Occurrence and Role of Selected RNA-Viruses as Potential Causative Agents of Watery Droppings in Pigeons
Abstract
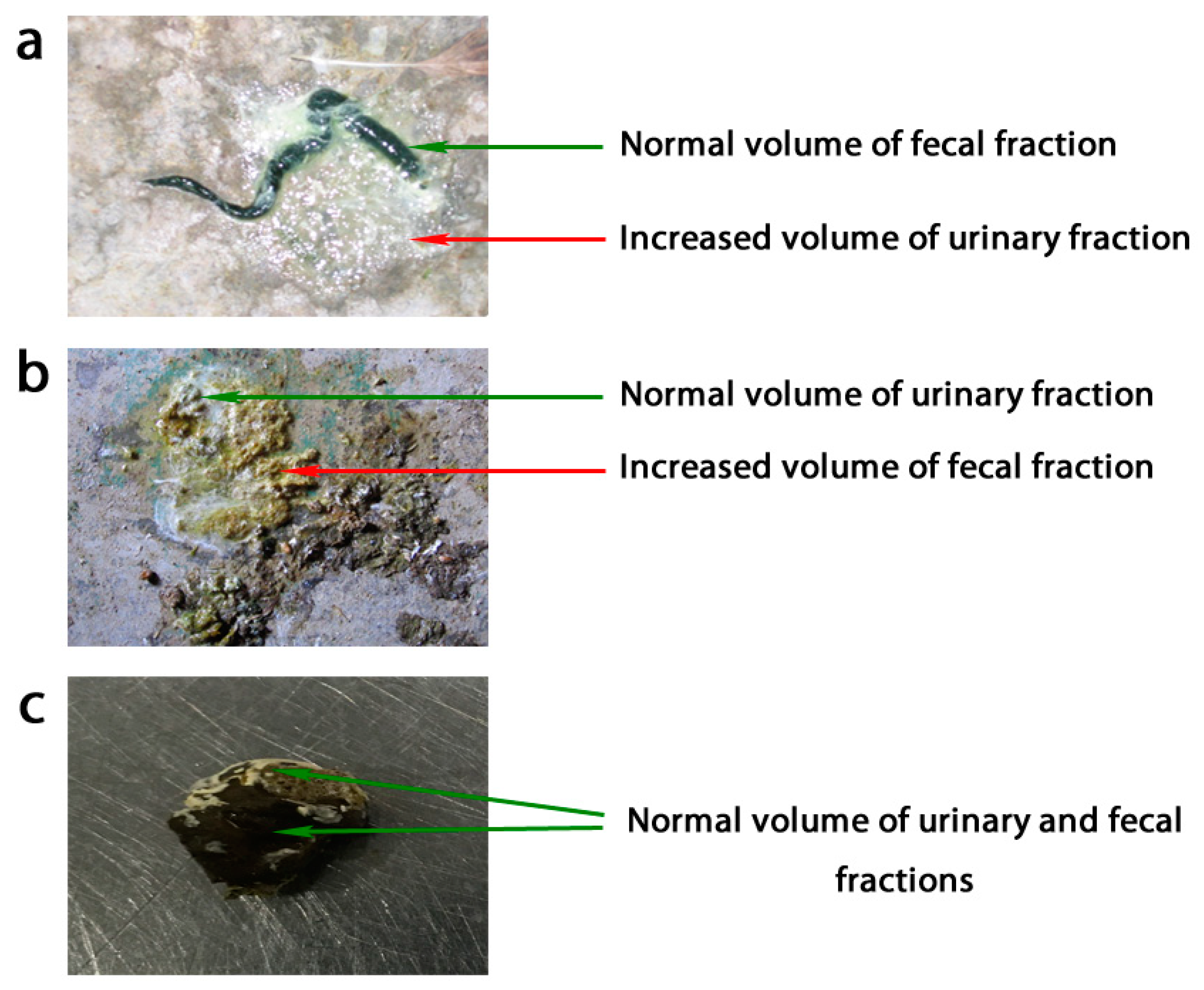
1. Introduction
2. Astroviruses
3. Picornaviruses
4. Coronaviruses
5. Rotaviruses
6. Viscerotropic Avulaviruses
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pigeons. IOC World Bird List Version 10.1. Gill, F., Donsker, D., Rasmussen, P., Eds.; Available online: https://www.worldbirdnames.org/bow/pigeons/ (accessed on 27 May 2020).
