Toronto (please refer to Section 3.3). The properties of each dataset are different; therefore, the scope of experiments it can provide is also different. Since the Artificial World dataset does not restrict the use of data in nature, it can give a broader scope of research. This study explores it from four perspectives: continuous learning, adaptive learning, transfer learning, and cross-task learning. In the Toronto dataset, this study first conducts adaptive learning and then progressive rehabilitation learning for stroke patients.
4.1. Artificial World Dataset Experiments
The experiments for the Artificial World dataset are summarized in Table 2 and divided into four parts:

Table 2.
The four experimental designs integrate the Artificial World dataset.
- I.
- Focus on the system’s ability to learn autonomously and continuously in an unknown environment.
- II.
- Allow the ANM system to learn arm movements with reduced degrees of freedom, considering how to minimize the number of muscle groups stimulated during movements.
- III.
- Explore how to repeatedly use knowledge from previous tasks to improve learning performance on another related task.
- IV.
- Investigate how to apply the current learning results of the ANM system to different tasks, testing whether the system can adapt to movement transitions.
4.1.1. Perpetual Learning
This experiment investigates the ANM system’s autonomous and continuous learning abilities. Simulating random arm movements of healthy people using the humanoid robotic arm provides the ANM system with arm movements of different degree-of-freedom combinations to learn. This tests whether the ANM system can stably and continuously learn target tasks in an unknown environment. In terms of assisting stroke patient rehabilitation, if the ANM system possesses autonomous learning ability and can continue learning in uncertain environments, it will provide robustness to the rehabilitation process.
The results are shown in Table 3, which primarily records the difference values between the learned trajectories and the target trajectories, using DTW as the performance indicators. The improvement rate calculation is shown in Equation (4). Additionally, this study uses the R2 Score (coefficient of determination) to evaluate the learning performance of the ANM system. R2 ranges from 0 to 1, 1 indicating excellent regression model performance. The experiment designed trajectories with single, two, three, and four degrees of freedom for the ANM system to learn to increase the model’s generalization ability. The results in Table 3 are from 600 generations of system learning, but the study allowed the system to continue learning up to 2000 generations. The results show that the differences between most rehabilitation movements and target trajectories become smaller after a learning period. Still, some two-degree and single-degree-of-freedom movements perform poorly during learning. This study speculates that the ANM system has characteristics similar to biological systems adapting to their environment and changing their structure and function. In each round of learning, a genetic algorithm is combined for evaluation, copy, and mutation. Therefore, when the combination of neurons in that round of mutations performs poorly, it may result in less than satisfactory performance.

Table 3.
DTW, improvement rate, and R2 Score for each rehabilitation movement during the later learning stage.
In addition to the previous discussion, this study further analyzes the experimental results in Table 3. From Table 3, it can be seen that when the learning tasks include up-lifting, the learning performance improves significantly. From the perspective of assisting stroke patients in rehabilitation, the ultimate goal is to help patients achieve rehabilitation outcomes with minimal effort. Suppose the four-movement combination is considered to represent the movements of stroke patients. In that case, the ANM system’s learning results suggest that up-lifting is the most suitable rehabilitation movement for stroke patients. This proves that the ANM system can find the most appropriate movements for patient rehabilitation after continuous learning.
represents the initial DTW of the ANM system’s learning, and represents the DTW in the later stages of system learning.
4.1.2. Adaptive Learning
The motivation for this experiment is from the perspective of stroke patient rehabilitation. As mentioned earlier, stroke patients often experience “abnormal coordinated movements” in their upper limbs, meaning that one movement involves multiple muscle groups, similar to “a small change affects everything”. If stroke patients can reduce the possibility of simultaneously activating numerous muscle groups, they can complete the required movements more smoothly, thereby improving rehabilitation efficiency.
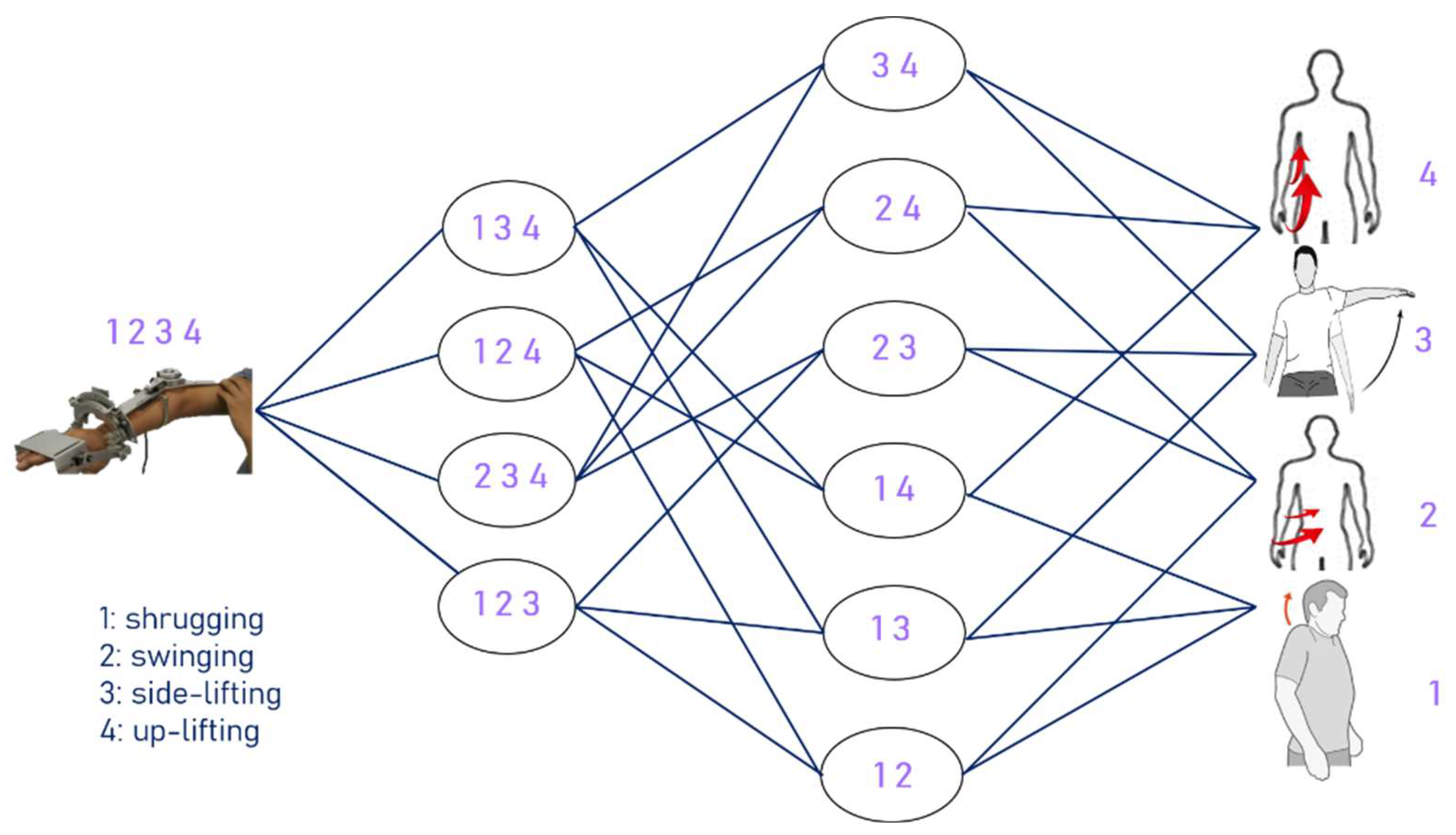
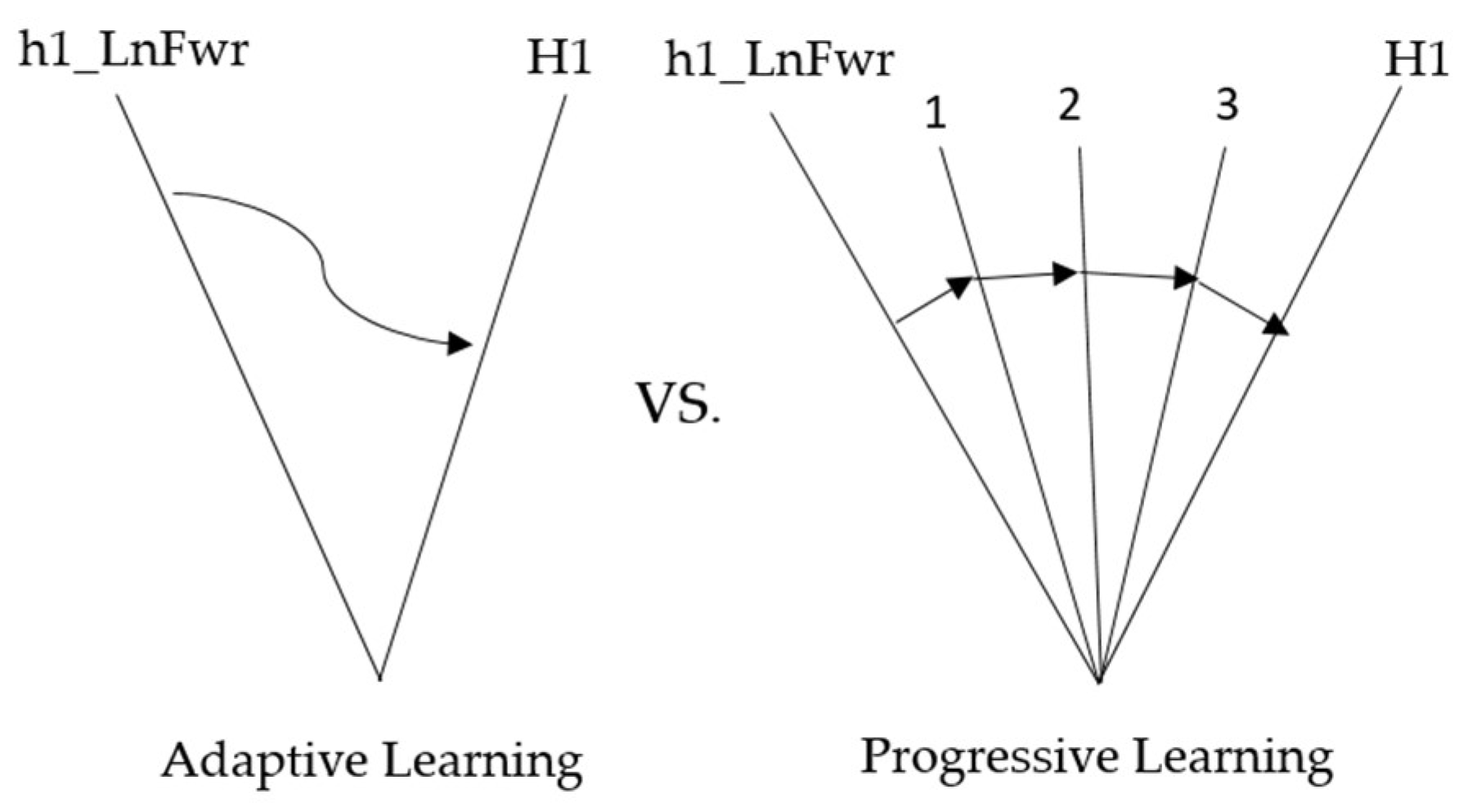
The method adopted in this experiment lets the ANM system learn from movements with relatively higher degrees of freedom to those with relatively lower degrees of freedom, from complex movements to single movements. The experiment is designed to let the ANM system learn from four-degrees-of-freedom movements to three-degrees-of-freedom movements, then learn two-degrees-of-freedom movements, and finally, single-degree-of-freedom movements. The structure of adaptive learning is shown in Figure 9, representing high degrees of freedom (complex movement combinations) to low degrees of freedom (single movements) from left to right.
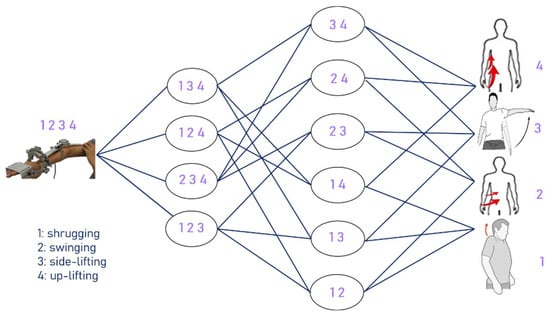
Figure 9.
The structure of adaptive learning.
The experimental results in Table 4 show that the ANM system can successfully assist in improving from four-degrees-of-freedom to three-degrees-of-freedom movements. From the perspective of helping stroke patients, the ANM system can effectively reduce the possibility of simultaneously stimulating multiple muscle groups, meaning patients can more smoothly achieve the required movements. Table 5 shows the improvement from four-degrees-of-freedom movements to three-, two-, and single-degree movements. Table 6 shows the improvement from three-degrees-of-freedom movements to two-degree and single-degree-of-freedom movements. Table 7 shows the improvement from two-degree to single-degree-of-freedom movements. Table 4, Table 5, Table 6 and Table 7 use DTW as the performance indicators.

