Collateral Impact of Community-Directed Treatment with Ivermectin (CDTI) for Onchocerciasis on Parasitological Indicators of Loa loa Infection
Abstract
1. Introduction
2. Results
2.1. Prevalence and Intensity of Loa loa Infection
2.2. Clinical Signs Attributable to Loiasis
2.3. Adherence to Ivermectin Yearly Mass Distributions
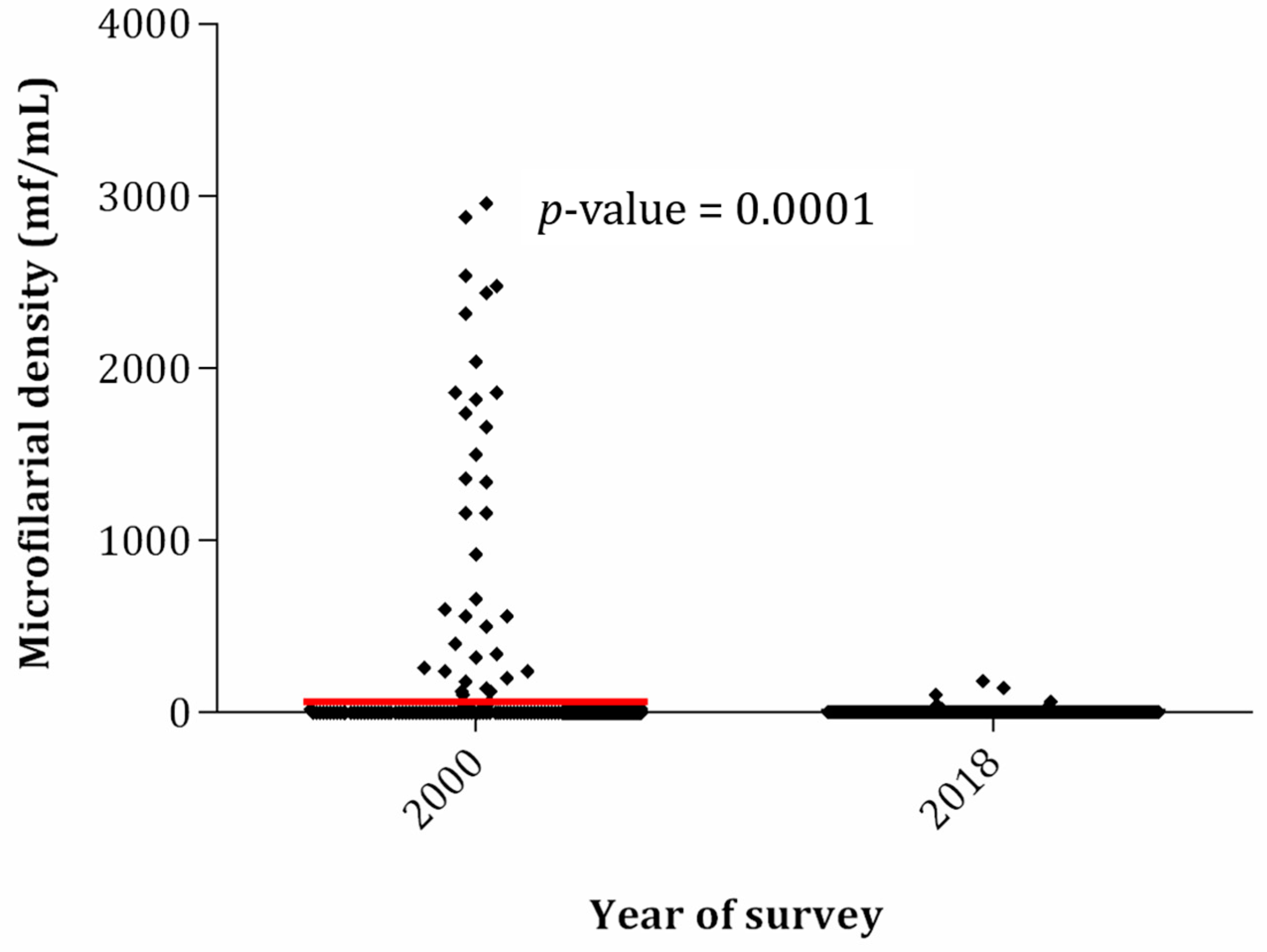
2.4. Eighteen-Year Trends in Prevalence and Intensity of Loa loa Infection
3. Discussion
4. Materials and Methods
4.1. Ethics Approval and Consent to Participate
4.2. Study Area and Population
4.3. Baseline Data and History of CDTI
4.4. Study Design
4.5. Clinical Examination
4.6. Parasitological Examination
4.7. History of Migration and Adherence to Mass Treatment
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALB | albendazole |
| CDTI | community-directed treatment with ivermectin |
| CI | confidence interval |
| IVM | ivermectin |
| mf/mL | microfilariae per milliliter of blood |
| mf | microfilariae |
| PC | preventive chemotherapy |
| SAE | severe adverse event |
| SD | standard deviation |
References
- Zoure, H.; Wanji, S.; Noma, M.; Amazigo, U.; Diggle, P.; Tekle, A.H.; Remme, J.H.F. The Geographic Distribution of Loa loa in Africa: Results of Large-Scale Implementation of the Rapid Assessment Procedure for Loiasis (RAPLOA). PLoS Negl. Trop. Dis. 2011, 5, e1210. [Google Scholar] [CrossRef]
- Metzger, W.G.; Mordmüller, B. Loa loa-does it deserve to be neglected? Lancet Infect. Dis. 2014, 14, 353–357. [Google Scholar] [CrossRef]
- Kamgno, J.; Nana-Djeunga, H.C.; Kouam-Kenmogne, M. Loiasis. In Neglected Tropical Diseases-Sub-Saharan Africa, Neglected Tropical Diseases; Gyapong, J., Boatin, B., Eds.; Springer International Publishing: Geneva, Switzerland, 2016; pp. 135–157. [Google Scholar]
- Chesnais, C.B.; Takougang, I.; Paguele, M.; Pion, S.D.; Boussinesq, M. Excess mortality associated with loiasis: A retrospective population-based cohort study. Lancet Infect. Dis. 2017, 17, 108–116. [Google Scholar] [CrossRef]
- Fischer, P.U. Filarial infection deserves attention as neglected tropical disease. Lancet Infect. Dis. 2017, 17, 12–13. [Google Scholar] [CrossRef]
- Omura, S.; Crump, A. The life and times of ivermectin-a success story. Nat. Rev. Microbiol. 2004, 2, 984–989. [Google Scholar] [CrossRef]
- Boussinesq, M. Ivermectine. Med. Trop. 2005, 65, 69–79. [Google Scholar]
- Geary, T.G. Ivermectin 20 years on: Maturation of a wonder drug. Trends Parasitol. 2005, 21, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Omura, S. Ivermectin: 25 years and still going strong. Int. J. Antimicrob. Agents 2008, 31, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Dadzie, K.Y. Onchocerciasis control: The APOC strategy. Afr. Health 1997, 19, 13–15. [Google Scholar] [PubMed]