- Zhao, W.; Zhu, A.L.; Yuan, C.L.; Yu, Y.; Zhu, C.X.; Lan, D.L.; Yang, Z.B.; Cui, L.; Hua, X.G. Detection of astrovirus infection in pigeons (Columbia livia) during an outbreak of diarrhea. Avian Pathol. 2011, 40, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Felippe, P.A.N.; da Silva, L.H.A.; Santos, M.M.A.B.; Spilki, F.R.; Arns, C.W. Genetic Diversity of Avian Infectious Bronchitis Virus Isolated from Domestic Chicken Flocks and Coronaviruses from Feral Pigeons in Brazil Between 2003 and 2009. Avian Dis. 2010, 54, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.M.; Cavanagh, D. The molecular biology of coronaviruses. Adv. Virus Res. 1997, 48, 1–100. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.; Wong, E.; Tsang, C.C.; Ahmed, S.S.; Au-Yeung, R.; Yuen, K.Y.; Wernery, U.; Woo, P. Discovery and Sequence Analysis of Four Deltacoronaviruses from Birds in the Middle East Reveal Interspecies Jumping with Recombination as a Potential Mechanism for Avian-to-Avian and Avian-to-Mammalian Transmission. J. Virol. 2018, 92, e00265-18. [Google Scholar] [CrossRef]
- Ujvári, D.; Wehmann, E.; Kaleta, E.F.; Werner, O.; Savić, V.; Nagy, E.; Czifraf, G.; Lomniczi, B. Phylogenetic analysis reveals extensive evolution of avian paramyxovirus type 1 strains of pigeons (Columba livia) and suggests multiple species transmission. Virus Res. 2003, 96, 63–73. [Google Scholar] [CrossRef]
- Domańska-Blicharz, K.; Jacukowicz, A.; Bocian, L.; Minta, Z. Astroviruses in Polish Commercial Turkey Farms in 2009–2012. Avian Dis. 2014, 58, 158–164. [Google Scholar] [CrossRef]
- Domańska-Blicharz, K.; Bocian, Ł.; Lisowska, A.; Jacukowicz, A.; Pikuła, A.; Minta, Z. Cross-sectional survey of selected enteric viruses in Polish turkey flocks between 2008 and 2011. BMC Vet. Res. 2017, 13, 108. [Google Scholar] [CrossRef]
- Day, J.M.; Spackman, E.; Pantin-Jackwood, M. A multiplex RT-PCR test for the differential identification of turkey astrovirus type 1, turkey astrovirus type 2, chicken astrovirus, avian nephritis virus, and avian rotavirus. Avian Dis. 2007, 51, 681–684. [Google Scholar] [CrossRef]
- Day, J.M.; Zsak, L. Recent Progress in the Characterization of Avian Enteric Viruses. Avian Dis. 2013, 57, 573–580. [Google Scholar] [CrossRef]
- Moura-Alvarez, J.; Chacon, J.V.; Scanavini, L.S.; Nunez, L.F.N.; Astolfi-Ferreira, C.S.; Jones, R.C.; Piantino Ferreira, A.J. Enteric viruses in Brazilian turkey flocks: Single and multiple virus infection frequency according to age and clinical signs of intestinal disease. Poult. Sci. 2013, 92, 945–955. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Day, J.M.; Jackwood, M.W.; Spackman, E. Enteric viruses detected by molecular methods in commercial chicken and turkey flocks in the United States between 2005 and 2006. Avian Dis. 2008, 52, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Pantin-Jackwood, M.J.; Spackman, E.; Day, J.M.; Rives, D. Periodic monitoring of commercial turkeys for enteric viruses indicates continuous presence of astrovirus and rotavirus on the farms. Avian Dis. 2007, 51, 674–680. [Google Scholar] [CrossRef]
- Erfan, A.M.; Selim, A.A.; Moursi, M.K.; Nasef, S.A.; Abdelwhab, E.M. Epidemiology and molecular characterisation of duck hepatitis A virus from different duck breeds in Egypt. Vet. Microbiol. 2005, 177, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhu, D.; Cheng, A.; Wang, M.; Chen, S.; Jia, R.; Liu, M.; Sun, K.; Zhao, X.; Yang, Q.; et al. Molecular epidemiology of duck hepatitis a virus types 1 and 3 in China, 2010-2015. Transbound. Emerg. Dis. 2018, 65, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.D.; Desai, P.T.; Zhang, Y.; Scharber, S.K.; Baller, J.; Xing, Z.S.; Cardona, C.J. Development of the Intestinal RNA Virus Community of Healthy Broiler Chickens. PLoS ONE 2016, 11, e0150094. [Google Scholar] [CrossRef]
- Lima, D.A.; Cibulski, S.P.; Finkler, F.; Teixeira, T.F.; Varela, A.P.M.; Cerva, C.; Loiko, M.R.; Scheffer, C.M.; Dos Santos, H.F.; Mayer, F.Q.; et al. Faecal virome of healthy chickens reveals a large diversity of the eukaryote viral community, including novel circular ssDNA viruses. J. Gen Virol. 2017, 98, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Pohjola, L.; Tammiranta, N.; Ek-Kommonen, C.; Soveri, T.; Hänninen, M.L.; Fredriksson Ahomaa, M.; Huovilainen, A. A survey for selected avian viral pathogens in backyard chicken farms in Finland. Avian Pathol. 2017, 46, 166–172. [Google Scholar] [CrossRef]