Table 4.
The improvement from four to three degrees of freedom.

Table 5.
The improvement from four to three degrees of freedom, then to two, and finally to one.

Table 6.
The rate of improvement from three to two degrees of freedom and finally to one.

Table 7.
The rate of improvement from two degrees of freedom to a single action.
4.1.3. Transfer Learning
Transfer learning (TL), proposed in 1995, is a machine learning technique that uses a model trained on one task to help learn another related task. Its basic principle is that there is often some common knowledge or features between two associated tasks. Therefore, expertise or features learned from the first task can be directly or indirectly applied to the second task to improve learning efficiency and effectiveness. From the perspective of intelligent systems assisting patient rehabilitation, this means the possibility of using the learning results of some previous rehabilitation movements to learn different but related rehabilitation movements more easily. This part aims to solve two problems: (1) Does the ANM system using transfer learning perform better than the system that did not use transfer learning at the beginning of learning? (2) After the system spends the same learning time (e.g., learning for 800 rounds), does the system using transfer learning perform better on the target task than when not using transfer learning?
According to the transfer learning results in Table 8, the ANM system using transfer learning performs better than the system that does not use transfer learning at the beginning of learning in most learning tasks. However, when the learning task includes shrugging, swinging, and side-lifting movements, the learning performance of the ANM system is relatively poor. Similar results were found in the fixed learning time (learning for 800 cycles) of the ANM system. Table 8 compares the improvement rates using and not using transfer learning, evaluating model learning performance with DTW.

Table 8.
Comparison of improvement rates with and without transfer learning.
4.1.4. Cross-Task Learning
Based on transfer learning, cross-task learning focuses on learning transitions between different movements. The experiment has two purposes: (1) Understand the system’s ability to adapt to problem (or task) changes. (2) Test whether the system can reproduce its ability to adapt to problem changes through previous learning results. From a rehabilitation perspective, the purpose of this experiment can be understood as first letting stroke patients learn a set of movements, then changing the required rehabilitation movements to know how patients adapt to changes with the help of the system.
The experimental results in Table 9, Table 10 and Table 11 mainly record the first generation of learning results for the current task based on the ANM system’s previous learning results. Since this experiment aims to test the system’s ability to adapt to problem changes and whether it can reproduce the ability to adjust to problem changes through previous learning results, it only needs to observe the first generation of learning results to know which movement combinations are more similar under movement of the same degree of freedom. From a rehabilitation perspective, this means that after a stroke, patients learn a set of movements, save the results of that learned movement, then change the required rehabilitation movement, and then adapt to the changes with the help of the system. Table 9, Table 10 and Table 11 show the adaptability results of changes in the degree of freedom of movement, evaluating model learning performance with DTW.

Table 9.
The result of the adaptability in confronting changes of one degree of freedom of movement.

Table 10.
The result of the adaptability in confronting changes of two degrees of freedom of movement.