- Homeida, M.; Braide, E.; Elhassan, E.; Amazigo, U.V.; Liese, B.; Benton, B.; Noma, M.; Etya’Ale, D.; Dadzie, K.Y.; Kale, O.O.; et al. APOC’s strategy of community-directed treatment with ivermectin (CDTi) and its potential for providing additional health services to the poorest populations. Ann. Trop. Med. Parasitol. 2002, 96 (Suppl. 1), S93–S104. [Google Scholar] [CrossRef] [PubMed]
- Amazigo, U. The African Programme for Onchocerciasis Control (APOC). Ann. Trop. Med. Parasitol. 2008, 102 (Suppl. 1), 19–22. [Google Scholar] [CrossRef] [PubMed]
- Diawara, L.; Traoré, M.O.; Badji, A.; Bissan, Y.; Doumbia, K.; Goita, S.F.; Konaté, L.; Mounkoro, K.; Sarr, M.D.; Seck, A.F.; et al. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: First evidence from studies in Mali and Senegal. PLoS Negl. Trop. Dis. 2009, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Traore, M.O.; Sarr, M.D.; Badji, A.; Bissan, Y.; Diawara, L.; Doumbia, K.; Goita, S.F.; Konate, L.; Mounkoro, K.; Seck, A.F.; et al. Proof-of-principle of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: Final results of a study in Mali and Senegal. PLoS Negl. Trop. Dis. 2012, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Tekle, A.H.; Elhassan, E.; Isiyaku, S.; Amazigo, U.V.; Bush, S.; Noma, M.; Cousens, S.; Abiose, A.; Remme, J.H.F. Impact of long-term treatment of onchocerciasis with ivermectin in Kaduna State, Nigeria: First evidence of the potential for elimination in the operational area of the African Programme for Onchocerciasis Control. Parasites Vectors 2012, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Tekle, A.H.; Zouré, H.G.; Noma, M.; Boussinesq, M.; Coffeng, L.E.; Stolk, W.A.; Remme, J.H.F. Progress towards onchocerciasis elimination in the participating countries of the African Programme for Onchocerciasis Control: Epidemiological evaluation results. Infect. Dis. Poverty 2016, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Zarroug, I.M.A.; ElMubark, W.A.; Aziz, N.; Higazi, T.B.; Elnojomi, N.A.A.; MacKenzie, C.D.; Shumo, Z.A.I.; Hashim, K.; Hassan, H.K.; Katabarwa, M.; et al. The First Confirmed Elimination of an Onchocerciasis Focus in Africa: Abu Hamed, Sudan. Am. J. Trop. Med. Hyg. 2016, 95, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Zouré, H.G.; Noma, M.; Tekle, A.H.; Amazigo, U.V.; Diggle, P.J.; Giorgi, E.; Remme, J.H.F. The geographic distribution of onchocerciasis in the 20 participating countries of the African Programme for Onchocerciasis Control: (2) pre-control endemicity levels and estimated number infected. Parasites Vectors 2014, 7, 326. [Google Scholar] [CrossRef]
- Cano, J.; Basáñez, M.G.; O’Hanlon, S.J.; Tekle, A.H.; Wanji, S.; Zouré, H.G.M.; Rebollo, M.P.; Pullan, R.L. Identifying co-endemic areas for major filarial infections in sub-Saharan Africa: Seeking synergies and preventing severe adverse events during mass drug administration campaigns. Parasites Vectors 2018, 11, 70. [Google Scholar] [CrossRef]
- Gardon, J.; Gardon-Wendel, N.; Demanga-Ngangue; Kamgno, J.; Chippaux, J.P.; Boussinesq, M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet 1997, 350, 18–22. [Google Scholar] [CrossRef]