- Andreopoulou, M.; Franzo, G.; Tucciarone, C.M.; Prentza, Z.; Koutoulis, K.C.; Cecchinato, M.; Chaligianni, I. Molecular epidemiology of infectious bronchitis virus and avian metapneumovirus in Greece. Poult Sci. 2019, 98, 5374–5384. [Google Scholar] [CrossRef]
- Bolfa, P.; Callanan, J.J.; Ketzis, J.; Marchi, S.; Cheng, T.; Huynh, H.; Lavinder, T.; Boey, K.; Hamilton, C.; Kelly, P. Infections and pathology of free-roaming backyard chickens on St. Kitts, West Indies. J. Vet. Diagn. Investig. 2019, 31, 343–349. [Google Scholar] [CrossRef]
- Apopo, A.A.; Kariithi, H.M.; Ateya, L.O.; Binepal, Y.S.; Sirya, J.H.; Dulu, T.D.; Welch, C.N.; Hernandez, S.M.; Afonso, C.L. A retrospective study of Newcastle disease in Kenya. Trop. Anim. Health Prod. 2020, 52, 699–710. [Google Scholar] [CrossRef]
- Shabbir, M.Z.; Zohari, S.; Yaqub, T.; Nazir, J.; Shabbir, M.A.; Mukhtar, N.; Shafee, M.; Sajid, M.; Anees, M.; Abbas, M.; et al. Genetic diversity of Newcastle disease virus in Pakistan: A countrywide perspective. Virol. J. 2013, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Kite, V.G.; Boyle, D.B.; Heine, H.G.; Pritchard, I.; Garner, M.G.; East, I.J. A serological and virological survey for evidence of infection with Newcastle disease virus in Australian chicken farms. Aust. Vet. J. 2007, 85, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.J. Newcastle disease in the European Union 2000 to 2009. Avian Pathol. 2011, 40, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Walker, C. The Flying Vet’s Pigeon Health and Management, 1st ed.; Dropping Interpretation; Colin Walker, Knox Veterinary Clinic: Victoria, Australia, 2000; pp. 251–259. ISBN 978-187-667-791-6. [Google Scholar]
- Matsui, S.M.; Greenberg, H.B. Astroviruses. In Fields Virology, 4th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001; Volume 1, pp. 875–893. ISBN 978-078-171-832-5. [Google Scholar]
- ICTV Virus Taxonomy: 2019 Release. Available online: https://talk.ictvonline.org/taxonomy/ (accessed on 24 November 2020).
- Fernández-Correa, I.; Truchado, D.A.; Gomez-Lucia, E.; Doménech, A.; Pérez-Tris, J.; Schmidt-Chanasit, J.; Cadar, D.; Benítez, L. A novel group of avian astroviruses from Neotropical passerine birds broaden the diversity and host range of Astroviridae. Sci. Rep. 2019, 9, 9513. [Google Scholar] [CrossRef] [PubMed]
- Donato, C.; Vijaykrishna, D. The Broad Host Range and Genetic Diversity of Mammalian and Avian Astroviruses. Viruses 2017, 9, 102. [Google Scholar] [CrossRef]
- Xue, J.; Han, T.; Zhao, Y.; Yang, H.; Zhang, G. Complete genome sequence and phylogenetic analysis of novel avastroviruses circulating in China from 2016 to 2018. Virus Res. 2020, 278, 197858. [Google Scholar] [CrossRef]
- Reynolds, D.L.; Saif, Y.M. Astrovirus: A Cause of an Enteric Disease in Turkey Poults. Avian Dis. 1986, 30, 728–735. [Google Scholar] [CrossRef]
- Shirai, J.; Nakamura, K.; Shinohara, K.; Kawamura, H. Pathogenicity and Antigenicity of Avian Nephritis Isolates. Avian Dis. 1991, 35, 49–54. [Google Scholar] [CrossRef]
- Imada, T.; Yamaguchi, S.; Mase, M.; Tsukamoto, K.; Kubo, M.; Morooka, A. Avian nephritis virus (ANV) as a new member of the family Astroviridae and construction of infectious ANV cDNA. J. Virol. 2000, 74, 8487–8493. [Google Scholar] [CrossRef]
- Baxendale, W.; Mebatsion, T. The isolation and characterisation of astroviruses from chickens. Avian Pathol. 2004, 33, 364–370. [Google Scholar] [CrossRef]
- Singh, A.; Mor, S.K.; Jindal, N.; Patnayak, D.; Sobhy, N.M.; Luong, N.T.; Goyal, S.M. Detection and molecular characterization of astroviruses in turkeys. Arch. Virol. 2016, 161, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.I.; Linnemann, E.; Icard, A.H.; Durairaj, V.; Mundt, E.; Sellers, H. Chicken astrovirus as an aetiological agent of runting-stunting syndrome in broiler chickens. J. Gen. Virol. 2018, 99, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Kofstad, T.; Jonassen, C.M. Screening of Feral and Wood Pigeons for Viruses Harbouring a Conserved Mobile Viral Element: Characterization of Novel Astroviruses and Picornaviruses. PLoS ONE 2011, 6, e25964. [Google Scholar] [CrossRef] [PubMed]