Table 11.
The result of the adaptability in confronting changes of three degrees of freedom of movement.
4.2. Toronto Rehabilitation Dataset Experiments
The purpose of the experiments designed for this dataset is to demonstrate the ANM system’s ability to assist stroke patients in progressive rehabilitation. This research divides the experiment into three parts:
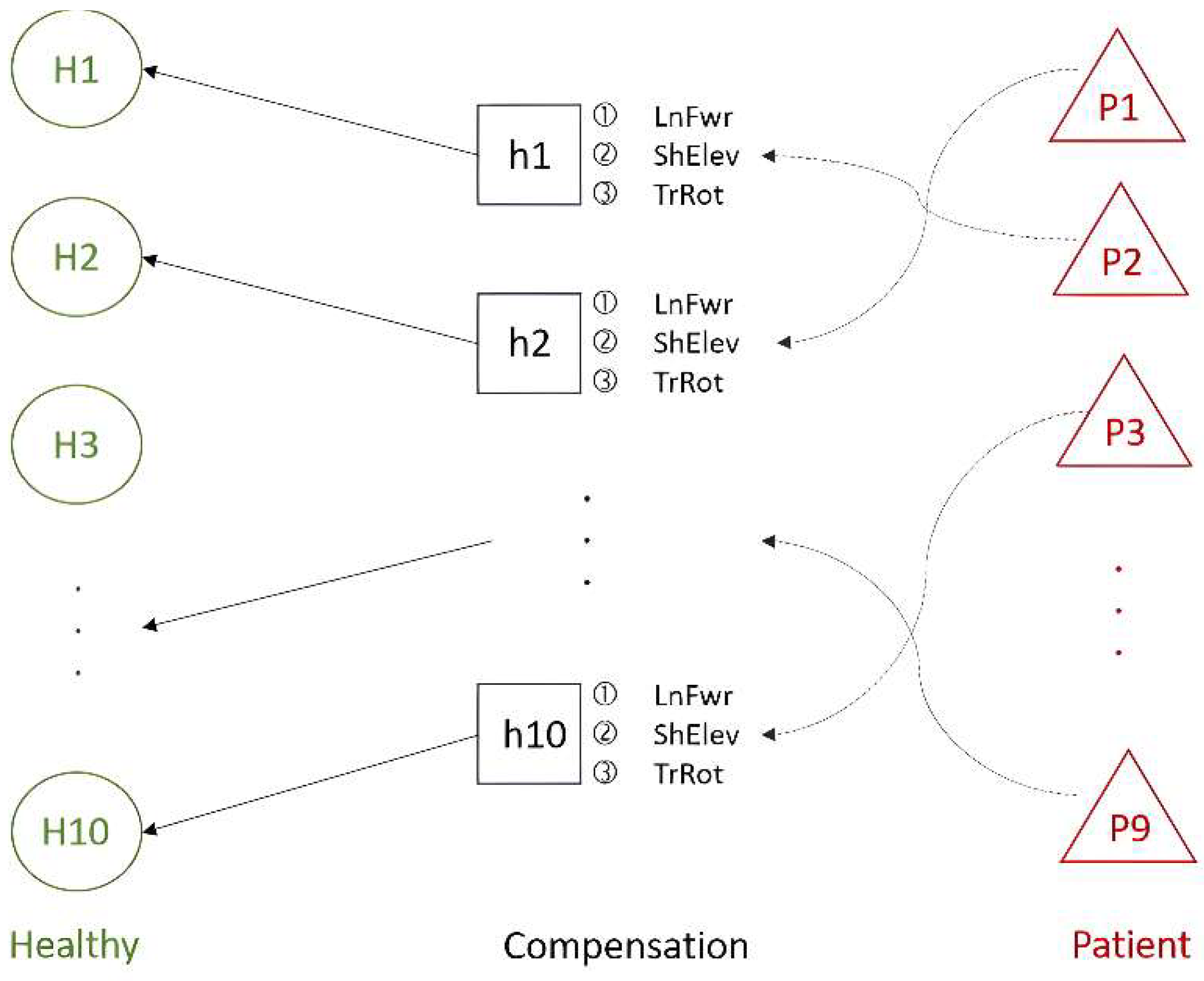
(1) Ten healthy people performed two regular rehabilitation movements (Healthy) types in the dataset. These movements were clustered using hierarchical clustering, and the results were analyzed. Subsequently, compensatory movements (Compensation) performed by these healthy people were correlated with the clusters of regular movements to achieve adaptive learning. In this process, each healthy person’s compensatory movements were matched with their corresponding group, as shown in the Healthy–Compensation relationship in Figure 10.
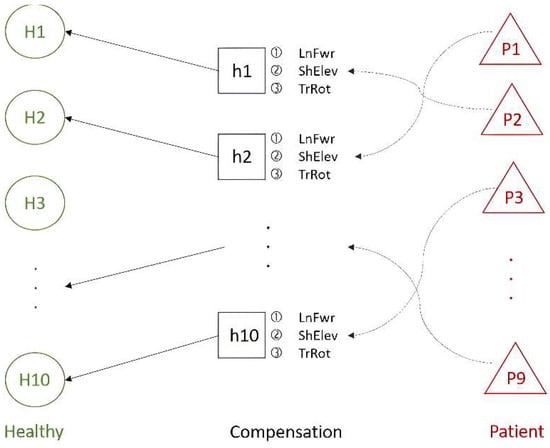
Figure 10.
The relationship between healthy people and patients in the Toronto Rehabilitation dataset.
(2) The similarity between the compensatory movements of the healthy people and those of nine stroke patients (Patients) was analyzed. The compensatory movements of healthy people with higher similarity to the stroke patients’ movements were then paired accordingly, as illustrated in the Compensation–Patient relationship in Figure 10. The results of this analysis serve as the foundation for progressive rehabilitation learning.
(3) The experiments from the previous two phases were combined. The compensatory movements performed by the healthy people acted as a bridge between regular movements and the movements of stroke patients. This connection between healthy individuals and stroke patients, facilitated through compensatory movements, supports subsequent progressive rehabilitation learning. Both upper limbs perform the two types of rehabilitation movements independently. In the following experiments, the compensatory movements simulated by healthy people post-stroke all use the lean-forward (LnFwr) compensation action.
4.2.1. Adaptive Learning in Rehabilitation
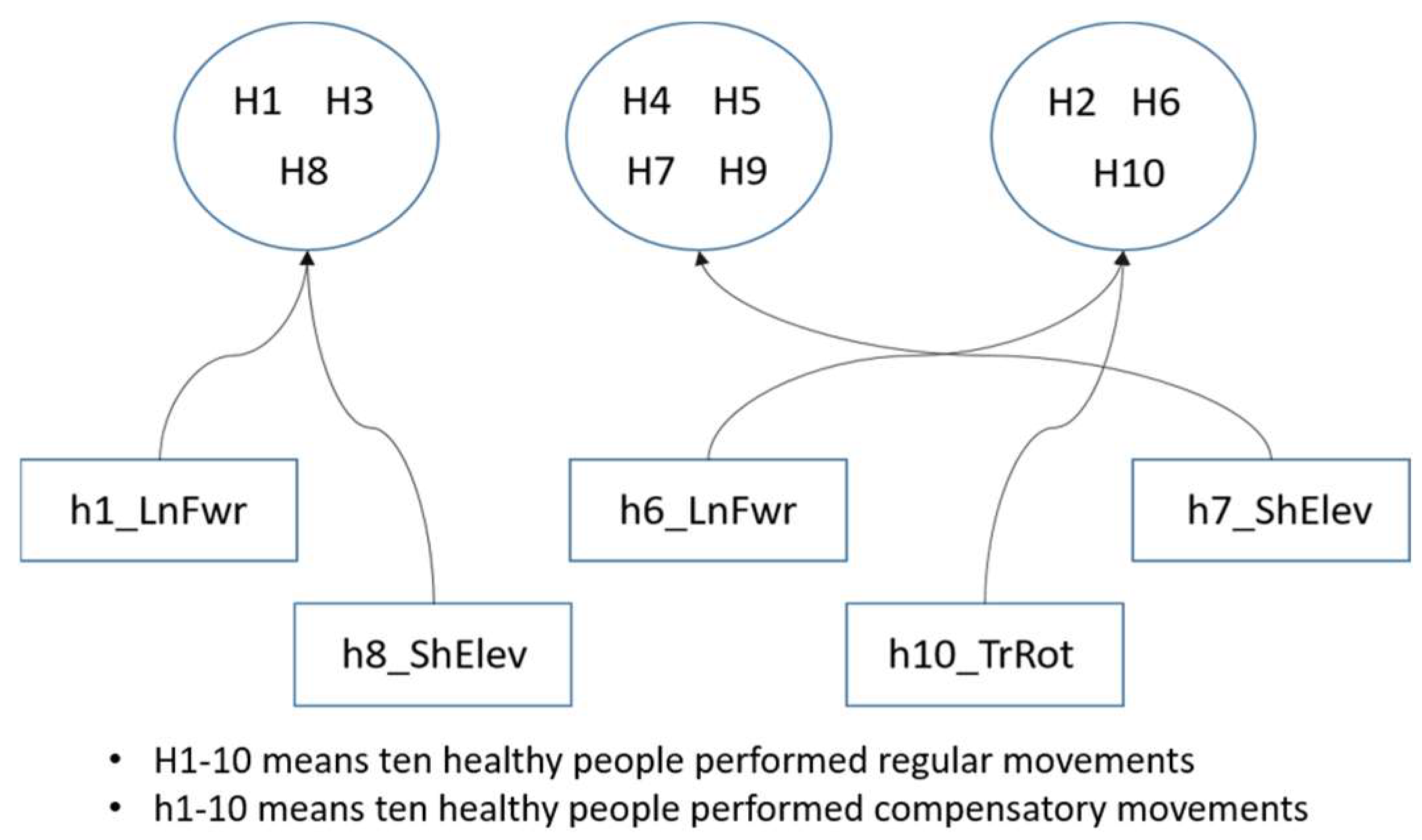
This experiment utilized motion data from two sets within the Toronto Rehabilitation dataset: regular rehabilitation movements and compensatory actions performed by ten healthy people. Initially, the experiment identified trajectory data for the two regular rehabilitation movements repeatedly performed by the ten healthy people. The average of multiple movement trajectories was calculated to establish a central point for each movement, similar to finding centroids in clustering. As the arm trajectories of the healthy people showed minimal variation when performing the same