- Mectizan Expert Committee and Technical Consultative Committee. Recommendations for the treatment of onchocerciasis with Mectizan in areas co-endemic for onchocerciasis and loiasis. 2004. Available online: http://www.who.int/apoc/publications/englishmectccloarecs-june04.pdf (accessed on 4 December 2020).
- de Vlas, S.J. Health Impact Assessment of APOC-Update 2011; Department of Public Health, Erasmus MC, University Medical Center Rotterdam: Rotterdam, The Netherlands, 2011. [Google Scholar]
- Coffeng, L.E.; Stolk, W.A.; Zouré, H.G.M.; Veerman, J.L.; Agblewonu, K.B.; Murdoch, M.E.; Noma, M.; Fobi, G.; Richardus, J.H.; Bundy, D.A.P.; et al. African Programme for Onchocerciasis Control 1995–2015: Updated health impact estimates based on new disability weights. PLoS Negl. Trop. Dis. 2014, 8, e2759. [Google Scholar] [CrossRef]
- Chippaux, J.P.; Bouchite, B.; Boussinesq, M.; Ranque, S.; Baldet, T.; Demanou, M. Impact of repeated large scale ivermectin treatments on the transmission of Loa loa. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 454–458. [Google Scholar] [CrossRef]
- Wanji, S.; Ndongmo, W.P.C.; Fombad, F.F.; Kengne-Ouafo, J.A.; Njouendou, A.J.; Tchounkeu, Y.F.L.; Koudou, B.; Bockarie, M.; Fobi, G.; Roungou, J.B.; et al. Impact of repeated annual community directed treatment with ivermectin on loiasis parasitological indicators in Cameroon: Implications for onchocerciasis and lymphatic filariasis elimination in areas co-endemic with Loa loa in Africa. PLoS Negl. Trop. Dis. 2020, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Kouam, M.K.; Tchatchueng-Mbougua, J.B.; Demanou, M.; Boussinesq, M.; Pion, S.D.; Kamgno, J. Impact of repeated ivermectin treatments against onchocerciasis on the transmission of loiasis: An entomologic evaluation in central Cameroon. Parasites Vectors 2013, 6, 283. [Google Scholar] [CrossRef] [PubMed]
- Kamgno, J.; Gardon, J.; Boussinesq, M. Analysis of the prevention of post-ivermectin Loa loa encephalopathy by administration of initial low dose. Med. Trop. 2000, 60, 275–277. [Google Scholar]
- Pion, S.D.; Tchatchueng-Mbougua, J.B.; Chesnais, C.B.; Kamgno, J.; Gardon, J.; Chippaux, J.-P.; Ranque, S.; Ernould, J.-C.; Garcia, A.; Boussinesq, M. Effect of a Single Standard Dose (150–200 μg/kg) of Ivermectin on Loa loa Microfilaremia: Systematic Review and Meta-analysis. Open Forum Infect. Dis. 2019, 6, ofz019. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Pion, S.D.S.; Fang, H.; Gardon, J.; Kamgno, J.; Basáñez, M.-G.; Boussinesq, M. Macrofilaricidal Efficacy of Repeated Doses of Ivermectin for the Treatment of River Blindness. Clin. Infect. Dis. 2017, 65, 2026–2034. [Google Scholar] [CrossRef]
- Takougang, I.; Meremikwu, M.; Wanji, S.; Yenshu, E.V.; Aripko, B.; Lamlenn, S.B.; Eka, B.L.; Enyong, P.; Meli, J.; Kale, O.; et al. Rapid assessment method for prevalence and intensity of Loa loa infection. Bull. World Health Organ. 2002, 80, 11. [Google Scholar]