- Toplu, N.; Alcigir, G. Avian encephalomyelitis in naturally infected pigeons in Turkey. Avian Pathol. 2004, 33, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, K.V.; Pomeroy, B.S. Coronaviral enteritis of turkeys (bluecomb disease). In Diseases of Poultry, 10th ed.; Calnek, B.W., Barnes, H.J., Beard, C.W., McDougald, L.R., Saif, Y.M., Eds.; IowaState University Press: Ames, IA, USA, 1997; pp. 686–692. ISBN 978-0813804279. [Google Scholar]
- Pohl, R. The histopathogenesis of the nephrosis-nephritis syndrome. Avian Pathol. 1974, 3, 1–13. [Google Scholar] [CrossRef]
- Qian, D.H.; Zhu, G.J.; Wu, L.Z.; Hua, G.X. Isolation and characterization of a coronavirus from pigeons with pancreatitis. Am. J. Vet. Res. 2006, 67, 1575–1579. [Google Scholar] [CrossRef]
- McCowan, C.; Crameri, S.; Kocak, A.; Shan, S.; Fegan, M.; Forshaw, D.; Rubbenstroth, D.; Chen, H.; Holmes, C.; Harper, J.; et al. A novel group A rotavirus associated with acute illness and hepatic necrosis in pigeons (Columba livia), in Australia. PLoS ONE 2018, 13, e0203853. [Google Scholar] [CrossRef]
- Rubbenstroth, D.; Peus, E.; Schramm, E.; Kottmann, D.; Bartels, H.; McCowan, C.; Schulze, C.; Akimkin, V.; Fischer, N.; Wylezich, C.; et al. Identification of a novel clade of group A rotaviruses in fatally diseased domestic pigeons in Europe. Transbound. Emerg. Dis. 2019, 66, 552–561. [Google Scholar] [CrossRef]
- Otto, P.; Liebler-Tenorio, E.M.; Elschner, M.; Reetz, J.; Löhren, U.; Diller, R. Detection of rotaviruses and intestinal lesions in broiler chicks from flocks with runting and stunting syndrome (RSS). Avian Dis. 2006, 50, 411–418. [Google Scholar] [CrossRef]
- Pestka, D.; Stenzel, T.; Koncicki, A. Occurrence, characteristics and control of pigeon paramyxovirus type 1 in pigeons. Pol. J. Vet. Sci. 2014, 17, 379–384. [Google Scholar] [CrossRef]
- Alexander, D.J.; Morris, H.T.; Pollitt, W.J.; Sharpe, C.E.; Eckford, R.L.; Sainsbury, R.M.; Mansley, L.M.; Gough, R.E.; Parsons, G. Newcastle disease outbreaks in domestic fowl and turkeys in Great Britain during 1997. Vet. Rec. 1998, 143, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Smyth, V.J.; Jewhurst, H.L.; Adair, B.M.; Todd, D. Detection of chicken astrovirus by reverse transcriptase-polymerase chain reaction. Avian Pathol. 2009, 38, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Vo, N.P.; Boros, Á.; Pankovics, P.; Reuter, G.; Li, O.T.W.; Wang, C.; Deng, X.; Poon, L.L.M.; Delwart, E. The Viruses of Wild Pigeon Droppings. PLoS ONE 2013, 8, e72787. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, C.M.; Kofstad, T.; Larsen, I.L.; Løvland, A.; Handeland, K.; Follestad, A.; Lillehaug, A. Molecular identification and characterization of novel coronaviruses infecting graylag geese (Anser anser), feral pigeons (Columbia livia) and mallards (Anas platyrhynchos). J. Gen. Virol. 2005, 86, 1597–1607. [Google Scholar] [CrossRef]
- Wise, M.G.; Suarez, D.L.; Seal, B.S.; Pedersen, J.C.; Senne, D.A.; King, D.J.; Kapczynski, D.R.; Spackam, E. Development of a real-time reverse-transcription PCR for detection of Newcastle disease virus RNA in clinical samples. J. Clin. Microbiol. 2004, 42, 329–338. [Google Scholar] [CrossRef]
- Boros, A.; Pankovics, P.; Reuter, G. Avian picornaviruses: Molecular evolution, genome diversity and unusual genome features of a rapidly expanding group of viruses in birds. Infect. Genet. Evol. 2014, 28, 151–166. [Google Scholar] [CrossRef]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Report Consortium. ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef]
- Tannock, G.A.; Shafren, D.R. Avian encephalomyelitis: A review. Avian Pathol. 1994, 23, 603–620. [Google Scholar] [CrossRef]
- Shi, S.; Chen, H.; Chen, Z.; Fu, G.; Wan, C.; Huang, Y.; Lin, S.; Cheng, L.; Fu, Q.; Lin, J.; et al. Complete Genome Sequence of a Duck Hepatitis A Virus 1 Isolated from a Pigeon in China. Genome Announc. 2013, 1, e00451-13. [Google Scholar] [CrossRef]
- Honkavuori, K.S.; Shivaprasad, H.L.; Briese, T.; Street, C.; Hirschberg, D.L.; Hutchison, S.K.; Lipkin, W.I. Novel picornavirus in Turkey poults with hepatitis, California, USA. Emerg. Infect. Dis. 2011, 17, 480–487. [Google Scholar] [CrossRef]
- Farkas, T.; Fey, B.; Hargitt, E., 3rd; Parcells, M.; Ladman, B.; Murgia, M.; Saif, Y. Molecular detection of novel picornaviruses in chickens and turkeys. Virus Genes 2012, 44, 262–272. [Google Scholar] [CrossRef] [PubMed]