rehabilitation movements, the centroids were computed using an averaging method. Subsequently, the trajectory data for the two rehabilitation movements performed by the ten healthy people were clustered using a hierarchical clustering algorithm. Each person’s compensatory movements were then mapped to the centroids of the regular movements. From the clustering results of the regular movements, one healthy person from each group was selected to represent the cluster. The compensatory movement of this representative person was used as the input for the ANM system, with the regular movement serving as the model’s output (learning target), facilitating the learning of associations between the two movements.
Adaptive rehabilitation learning mainly involves selecting one healthy person from each group to start training from scratch, while the learning tasks of other people in the group use transfer learning with the learning weights of the representative person. This approach saves learning time and improves training efficiency. It also allows for observing the group’s cohesion through improvement rates with and without transfer learning. The concept of adaptive rehabilitation learning clustering is shown in Figure 11, though this is not the actual clustering result.
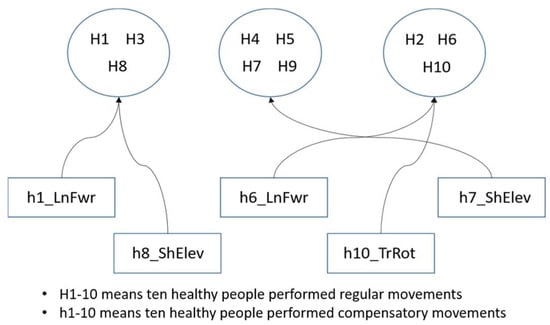
Figure 11.
The concept of clustering in adaptive learning.
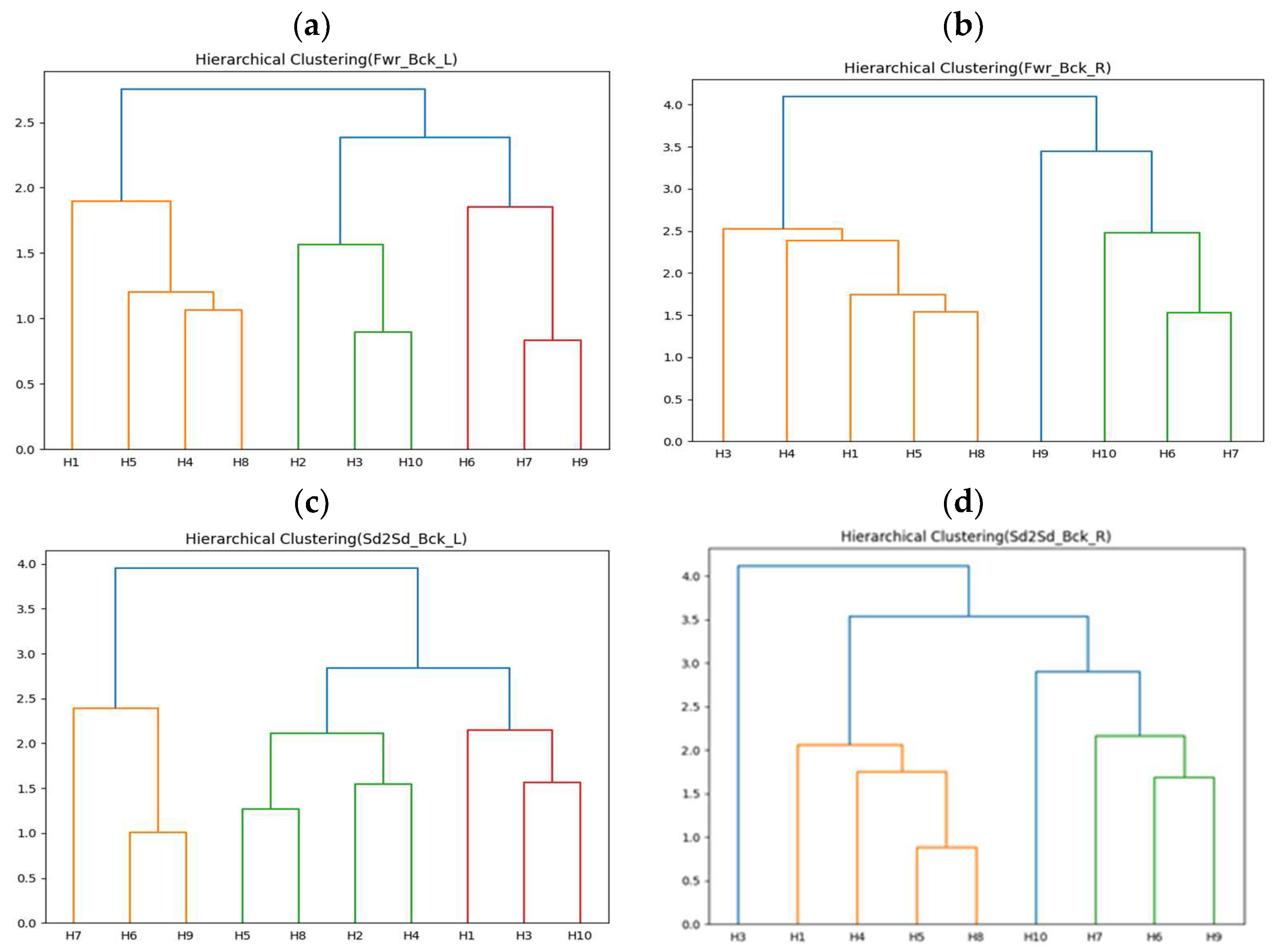
The first-stage clustering results of this experiment are shown in Figure 12a–d, representing forward–backward with left arm (Fwr_Bck_L), forward–backward with right arm (Fwr_Bck_R), side-to-side with left arm (Sd2Sd_Bck_L), and side-to-side with right arm (Sd2Sd_Bck_R), respectively. H2 did not collect correct arm data for the two rehabilitation movements. The clustering results in Figure 12 show that the movements can be roughly divided into three or four groups. The second-stage experimental results are shown in Table 12, Table 13, Table 14, Table 15, Table 16, Table 17, Table 18 and Table 19. All upper limb rehabilitation movements show good learning performance during training, using DTW as the performance evaluation indicator. Among the different upper limb rehabilitation movement clusters, the first group of forward–backward with left arm achieved the best learning efficiency and performance through transfer learning, also showing the highest cohesion.
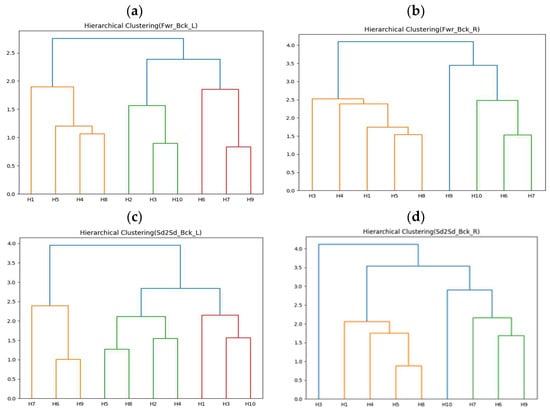
Figure 12.
(a) Clustering results of ten healthy people moving forward–backward with left arm. (b) Clustering results of nine healthy people moving forward–backward with right arm. (c) Clustering results of ten healthy people moving side-to-side with left arm. (d) Clustering results of nine healthy people moving side-to-side with right arm.