- Eveland, L.K.; Yermakov, V.; Kenney, M. Loa loa infection without microfilaraemia. Trans. R. Soc. Trop. Med. Hyg. 1975, 69, 354–355. [Google Scholar] [CrossRef]
- Hovette, P.; Debonne, J.M.; Touze, J.E.; Gaxotte, P.; Imbert, P.; Fourcade, L.; Laroche, R. Efficacy of ivermectin treatment of Loa loa filariasis patients without microfilaraemias. Ann. Trop. Med. Parasitol. 1994, 88, 93–94. [Google Scholar] [CrossRef]
- Kamga, G.R.; Dissak-Delon, F.N.; Nana-Djeunga, H.C.; Biholong, B.D.; Ghogomu, S.; Souopgui, J.; Zouré, H.G.M.; Boussinesq, M.; Kamgno, J.; Robert, A. Still mesoendemic onchocerciasis in two Cameroonian community-directed treatment with ivermectin projects despite more than 15 years of mass treatment. Parasites Vectors 2016, 9, 581. [Google Scholar] [CrossRef]
- Wanji, S.; Tendongfor, N.; Esum, M.E.; Enyong, P. Chrysops silacea biting densities and transmission potential in an endemic area of human loiasis in south-west Cameroon. Trop. Med. Int. Health 2002, 7, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Kamgno, J.; Pion, S.D.; Chesnais, C.B.; Bakalar, M.H.; D’Ambrosio, M.V.; MacKenzie, C.D.; Nana-Djeunga, H.C.; Gounoue-Kamkumo, R.; Njitchouang, G.-R.; Nwane, P.; et al. A Test-and-Not-Treat Strategy for Onchocerciasis in Loa loa-Endemic Areas. N. Engl. J. Med. 2017, 377, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Boussinesq, M.; Fobi, G.; Kuesel, A.C. Alternative treatment strategies to accelerate the elimination of onchocerciasis. Int. Health 2018, 10, 40–48. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Population Denominators 2017–Cameroon; World Health Organ: Yaounde, Cameroon, 2017; p. 98. [Google Scholar]
- Kamgno, J.; Pion, S.D.; Mackenzie, C.D.; Thylefors, B.; Boussinesq, M. Loa loa microfilarial periodicity in ivermectin-treated patients: Comparison between those developing and those free of serious adverse events. Am. J. Trop. Med. Hyg. 2009, 81, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- WHO. Basic Laboratory Methods in Medical Parasitology; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
| Variables | Baseline (Year 2000) | Follow-Up (Year 2018) | % Difference | ||
|---|---|---|---|---|---|
| N Examined | N Infected (%) | N Examined | N Infected (%) | ||
| Genders | |||||
| Female | 282 | 50 (17.7) | 210 | 8 (4.8) | −72.9 |
| Male | 355 | 29 (8.2) | 166 | 6 (2.9) | −64.6 |
| Age Groups | |||||
| 5–20 | 45 | 1 (2.2) | 147 | 2 (1.4) | −36.4 |
| 20–35 | 126 | 15 (11.9) | 48 | 2 (4.2) | −64.7 |
| 35–50 | 153 | 21 (13.7) | 68 | 3 (4.4) | −67.9 |
| 50–90 | 313 | 42 (13.4) | 113 | 7 (6.2) | −53.7 |
| Communities | |||||
| Longtoka Health Area | |||||
| Longtoka | 114 | 13 (11.4) | 59 | 2 (3.4) | −70.1 |
| Ndogpo | 39 | 3 (7.7) | 58 | 5 (8.6) | 11.7 |
| Nkogmalan | 84 | 14 (16.7) | 45 | 2 (4.4) | −73.6 |
| Yabassi Centre Health Area | |||||
| Bodiman | 81 | 6 (7.4) | 73 | 1 (1.4) | −81.0 |