- De Groot, R.J.; Baker, S.C.; Baric, R.; Enjuanes, L.; Gorbalenya, A.E.; Holmes, K.V.; Perlman, S.; Poon, L.; Rottier, P.J.M.; Talbot, P.J.; et al. Coronaviridae. In Virus Taxonomy. Ninth Report of the International Committee on Taxonomy of Viruses, 1st ed.; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier Academic Press: London, UK, 2012; pp. 806–828. ISBN 978-012-384-684-6. [Google Scholar]
- Woo, P.C.; Lau, S.K.; Lam, C.S.; Lau, C.C.; Tsang, A.K.; Lau, J.H.; Bai, R.; Teng, J.L.; Tsang, C.C.; Wang, M.; et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [CrossRef] [PubMed]
- Breslin, J.J.; Smith, L.G.; Fuller, F.J.; Guy, J.S. Sequence analysis of the turkey coronavirus nucleocapsid protein gene and 3′ untranslated region identifies the virus as a close relative of infectious bronchitis virus. Virus Res. 1999, 65, 187–193. [Google Scholar] [CrossRef]
- Cavanagh, D.; Mawditt, K.; Welchman, D.; De, B.; Britton, P.; Gough, R.E. Coronaviruses from pheasants (Phasianus colchicus) are genetically closely related to coronaviruses of domestic fowl (infectious bronchitis virus) and turkeys. Avian Pathol. 2002, 31, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Gough, R.E.; Drury, S.E.; Culver, F.; Britton, P.; Cavanagh, D. Isolation of a coronavirus from a green-cheeked Amazon parrot (Amazon viridigenalis Cassin). Avian Pathol. 2006, 35, 122–126. [Google Scholar] [CrossRef]
- Cavanagh, D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007, 38, 281–297. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Lam, C.S.; Lai, K.K.; Huang, Y.; Lee, P.; Luk, G.S.; Dyrting, K.C.; Chan, K.H.; Yuen, K.Y. Comparative analysis of complete genome sequences of three avian coronaviruses reveals a novel group 3c coronavirus. J. Virol. 2009, 83, 908–917. [Google Scholar] [CrossRef]
- Suryaman, G.K.; Soejoedono, R.D.; Setiyono, A.; Poetri, O.N.; Handharyani, E. Isolation and characterization of avian coronavirus from healthy Eclectus parrots (Eclectus roratus) from Indonesia. Vet. World 2019, 12, 1797–1805. [Google Scholar] [CrossRef]
- Uenaka, T.; Kishimoto, I.; Sato, S.; Animas, S.B.; Ito, T.; Otsuki, K.; Cook, J.K.A. Intracloacal infection with avian infectious bronchitis virus. Avian Pathol. 1998, 27, 309–312. [Google Scholar] [CrossRef]
- Otsuki, K.; Nakamura, T.; Kubota, N.; Kawaoka, Y.; Tsubokura, M. Comparison of two strains of avian infectious bronchitis virus for their interferon induction, viral growth and development of virus-neutralizing antibody in experimentally-infected chickens. Vet. Microbiol. 1987, 15, 31–40. [Google Scholar] [CrossRef]
- Barr, D.A.; Reece, R.L.; O’Rourke, D.; Button, C.; Faragher, J.T. Isolation of infectious bronchitis virus from a flock of racing pigeons. Aust. Vet. J. 1988, 65, 228. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.C.; Caserta, L.C.; dos Santos, M.M.A.B.; Barnabé, A.C.S.; Durães-Carvalho, R.; Padilla, M.A.; Simão, R.M.; Rizotto, L.S.; Simas, P.V.M.; Bastos, J.C.S.; et al. Avian coronavirus isolated from a pigeon sample induced clinical disease, tracheal ciliostasis, and a high humoral response in day-old chicks. Avian Pathol. 2018, 47, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Liu, S.; Zhang, X.; Jiang, W.; Wang, K.; Wang, S.; Peng, C.; Hou, G.; Li, J.; Yu, X.; et al. Surveillance and taxonomic analysis of the coronavirus dominant in pigeons in China. Transbound. Emerg. Dis. 2020, 1–10. [Google Scholar] [CrossRef]
- Wille, M.; Holmes, E.C. Wild birds as reservoirs for diverse and abundant gamma- and deltacoronaviruses. FEMS Microbiol. Rev. 2020, 44, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Attoui, H.; Mertens, P.P.C.; Becnel, J.; Belaganahalli, S.; Bergoin, M.; Brussaard, C.P.; Chappell, J.D.; Ciarlet, M.; del Vas, M.; Dermody, T.S.; et al. Reoviridae. In Virus Taxonomy. Ninth Report of the International Committee on Taxonomy of Viruses, 1st ed.; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier Academic Press: London, UK, 2012; pp. 541–637. ISBN 978-012-384-684-6. [Google Scholar]
- Kindler, E.; Trojnar, E.; Heckel, G.; Otto, P.H.; Johne, R. Analysis of rotavirus species diversity and evolution including the newly determined full-length genome sequences of rotavirus F and G. Infect. Genet. Evol. 2013, 14, 58–67. [Google Scholar] [CrossRef]