Table 12.
The results of forward–backward with left arm under adaptive learning in rehabilitation.

Table 13.
Comparison of improvement rates for forward–backward-with-left-arm learning tasks with and without transfer learning.

Table 14.
The results of forward–backward with right arm under adaptive learning in rehabilitation.

Table 15.
Comparison of improvement rates for forward–backward-with-right-arm learning tasks with and without transfer learning.

Table 16.
The results of side-to-side with left arm under adaptive learning in rehabilitation.

Table 17.
Comparison of improvement rates for side-to-side-with-left-arm learning tasks with and without transfer learning.

Table 18.
The results of side-to-side with right arm under adaptive learning in rehabilitation.

Table 19.
Comparison of improvement rates for side-to-side-with-right-arm learning tasks with and without transfer learning.
4.2.2. Correlation Analysis between Stroke Patients and Compensatory Movements of Healthy People
This experiment first compares the similarity between two rehabilitation movements (forward–backward and side-to-side) performed by nine stroke patients and compensatory rehabilitation movements performed by ten healthy people. The upper limbs perform the two types of rehabilitation movements independently. Each stroke patient’s motions are compared with compensatory movements performed by ten healthy people for similarity, using Euclidean distance to calculate the distance and represent similarity. Ideally, this would result in a 9 × 10 distance matrix. However, due to some stroke patients being unable to perform multiple rehabilitation movements during the test process due to fatigue or personal factors, the actual 9 × 10 distance matrix has missing values. This study clusters the distance matrix using a hierarchical clustering algorithm.
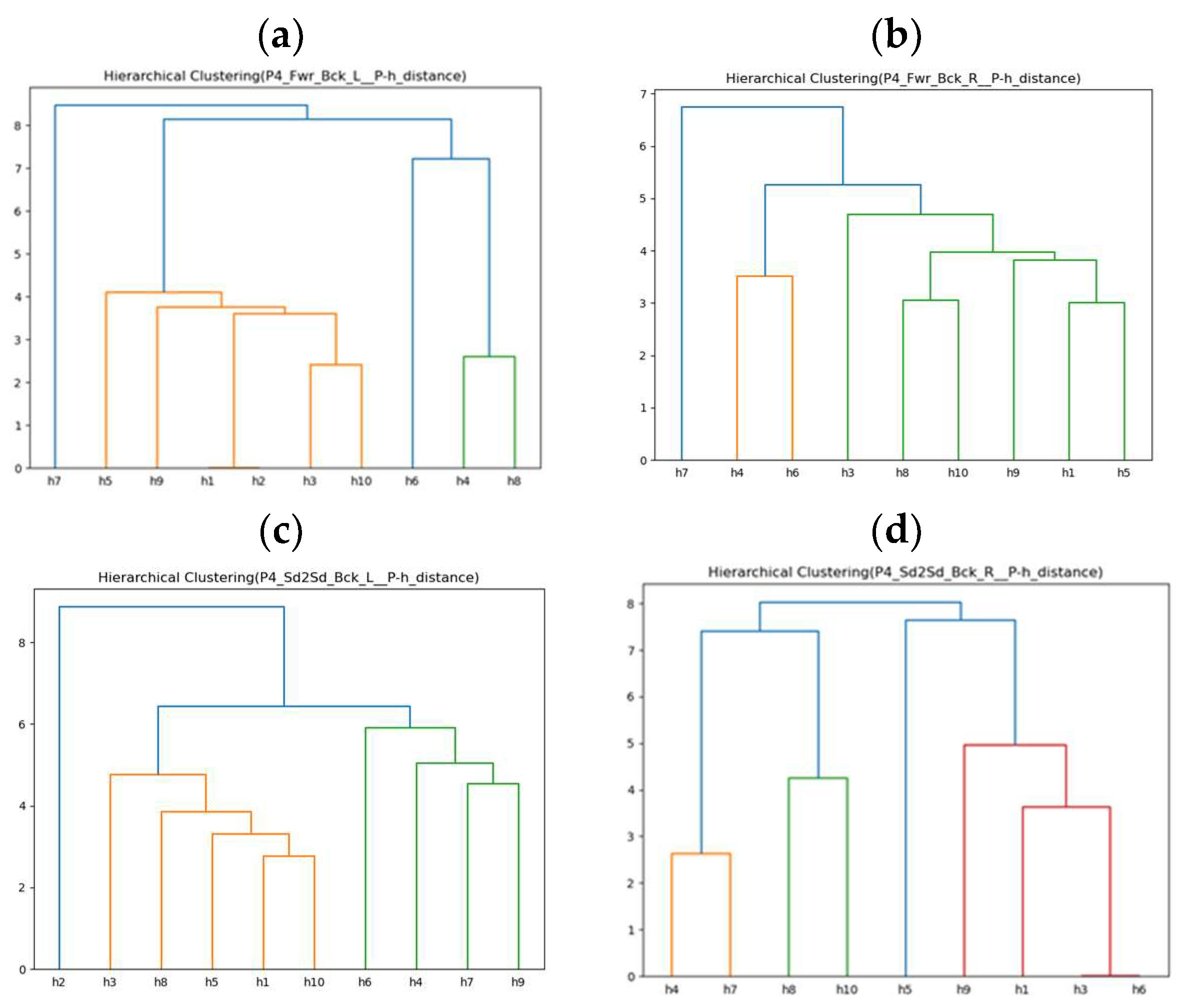
Since the previous adaptive rehabilitation learning has already established a connection between compensatory movements and regular movements performed by healthy people and input them into the ANM system for learning, this experiment only needs to establish the relationship between stroke patients’ movements and compensatory movements of healthy people. This allows us to use the previous ANM system learning results for transfer learning, providing progressive rehabilitation learning. The experimental results are shown in Figure 13a–d, which only present the clustering results of ten healthy people simulating lean-forward compensatory movements corresponding to two rehabilitation movements performed by the fourth stroke patient (P4).
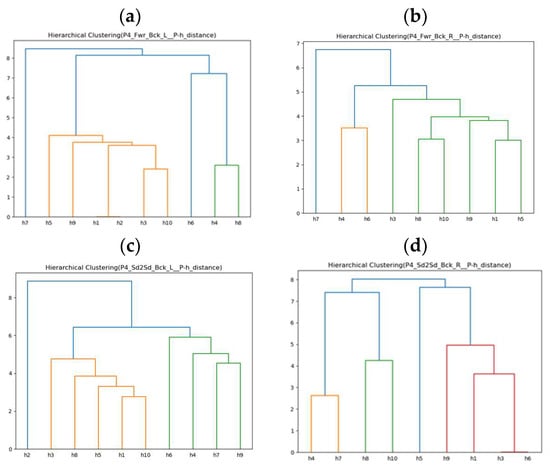
Figure 13.
(a) Similarity clustering of ten healthy people’s compensatory actions (Fwr_Bck_L) for P4. (b) Similarity clustering of nine healthy people’s compensatory actions (Fwr_Bck_R) for P4. (c) Similarity clustering of ten healthy people’s compensatory actions (Sd2Sd_Bck_L) for P4. (d) Similarity clustering of nine healthy people’s compensatory actions (Sd2Sd_Bck_R) for P4. We note that different colors of lines are for better visualization.
Taking the fourth stroke patient (P4) as an example, before proceeding with progressive rehabilitation learning, it is necessary to find the similarity between the two rehabilitation movements performed by P4 and the lean-forward compensatory movements simulated by ten healthy people. This study uses Euclidean distance as a similarity measure. The smaller the difference in movement trajectories, the more similar a movement is to the compensatory movement simulated by the healthy individual. This establishes a connection with the two regular rehabilitation movements healthy people perform. This is achieved using the ANM network’s learning results for subsequent application in transfer learning for progressive rehabilitation. As shown in Table 20, the black bold text indicates the most minor difference (highest similarity). It can be observed that for the fourth stroke patient (P4), in the forward–backward-with-left-arm movement, they are closest to the tenth healthy individual (h10); in the forward–backward-with-right-arm movement, they are closest to the seventh healthy individual (h7); in the side-to-side-with-left-arm movement, they are closest to the fourth healthy individual (h4); and in the side-to-side-with-right-arm movement, they are closest to the third and sixth healthy people (h3, h6).