| Ndogbele | 207 | 30 (14.5) | 102 | 4 (3.9) | −73.1 |
| Yabassi | 112 | 13 (11.6) | 39 | 0 (0.0) | −100 |
| Overall | 637 | 79 (12.4) | 376 | 14 (3.7) | −70.2 |
| Variables | Baseline (Year 2000) | Follow Up (Year 2018) | % Reduction | ||
|---|---|---|---|---|---|
| N Examined | Microfilarial Density (SD) | N Examined | Microfilarial Density (SD) | ||
| Genders | |||||
| Female | 282 | 1517.0 (9410.3) | 210 | 1.7 (10.0) | −99.9 |
| Male | 355 | 301 (1930.2) | 166 | 1.9 (15.9) | −99.9 |
| Age Groups | |||||
| 5–20 | 45 | 0.44 (3.0) | 147 | 0.3 (2.3) | −31.8 |
| 20–35 | 126 | 1975.5 (12,737.5) | 48 | 0.4 (2.9) | −99.9 |
| 35–50 | 153 | 610.5 (4169.1) | 68 | 3.8 (20.8) | −99.4 |
| 50–90 | 313 | 614.3 (3249.4) | 113 | 3.2 (18.4) | −99.5 |
| Communities | |||||
| Longtoka Health Area | |||||
| Longtoka | 114 | 754.0 (4223.1) | 59 | 1.4 (8.2) | −99.8 |
| Ndogpo | 39 | 17.9 (91.2) | 58 | 5.2 (22.7) | −70.9 |
| Nkogmalan | 84 | 1091.4 (3963.0) | 45 | 0.9 (4.2) | −99.9 |
| Yabassi Centre Health Area | |||||
| Bodiman | 81 | 2.7 (12.6) | 73 | 0.3 (2.3) | −88.9 |
| Ndogbele | 207 | 1449.2 (10,342.2) | 102 | 2.4 (18.4) | −99.8 |
| Yabassi | 112 | 500.7 (2851.9) | 39 | 0.0 (0.0) | −100.0 |
| Overall | 637 | 839.3 (6447.1) | 376 | 1.8 (13.6) | −99.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nana-Djeunga, H.C.; Lenou-Nanga, C.G.; Donfo-Azafack, C.; Djune-Yemeli, L.; Fossuo-Thotchum, F.; Domche, A.; Litchou-Tchuinang, A.V.; Bopda, J.; Mbickmen-Tchana, S.; Nkoa, T.; et al. Collateral Impact of Community-Directed Treatment with Ivermectin (CDTI) for Onchocerciasis on Parasitological Indicators of Loa loa Infection. Pathogens 2020, 9, 1043. https://doi.org/10.3390/pathogens9121043
Nana-Djeunga HC, Lenou-Nanga CG, Donfo-Azafack C, Djune-Yemeli L, Fossuo-Thotchum F, Domche A, Litchou-Tchuinang AV, Bopda J, Mbickmen-Tchana S, Nkoa T, et al. Collateral Impact of Community-Directed Treatment with Ivermectin (CDTI) for Onchocerciasis on Parasitological Indicators of Loa loa Infection. Pathogens. 2020; 9(12):1043. https://doi.org/10.3390/pathogens9121043
Chicago/Turabian StyleNana-Djeunga, Hugues C., Cédric G. Lenou-Nanga, Cyrille Donfo-Azafack, Linda Djune-Yemeli, Floribert Fossuo-Thotchum, André Domche, Arsel V. Litchou-Tchuinang, Jean Bopda, Stève Mbickmen-Tchana, Thérèse Nkoa, and et al. 2020. "Collateral Impact of Community-Directed Treatment with Ivermectin (CDTI) for Onchocerciasis on Parasitological Indicators of Loa loa Infection" Pathogens 9, no. 12: 1043. https://doi.org/10.3390/pathogens9121043
APA StyleNana-Djeunga, H. C., Lenou-Nanga, C. G., Donfo-Azafack, C., Djune-Yemeli, L., Fossuo-Thotchum, F., Domche, A., Litchou-Tchuinang, A. V., Bopda, J., Mbickmen-Tchana, S., Nkoa, T., Penlap, V., Ntoumi, F., & Kamgno, J. (2020). Collateral Impact of Community-Directed Treatment with Ivermectin (CDTI) for Onchocerciasis on Parasitological Indicators of Loa loa Infection. Pathogens, 9(12), 1043. https://doi.org/10.3390/pathogens9121043