- Mihalov-Kovács, E.; Gellért, Á.; Marton, S.; Farkas, S.L.; Fehér, E.; Oldal, M.; Jakab, F.; Martella, V.; Bányai, K. Candidate new rotavirus species in sheltered dogs, Hungary. Emerg. Infect. Dis. 2015, 21, 660–663. [Google Scholar] [CrossRef]
- Bányai, K.; Kemenesi, G.; Budinski, I.; Földes, F.; Zana, B.; Marton, S.; Varga-Kugler, R.; Oldal, M.; Kurucz, K.; Jakab, F. Candidate new rotavirus species in Schreiber’s bats, Serbia. Infect. Genet. Evol. 2017, 48, 19–26. [Google Scholar] [CrossRef]
- McNulty, M.S.; Todd, D.; Allan, G.M.; McFerran, J.B.; Greene, J.A. Epidemiology of rotavirus infection in broiler chickens: Recognition of four serogroups. Arch. Virol. 1984, 81, 113–121. [Google Scholar] [CrossRef]
- Takase, K.; Nonaka, F.; Sakaguchi, M.; Yamada, S. Cytopathic avian rotavirus isolated from duck faeces in chicken kidney cell cultures. Avian Pathol. 1986, 15, 719–730. [Google Scholar] [CrossRef]
- Reynolds, D.L.; Theil, K.W.; Saif, Y.M. Demonstration of rotavirus and rotavirus-like virus from the intestinal contents of diarrheic pheasant chicks. Avian Dis. 1987, 31, 376–379. [Google Scholar] [CrossRef]
- Takehara, K.; Kiuchi, H.; Kuwahara, M.; Yanagisawa, F.; Mizukami, M.; Matsuda, H.; Yoshimura, M. Identification and characterization of a plaque forming avian rotavirus isolated from a wild bird in Japan. J. Vet. Med. Sci. 1991, 53, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Otto, P.; Ahmed, M.U.; Hotzel, H.; Machnowska, P.; Reetz, J.; Roth, B.; Trojnar, E.; Johne, R. Detection of avian rotaviruses of groups A, D, F and G in diseased chickens and turkeys from Europe and Bangladesh. Vet. Microbiol. 2012, 156, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Day, J.M. Rotavirus infections. In Diseases of Poultry, 13th ed.; Swayne, D.E., Ed.; Wiley-Blackwell: Ames, IA, USA, 2013; pp. 381–395. ISBN 978-111-871-973-2. [Google Scholar]
- Minamoto, N.; Oki, K.; Tomita, M.; Kinjo, T.; Suzuki, Y. Isolation and characterization of rotavirus from feral pigeon in mammalian cell cultures. Epidemiol. Infect. 1988, 100, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Rohwedder, A.; Schütz, K.I.; Minamoto, N.; Brüssow, H. Sequence Analysis of Pigeon, Turkey, and Chicken Rotavirus VP8* Identifies Rotavirus 993/83, Isolated from Calf Feces, as a Pigeon Rotavirus. Virology 1995, 210, 231–235. [Google Scholar] [CrossRef][Green Version]
- Mori, Y.; Sugiyama, M.; Takayama, M.; Atoji, Y.; Masegi, T.; Minamoto, N. Avian to mammal transmission of an avian rotavirus: Analysis of its pathogenicity in a heterologous mouse model. Virology 2001, 288, 63–70. [Google Scholar] [CrossRef]
- Blakey, J.; Crossley, B.; Rosenberger, J.K.; Rejmanek, D.; Markis, M.; Bickford, A.; Bland, M.; Woods, L.; Shivaprasad, H.L.; Goldsmith, D.; et al. Associated with Clinical Disease and Hepatic Necrosis in California Pigeons (Columba livia domestica). Avian Dis. 2019, 63, 651–658. [Google Scholar] [CrossRef]
- Raue, R.; Schmidt, V.; Freick, M.; Reinhardt, B.; Johne, R.; Kamphausen, L.; Kaleta, E.F.; Müller, H.; Krautwald-Junghanns, M.E. A disease complex associated with pigeon circovirus infection, young pigeon disease syndrome. Avian Pathol. 2005, 34, 418–425. [Google Scholar] [CrossRef]
- Stenzel, T.; Koncicki, A. The epidemiology, molecular characterization and clinical pathology of circovirus infections in pigeons—Current knowledge. Vet. Q. 2017, 37, 166–174. [Google Scholar] [CrossRef]
- Rubbenstroth, D.; Ulrich, R.; Wylezich, C.; Rautenschlein, S.; Beer, M.; Mohr, L. First experimental proof of Rotavirus A (RVA) genotype G18P[17] inducing the clinical presentation of ‘young pigeon disease syndrome’ (YPDS) in domestic pigeons (Columba livia). Transbound. Emerg. Dis. 2020, 67, 1507–1516. [Google Scholar] [CrossRef]
- Chen, F.; Knutson, T.P.; Porter, R.E.; Ciarlet, M.; Mor, S.K.; Marthaler, D.G. Genome characterization of Turkey Rotavirus G strains from the United States identifies potential recombination events with human Rotavirus B strains. J. Gen. Virol. 2017, 98, 2931–2936. [Google Scholar] [CrossRef]
- Lamb, R.; Parks, G. Paramyxoviridae: The viruses and their replication. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Griffin, D.E., Lamb, R.A., Martin, M.A., Roizman, B., Straus, S.E., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 1, pp. 1449–1496. ISBN 978-078-176-060-7. [Google Scholar]