Table 20.
Comparison of actions performed by P4 and compensation actions performed by ten healthy people.
4.2.3. Progressive Learning in Rehabilitation
This experiment is designed as a continuation based on the first two parts of the experimental design. From the perspective of assisting stroke patient rehabilitation, having stroke patients directly learn healthy people’s movements is a significant challenge. Due to movement coordination disorders in stroke patients, if a patient’s stroke is very severe, directly learning healthy people’s movements might cause secondary injury or poor rehabilitation outcomes. Therefore, it is more appropriate for stroke patients to undergo rehabilitation progressively. This study applies the above ideas in the experiment.
The first part of the experiment—adaptive rehabilitation learning—mainly involves inputting compensatory movements performed by healthy people into the ANM system to learn the relationship with everyday movements. Since adaptive rehabilitation learning allows the ANM system to directly learn healthy people’s movements by simulating stroke patients’ movements, this is permissible in a laboratory environment but may not be suitable for real-world applications. Therefore, this experiment aims to compare the results of progressive rehabilitation learning with adaptive rehabilitation learning to prove that the ANM system can still achieve good results when used for progressive rehabilitation learning. Suppose the feasibility of progressive rehabilitation learning is successfully verified. In that case, it can be combined with the second part of the experiment (which mainly finds the relationship between the movements of nine stroke patients and ten healthy people with compensatory actions) to allow stroke patients to undergo subsequent progressive rehabilitation learning based on the previous ANM system learning results. Table 21 and Figure 14 show the comparison table and schematic diagram of adaptive and progressive rehabilitation learning.

Table 21.
Comparison of adaptive and progressive learning in rehabilitation.
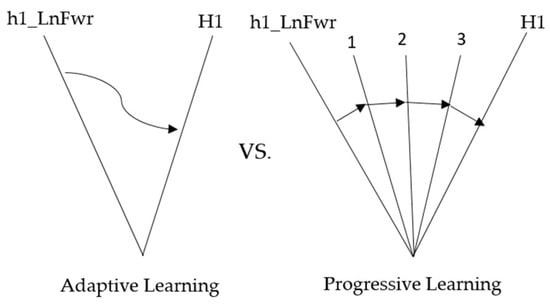
Figure 14.
Comparative diagram of adaptive and progressive learning in rehabilitation.
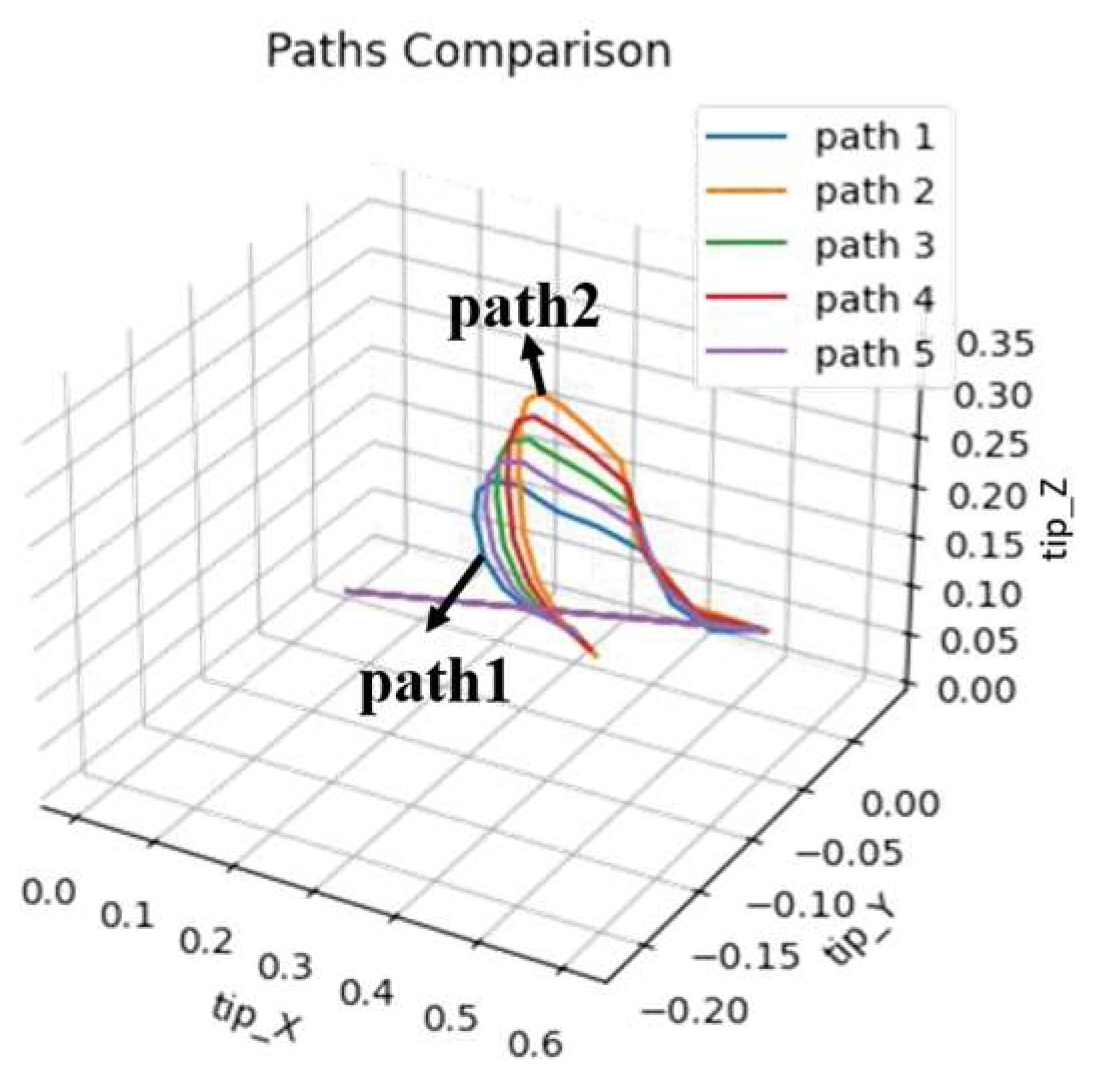
The concept of progressive learning mainly aims to find multiple movement trajectories between healthy people’s compensatory and normal movement trajectories, allowing the system to learn regular joint movements progressively. This concept is shown in Figure 15. Assuming trajectory 1 is the compensatory movement trajectory and trajectory 2 is the typical movement trajectory, the experiment will use linear interpolation to find three progressively changing movement trajectories between the two trajectories. This tests whether the system can help patients accelerate their rehabilitation process through this method. This experiment can also be compared with the time spent on rehabilitation learning in the first part, divided into two comparisons: (1) Compare the performance of rehabilitation learning and progressive learning under a fixed number of training generations. (2) Observe the performance and training time spent on rehabilitation and progressive learning.
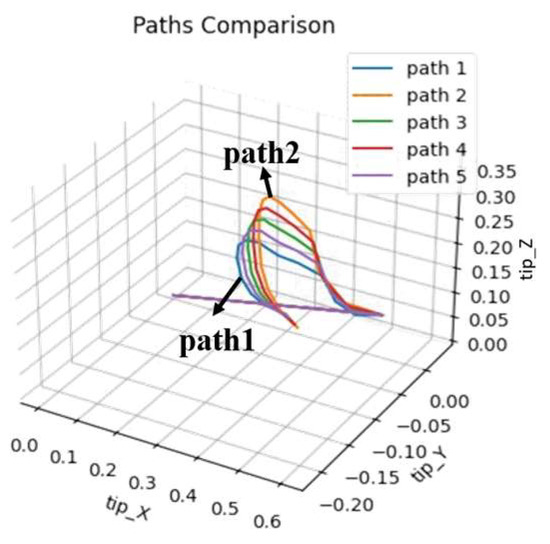
Figure 15.
The concept of progressive learning.
The experimental results are shown in Table 22, Table 23, Table 24 and Table 25, only recording the DTW of the initial and later stages of adaptive and progressive learning in rehabilitation. Since this experiment mainly aims to verify that using the ANM system for progressive learning can still achieve sound learning effects, the experiment only observes the learning performance of left arm movements for two types of rehabilitation movements as representatives. Adaptive and progressive rehabilitation learning of the ANM system start training from the first generation. Taking the learning of the fifth healthy individual (H5) in Table 22 as an example, adaptive rehabilitation learning only has two movement trajectories (h5_Fwr_Bck_LnFwr_L and H5_Fwr_Bck_L_central), which are single-stage learning. Progressive rehabilitation learning is multi-stage learning. Between h5_Fwr_Bck_LnFwr_L and H5_Fwr_Bck_L_central, two intermediate trajectories (path1 and path2) are found through linear interpolation. The result of h5_Fwr_Bck_LnFwr_L learning path1 is saved and provided as the movement trajectory after the first stage of learning, serving as the input for learning path2 in the second stage. Subsequent stages of progressive rehabilitation learning follow this pattern.