- Liu, Y.-P.; Kuo, S.-T.; Chiou, C.-J.; Terregino, C.; Tsai, H.-J. Novel avian metaavulavirus isolated from birds of the family Columbidae in Taiwan. Vet. Microbiol. 2019, 236, 108377. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, P.; Ganar, K.; Kumar, S. Avian Paramyxovirus: A Brief Review. Transbound. Emerg. Dis. 2015, 64, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Parede, L.; Young, P.L. The Pathogenesis of Velogenic Newcastle Disease Virus Infection of Chickens of Different Ages and Different Levels of Immunity. Avian Dis. 1990, 34, 803–808. [Google Scholar] [CrossRef]
- Alexander, D.J.; Allan, W.H. Newcastle disease virus pathotypes. Avian Pathol. 1974, 3, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Beard, C.W.; Hanson, R.P. Newcastle disease. In Diseases of Poultry, 8th ed.; Hofstad, M.S., Barnes, H.J., Calnek, B.W., Reid, W.M., Yoder, H.W., Eds.; Iowa State University Press: Ames, IA, USA, 1984; pp. 452–470. [Google Scholar]
- Kida, H.; Yanagawa, R. Isolation of a new avian paramyxovirus from a rock pigeon (Columba livia). Zentralbl. Bakteriol. Orig. A. 1979, 245, 421–428. [Google Scholar]
- Alexander, D.J.; Hinshaw, V.S.; Collins, M.S. Characterization of viruses from doves representing a new serotype of avian paramyxoviruses. Arch. Virol. 1981, 68, 265–269. [Google Scholar] [CrossRef]
- Gough, R.E.; Alexander, D.J. Isolation and preliminary characterisation of a paramyxovirus from collared doves (Streptopelia decaocto). Avian Pathol. 1983, 12, 125–134. [Google Scholar] [CrossRef]
- Kaleta, E.F.; Marschall, H.J. Newcastle disease in a zoo affecting demoiselle cranes (Anthropoides virgo), greater flamingos (Phoenicopterus ruber) and a pied imperial pigeon (Ducula bicolor). Avian Pathol. 1981, 10, 395–401. [Google Scholar] [CrossRef]
- Erickson, G.A.; Brugh, M.; Beard, C.W. Viscerotropic velogenic Newcastle disease in pigeons: Clinical disease and immunization. Avian Dis. 1980, 24, 257–267. [Google Scholar] [CrossRef]
- Ellakany, H.F.; Elbestawy, A.R.; Abd El-Hamid, H.S.; Zedan, R.E.; Gado, A.R.; Taha, A.E.; Soliman, M.A.; Abd El-Hack, M.E.; Swelum, A.A.; Saadeldin, I.M.; et al. Role of Pigeons in the Transmission of Avian Avulavirus (Newcastle Disease-Genotype VIId) to Chickens. Animals 2019, 9, 338. [Google Scholar] [CrossRef]
- Kaleta, E.F.; Alexander, D.J.; Russell, P.H. The first isolation of the avian PMV-1 virus responsible for the current panzootic in pigeons? Avian Pathol. 1985, 14, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.J.; Wilson, G.W.; Thain, J.A.; Lister, S.A. Avian Paramyxovirus type 1 infection of racing pigeons: 3. Epizootiological considerations. Vet. Rec. 1984, 115, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Marlier, D.; Vindevogel, H. Viral infections in pigeons. Vet. J. 2006, 172, 40–51. [Google Scholar] [CrossRef]
- Olszewska-Tomczyk, M.; Dolka, I.; Świętoń, E.; Śmietanka, K. Genetic Changes in Pigeon Paramyxovirus Type-1 Induced by Serial Passages in Chickens and Microscopic Lesions Caused by the Virus in Various Avian Hosts. J. Vet. Res. 2018, 62, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.J.; Wilson, G.W.; Russell, P.H.; Lister, S.A.; Parsons, G. Newcastle disease outbreaks in fowl in Great Britain during 1984. Vet. Rec. 1985, 117, 429–434. [Google Scholar] [CrossRef]
- Kommers, G.D.; King, D.J.; Seal, B.S.; Brown, C.C. Virulence of Pigeon-Origin Newcastle Disease Virus Isolates for Domestic Chickens. Avian Dis. 2001, 45, 906–921. [Google Scholar] [CrossRef]
- Dortmans, J.C.F.M.; Rottier, P.J.M.; Koch, G.; Peeters, B.P.H. Passaging of a Newcastle disease virus pigeon variant in chickens results in selection of viruses with mutations in the polymerase complex enhancing virus replication and virulence. J. Gen. Virol. 2011, 92, 336–345. [Google Scholar] [CrossRef]
- Dortmans, J.C.F.M.; Koch, G.; Rottier, P.J.M.; Peeters, B.P.H. A comparative infection study of pigeon and avian paramyxovirus type 1 viruses in pigeons: Evaluation of clinical signs, virus shedding and seroconversion. Avian Pathol. 2011, 40, 125–130. [Google Scholar] [CrossRef]
- Woolcock, P.R.; Moore, J.D.; McFarland, M.D.; Panigrahy, B. Isolation of Paramyxovirus Serotype 7 from Ostriches (Struthio camelus). Avian Dis. 1996, 40, 945–949. [Google Scholar] [CrossRef]