Table 22.
Comparison of adaptive and progressive learning performance in rehabilitation under fixed training generations (Fwr_Bck_L).

Table 23.
Comparison of adaptive and progressive learning performance in rehabilitation under fixed training generations (Sd2Sd_Bck_L).

Table 24.
Comparison of adaptive and progressive learning performance in rehabilitation (Fwr_Bck_L).

Table 25.
Comparison of adaptive and progressive learning performance in rehabilitation (Sd2Sd_Bck_L).
The experimental results show that under a fixed number of training generations, the performance of adaptive rehabilitation learning is better than that of progressive rehabilitation learning for both rehabilitation movements, as shown in Table 22 and Table 23. This study estimates that this may be due to multi-stage learning. Since the ANM system has biological characteristics that allow it to adapt to environmental changes, its structure/function also changes with different learning tasks. When conducting progressive rehabilitation learning, if the gene combination of neurons in one stage happens to be poor, it may affect the learning results. However, for adaptive rehabilitation learning, because there is only a single stage of training, it can continuously and stably learn the target task.
The comparison of adaptive and progressive rehabilitation learning performance in Table 24 and Table 25 shows that the ANM system performs better in learning the forward–backward-with-left-arm rehabilitation movement under adaptive rehabilitation learning. Still, the training time required is relatively longer. Under progressive rehabilitation learning, the performance of learning the side-to-side-with-left-arm rehabilitation movement is better, and the time spent is also shorter. Synthesizing the above results, although the performance of the ANM system in progressive learning under fixed training time is not as good as that of rehabilitation learning, progressive learning has advantages when time is sufficient, and it can also demonstrate the ability of biological systems to adapt to environmental changes. Therefore, this study contends that using the ANM system for progressive learning in rehabilitation tasks still has a certain degree of benefit.
5. Discussion
This study aims to establish an autonomous learning platform to help patients perform actions similar to those of healthy people. In other words, the platform must show that it can use autonomous learning to determine how to close these gaps in ancillary ways. The ANM system used in this study conducts an in-depth discussion on the issue of adaptability. Our experimental results show that the ANM system can continuously learn for problem-solving. In addition, our three experimental results of adaptive learning, transfer learning, and cross-task learning further confirm that the ANM system can use previously learned systems to complete the delivered tasks through autonomous learning (instead of learning from scratch). From this perspective, whether the patient data currently used have high accuracy is relatively unimportant. This is because when applied to accurate data in the future, the ANM system is still moving toward solving the problem.
As mentioned above, this research emphasizes the adaptive learning capabilities of the system. This is a significant difference between this study and other state-of-the-art research. In general, the lines of most advanced studies still belong to the Hebbian message processing style, that is, they have stagnant problems in the regional optimal solution. In response to this problem, these state-of-the-art studies may lead to a complete stagnation of learning or must start from the perspective of re-learning. In contrast, the ANM system used in this study can make the learning curve present a smooth improvement method through autonomous learning: continuous improvement (there is no complete stagnation of learning). When we let the system learn long enough, it can move toward solving the problem completely.
However, this study still has certain limitations. For example, this study only considers the movement information of the end of the robot arm. There is no doubt that this research must broaden the pursuit of movement trajectories, such as to the elbow or even the wrist, due to the needs of the problem area. However, from the perspective of assisting the patient’s arm movement, this approach seems to limit how the patient’s arm moves to some extent. In the future, in conjunction with the recommendations of rehabilitation physicians, the system developed in this study can take into account multi-trajectory movements according to the different needs of patients (age, gender, etc.). Another limitation of this study is that it is not yet mature enough to take patient age, gender, and physical factors into consideration. In the future, another research direction is to consider the operation (and even strength) of the fingers of the hand.
6. Conclusions
This study utilized the “Artificial World dataset” and the “Toronto Rehabilitation dataset” to validate the ANM system’s capability for continuous autonomous learning and adaptation and its potential in progressive rehabilitation assistance for stroke patients. Experimental results demonstrated the ANM system’s ability to continuously improve and adapt when facing complex problems, particularly excelling in continuous learning tasks involving arm elevation movements. The system effectively reduced single-degree-of-freedom movements to lower degrees, minimizing the likelihood of simultaneously stimulating multiple muscle groups, thereby enhancing rehabilitation efficiency. Through transfer learning, the ANM system outperformed non-transfer-learning approaches in most learning tasks, although it showed relatively poor performance in tasks involving shrugging, swinging, and side-lifting. In the Toronto Rehabilitation dataset, the study applied hierarchical clustering and transfer learning to establish relationships between regular and compensatory movements of healthy people. It analyzed the relationship between stroke patients and healthy people through compensatory movements. This approach identified the most similar healthy people’s model for each patient, establishing a progressive rehabilitation path. The findings revealed that given sufficient time, using the ANM system for progressive learning in rehabilitation can achieve effective learning outcomes, reduce the risk of secondary injuries, and, in some cases, demonstrate higher efficiency, aligning with the study’s goal of utilizing the ANM system to achieve progressive rehabilitation assistance for stroke patients.
Author Contributions
Conceptualization, J.-C.C. and H.-M.C.; methodology, J.-C.C. and H.-M.C.; software, J.-C.C.; validation, J.-C.C. and H.-M.C.; formal analysis, J.-C.C. and H.-M.C.; investigation, J.-C.C. and H.-M.C.; resources, J.-C.C.; data curation, J.-C.C. and H.-M.C.; writing—original draft preparation, J.-C.C.; writing—review and editing, J.-C.C.; visualization, J.-C.C. and H.-M.C.; supervision, J.-C.C.; project ad-ministration, J.-C.C.; funding acquisition, J.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly funded by the Taiwan Ministry of Science and Technology (Grant 113-2221-E-224-048).
Institutional Review Board Statement
The project has been certified for exemption from further review by the Human Research Ethics Committee at the National Cheng Kung University (Approval No.: NCKU HREC- 113-421, date: 11 June 2024).
Data Availability Statement
The data can be accessed through the following link: https://drive.google.com/drive/folders/1X7f5tptWtoB4q08G_y4WhYfOGEiHC5tM?usp=sharing (accessed on 2 August 2024).
Conflicts of Interest
The author has no conflicts of interest.
References
- Dewald, J.P.; Beer, R.F. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2001, 24, 273–283. [Google Scholar] [CrossRef]
- Ellis, M.D.; Holubar, B.G.; Acosta, A.M.; Beer, R.F.; Dewald, J.P. Modifiability of abnormal isometric elbow and shoulder joint torque coupling after stroke. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2005, 32, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Lum, P.S.; Reinkensmeyer, D.J.; Lehman, S.L. Robotic assist devices for bimanual physical therapy: Preliminary experiments. IEEE Trans. Rehabil. Eng. 1993, 1, 185–191. [Google Scholar] [CrossRef]