- Saif, Y.M.; Mohan, R.; Ward, L.; Senne, D.A.; Panigrahy, B.; Dearth, R.N. Natural and Experimental Infection of Turkeys with Avian Paramyxovirus-7. Avian Dis. 1997, 41, 326–329. [Google Scholar] [CrossRef]
- Warke, A.; Appleby, L.; Mundt, E. Prevalence of Antibodies to Different Avian Paramyxoviruses in Commercial Poultry in the United States. Avian Dis. 2008, 52, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Day, J.M.; Ballard, L.L.; Duke, M.V.; Scheffler, B.E.; Zsak, L. Metagenomic analysis of the turkey gut RNA virus community. Virol. J. 2010, 7, 313. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, T.; Dziewulska, D.; Tykałowski, B.; Koncicki, A. The clinical infection with pigeon circovirus (PiCV) leads to lymphocyte B apoptosis but has no effect on lymphocyte T subpopulation. Pathogens 2020, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- François, S.; Pybus, O.G. Towards an understanding of the avian virome. J. Gen. Virol. 2020, 101, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Mokili, J.L.; Rohwer, F.; Dutilh, B.E. Metagenomics and future perspectives in virus discovery. Cur. Opin. Virol. 2012, 2, 63–77. [Google Scholar] [CrossRef]
| Infection | Diarrhea/Polyuria Confirmed | Reference |
|---|---|---|
| Avian nephritis virus (ANV) | Yes, >2-week-old pigeons Yes, 3- to 7-day-old chickens | Zhao et al., 2011 [2] Shirai et al., 1991 [32] |
| Chicken astrovirus (CAstV) | Yes, >2-week-old pigeons Yes, 3-week-old chickens | Zhao et al., 2011 [2] Baxendale and Mebatsion, 2004 [34] |
| Turkey astrovirus (TAstV) | Yes, 1- to 4-week-old turkeys | Reynolds and Saif, 1986 [31] |
| Pigeon picornavirus (PiPV) | No | Kofstad and Jonassen, 2011 [37] |
| Avian encephalitis virus (AEV) | Yes, adult pigeons | Toplu and Alcigir, 2004 [38] |
| Turkey coronavirus (TCoV) | Yes, turkeys of all ages | Nagaraja and Pomeroy, 1997 [39] |
| Infectious bronchitis virus (IBV) | Yes, 4-week-old chickens | Pohl, 1974 [40] |
| Pigeon coronavirus strain PSH050513 | No | Qian et al., 2006 [41] |
| Rotavirus A (RVA) | Yes, domestic pigeons of all ages, but mainly young birds Yes, 5- to 14-day-old chickens | McCowan et al., 2018 [42] Rubbenstroth et al., 2019 [43] Otto et al., 2006 [44] |
| Pigeon paramyxovirus type 1 (PPMV-1) | Yes, in pigeons | Pestka et al., 2014 [45] |
| Avian avulavirus 1 (AAvV-1) | Yes, 3- and 6-week-old chickens Yes, 13- and 19-week-old turkeys | Alexander et al., 1998 [46] |
| Infection | Sample Type | Method | Amplified Gene | Reference |
|---|---|---|---|---|
| Avian nephritis virus (ANV) | Fecal sample | Reverse transcription polymerase chain reaction (RT-PCR) | ORF2 | Zhao et al., 2011 [2] |
| Rotavirus A (RVA) | Fecal sample, cloacal swab, tissue sample (liver, spleen, intestine, cloacal bursa, lung, kidney, heart, thymus, brain, vagal nerve) | RT-PCR | VP6, NSP4 | Rubbenstroth et al., 2019 [43] |
| Pigeon mesivirus 1 and 2 (MeV-B1, MeV-B2) | Fecal sample, cloacal swab | RT-PCR | RNA-dependent RNA polymerase | Phan et al., 2013 [48] |
| Pigeon coronavirus (PCoV) | Cloacal swab, tracheal swab | RT-PCR | Replicase gene | Jonassen et al., 2005 [49] |
| Avian avulavirus 1 (AAvV-1) | Tracheal, oropharyngeal or cloacal swabs | Reverse transcription quantitative polymerase chain reaction with TaqMan probes (RT-TaqMan qPCR) | Matrix protein (M) gene + fusion protein (F) gene | Wise et al. 2004 [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łukaszuk, E.; Stenzel, T. Occurrence and Role of Selected RNA-Viruses as Potential Causative Agents of Watery Droppings in Pigeons. Pathogens 2020, 9, 1025. https://doi.org/10.3390/pathogens9121025
Łukaszuk E, Stenzel T. Occurrence and Role of Selected RNA-Viruses as Potential Causative Agents of Watery Droppings in Pigeons. Pathogens. 2020; 9(12):1025. https://doi.org/10.3390/pathogens9121025
Chicago/Turabian StyleŁukaszuk, Ewa, and Tomasz Stenzel. 2020. "Occurrence and Role of Selected RNA-Viruses as Potential Causative Agents of Watery Droppings in Pigeons" Pathogens 9, no. 12: 1025. https://doi.org/10.3390/pathogens9121025
APA StyleŁukaszuk, E., & Stenzel, T. (2020). Occurrence and Role of Selected RNA-Viruses as Potential Causative Agents of Watery Droppings in Pigeons. Pathogens, 9(12), 1025. https://doi.org/10.3390/pathogens9121025