- Barbuto, S.; Stein, J. Rehabilitation Robotics for Stroke. In Stroke Rehabilitation; Elsevier: St. Louis, MI, USA, 2019; pp. 235–247. [Google Scholar]
- Gupta, M.; Bhatia, D.; Kumar, P. Advanced robotic rehabilitation. In Modern Intervention Tools for Rehabilitation; Academic Press: Cambridge, MA, USA, 2023; pp. 69–90. [Google Scholar]
- Stein, J.; Narendran, K.; McBean, J.; Krebs, K.; Hughes, R. Electromyography-controlled exoskeletal upper-limb–powered orthosis for exercise training after Stroke. Am. J. Phys. Med. Rehabil. 2007, 86, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Tong, K.; Hu, X.; Li, L. Assistive control system using continuous myoelectric signal in robot-aided arm training for patients after stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 371–379. [Google Scholar] [CrossRef]
- Su, H.; Hou, X.; Zhang, X.; Qi, W.; Cai, S.; Xiong, X.; Guo, J. Pneumatic Soft Robots: Challenges and Benefits. Actuators 2022, 11, 92. [Google Scholar] [CrossRef]
- Zaghloul, A.; Bone, G.M. Origami-Inspired Soft Pneumatic Actuators: Generalization and Design Optimization. Actuators 2023, 12, 72. [Google Scholar] [CrossRef]
- Pehlivan, A.U.; Celik, O.; O’Malley, M.K. Mechanical design of a distal arm exoskeleton for stroke and spinal cord injury rehabilitation. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011. [Google Scholar]
- Simonetti, D.; Tagliamonte, N.L.; Zollo, L.; Accoto, D.; Guglielmelli, E. Biomechatronic design criteria of systems for robot-mediated rehabilitation therapy. In Rehabilitation Robotics; Academic Press: Cambridge, MA, USA, 2018; pp. 29–46. [Google Scholar]
- Huang, V.S.; Krakauer, J.W. Robotic neurorehabilitation: A computational motor learning perspective. J. Neuroeng. Rehabil. 2009, 6, 5. [Google Scholar] [CrossRef]
- Loureiro, R.; Amirabdollahian, F.; Topping, M.; Driessen, B.; Harwin, W. Upper limb robot mediated stroke therapy—GENTLE/s approach. Auton. Robot. 2003, 15, 35–51. [Google Scholar] [CrossRef]
- Nef, T.; Mihelj, M.; Riener, R. ARMin: A robot for patient-cooperative arm therapy. Med. Biol. Eng. Comput. 2007, 45, 887–900. [Google Scholar] [CrossRef]
- Sanchez, R.J.; Wolbrecht, E.; Smith, R.; Liu, J.; Rao, S.; Cramer, S.; Reinkensmeyer, D.J. A pneumatic robot for re-training arm movement after stroke: Rationale and mechanical design. In Proceedings of the 2005 IEEE 9th International Conference on Rehabilitation Robotics, Chicago, IL, USA, 28 June–1 July 2005. [Google Scholar]
- Sugar, T.G.; He, J.; Koeneman, E.J.; Koeneman, J.B.; Herman, R.; Huang, H.; Ward, J.A. Design and control of RUPERT: A device for robotic upper extremity repetitive therapy. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 336–346. [Google Scholar] [CrossRef]
- Fazekas, G.; Horvath, M.; Troznai, T.; Toth, A. Robot-mediated upper limb physiotherapy for patients with spastic hemiparesis: A preliminary study. J. Rehabil. Med. 2007, 39, 580–582. [Google Scholar] [CrossRef]
- Krebs, H.I.; Hogan, N.; Volpe, B.T.; Aisen, M.L.; Edelstein, L.; Diels, C. Overview of clinical trials with MIT-MANUS: A robot-aided neuro-rehabilitation facility. Technol. Health Care 1999, 7, 419–423. [Google Scholar] [CrossRef]
- Cozens, J.A. Robotic assistance of an active upper limb exercise in neurologically impaired patients. IEEE Trans. Rehabil. Eng. 1999, 7, 254–256. [Google Scholar] [CrossRef]
- Hesse, S.; Schulte-Tigges, G.; Konrad, M.; Bardeleben, A.; Werner, C. Robot-assisted arm trainer for the passive and active practice of bilateral forearm and wrist movements in hemiparetic subjects. Arch. Phys. Med. Rehabil. 2003, 84, 915–920. [Google Scholar] [CrossRef]
- Brokaw, E.B.; Black, I.; Holley, R.J.; Lum, P.S. Hand spring operated movement enhancer (HandSOME): A portable, passive hand exoskeleton for stroke rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 391–399. [Google Scholar] [CrossRef]
- Yurkewich, A.; Kozak, I.J.; Ivanovic, A.; Rossos, D.; Wang, R.H.; Hebert, D.; Mihailidis, A. Myoelectric untethered robotic glove enhances hand function and performance on daily living tasks after stroke. J. Rehabil. Assist. Technol. Eng. 2020, 7, 2055668320964050. [Google Scholar] [CrossRef]
- Park, W.; Jeong, W.; Kwon, G.H.; Kim, Y.H.; Kim, L. A rehabilitation device to improve the hand grasp function of stroke patients using a patient-driven approach. In Proceedings of the IEEE 13th International Conference on Rehabilitation Robotics, Seattle, WA, USA, 24–26 June 2013. [Google Scholar]
- Sivan, M.; Gallagher, J.; Makower, S.; Keeling, D.; Bhakta, B.; O’Connor, R.J.; Levesley, M. Home-based computer assisted arm rehabilitation (hCAAR) robotic device for upper limb exercise after stroke: Results of a feasibility study in home setting. J. Neuroeng. Rehabil. 2014, 11, 163. [Google Scholar] [CrossRef]
- Idà, E.; Mattioni, V. Cable-driven parallel robot actuators: State of the art and novel servo-winch concept. Actuators 2022, 11, 290. [Google Scholar] [CrossRef]
- Rad, C.; Hancu, O.; Lapusan, C. Data-driven kinematic model of PneuNets bending actuators for soft grasping tasks. Actuators 2022, 11, 58. [Google Scholar] [CrossRef]
- Pagliarani, N.; Arleo, L.; Albini, S.; Cianchetti, M. Variable stiffness technologies for soft robotics: A comparative approach for the STIFF-FLOP manipulator. Actuators 2023, 12, 96. [Google Scholar] [CrossRef]
- Banyai, A.D.; Brișan, C. Robotics in physical rehabilitation: Systematic Review. Healthcare 2024, 12, 1720. [Google Scholar] [CrossRef]
- Akbari, A.; Haghverd, F.; Behbahani, S. Robotic Home-Based Rehabilitation Systems Design: From a Literature Review to a Conceptual Framework for Community-Based Remote Therapy During COVID-19 Pandemic. Front. Robot. AI 2021, 8, 612331. [Google Scholar] [CrossRef]
- Maciejasz, P.; Eschweiler, J.; Gerlach-Hahn, K.; Jansen-Troy, A.; Leonhardt, S. A survey on robotic devices for upper limb rehabilitation. J. NeuroEngineering Rehabil. 2014, 11, 3. [Google Scholar] [CrossRef]
- Kwakkel, G.; Kollen, B.J.; Krebs, H.I. Effects of Robot-Assisted Therapy on Upper Limb Recovery After Stroke: A Systematic Review. Neurorehabilit. Neural Repair 2008, 22, 111–121. [Google Scholar] [CrossRef]
- Marchal-Crespo, L.; Reinkensmeyer, D.J. Review of control strategies for robotic movement training after neurologic injury. J. Neuroeng. Rehabil. 2009, 6, 20. [Google Scholar] [CrossRef]
- Fu, J.; Rota, A.; Li, S.; Zhao, J.; Liu, Q.; Iovene, E.; Ferrigno, G.; De Momi, E. Recent Advancements in Augmented Reality for Robotic Applications: A Survey. Actuators 2023, 12, 323. [Google Scholar] [CrossRef]
- Chen, J.-C. Using Artificial Neuro-Molecular System in Robotic Arm Motion Control—Taking Simulation of Rehabilitation as an Example. Sensors 2022, 22, 2584. [Google Scholar] [CrossRef]
- Chen, J.-C.; Cheng, H.-M. Applying an Artificial Neuromolecular System to the Application of Robotic Arm Motion Control in Assisting the Rehabilitation of Stroke Patients—An Artificial World Approach. Biomimetics 2023, 8, 385. [Google Scholar] [CrossRef]
- Toronto Rehab Stroke Pose Dataset. Available online: https://www.kaggle.com/datasets/derekdb/toronto-robot-stroke-posture-dataset (accessed on 11 September 2017).
- BioDigital Human Explore the Body in 3D. Available online: https://www.biodigitalhuman.com/ (accessed on 5 November 2013).
- Dolatabadi, E.; Zhi, Y.X.; Ye, B.; Coahran, M.; Lupinacci, G.; Mihailidis, A.; Wang, R.; Taati, B. The Toronto rehab stroke poses dataset to detect compensation during stroke rehabilitation therapy. In Proceedings of the 11th EAI international Conference on Pervasive Computing Technologies for Healthcare, Barcelona, Spain, 23–26 May 2017. [Google Scholar]
- Zhi, Y.X.; Lukasik, M.; Li, M.H.; Dolatabadi, E.; Wang, R.H.; Taati, B. Automatic detection of compensation during robotic stroke rehabilitation therapy. IEEE J. Transl. Eng. Health Med. 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Salvador, S.; Chan, P. Toward accurate dynamic time warping in linear time and space. Intell. Data Anal. 2007, 11, 561–580. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).