The Functional Parasitic Worm Secretome: Mapping the Place of Onchocerca volvulus Excretory Secretory Products
Abstract
Highlights
- ➢
- As they are parasites, the biology of filariae involves dialogs with their hosts in which ESPs are main actors.
- ➢
- The immune microenvironment is dynamic across different tissues, and thus, activation of the immune response by nematode ESPs may also be stage- and niche-specific.
- ➢
- Understanding the evolution of the nematode secretome could improve our understanding of parasitic evolution in nematodes.
- ➢
- Addressing the function of nematode ESPs remains crucial for a proper understanding of host–parasite interactions.
1. Introduction
1.1. The Parasitic Way of Life
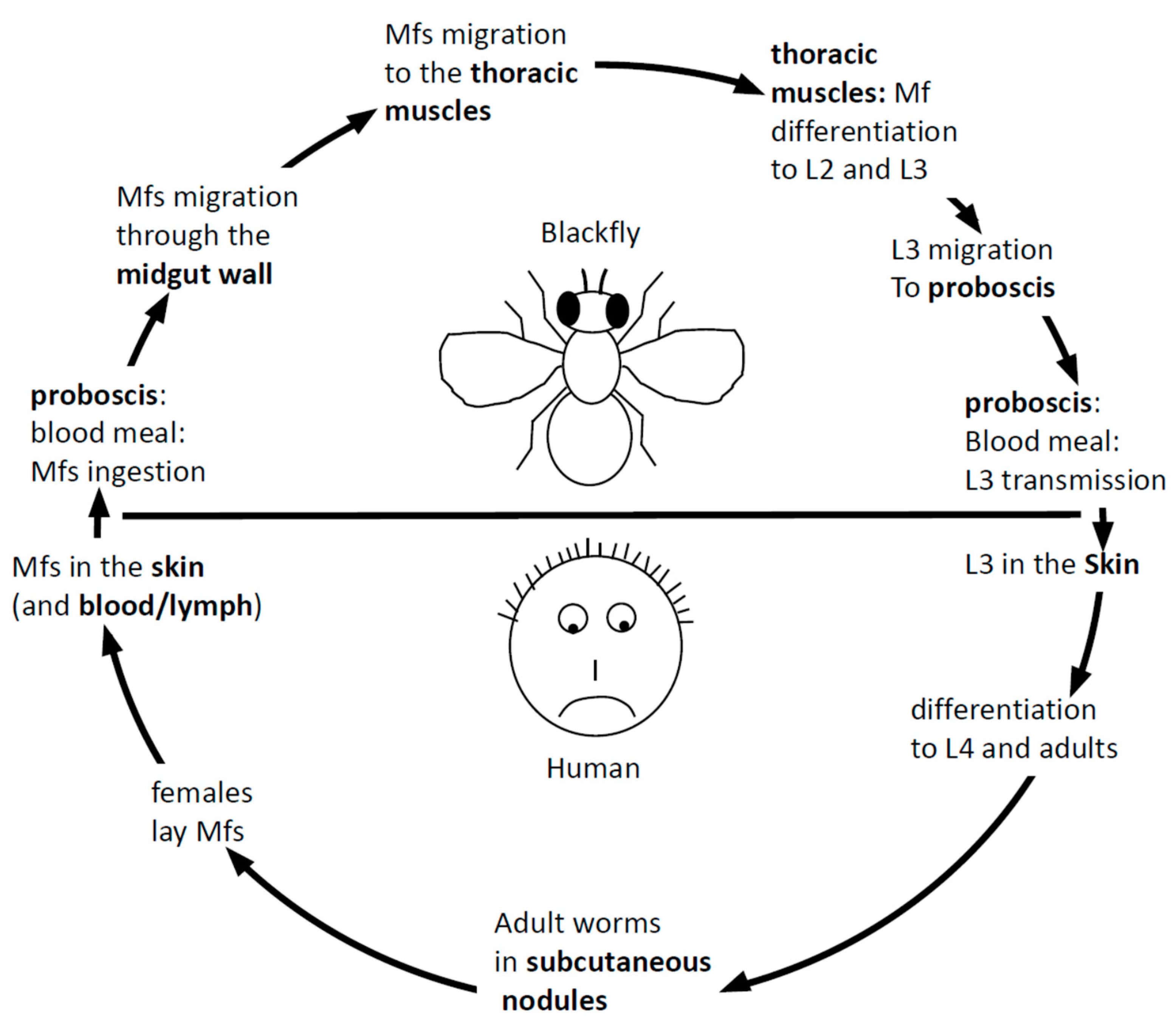
1.2. Onchocerciasis
| Published Antigens | Proposed Application | Function Domain | Protein Category | Publication |
|---|---|---|---|---|
| Ov-RAL-2/Ov-17 | Protective | Domain of Unknown Function | Predicted ESP | [28] |
| Ov-7, Ov-CPI-1, Ov-CPI-2, OC9.3, Onchocystatin | Protective | Cystatin | ESP | [29,30] |
| Ov-1CF | Potentially Diagnostic | Intermediate filament | ESP | [31,32] |
| OvSOD1 | Potentially Protective | Superoxide dismutase 1 | Cytosolic | [33,34] |
| Ov-20, Ov-FAR-1, OvMBP/11 | Chemotherapeutic | Fatty acid and retinol binding | ESP | [35] |
| Ov103, OvMSA-1 | Protective | Uncharacterized conserved protein | ESP | [36,37] |
| Ov-9M/Ov-CAL-1 | Protective | Calponin-like protein | Cytosolic | [38,39] |
| Ov-FBA-1 | Protective | Aldolase | Intracellular | [40] |
| Ov-ENO | Potentially protective | Enolase | Intracellular | [41] |
| Ov-16 | Diagnostic | Phosphatidylethanolamine binding protein | ESP | [23] |
| Ov-33, Ov-API-1, OC3.6 | Diagnostic | Aspartic Protease Inhibitor | ESP | [42] |
| OvB20 | Potentially protective | No functional annotation | Intracellular | [43] |
| MOv-14, OvTrop, Ov-TMY-1 | Protective | Tropomyosin | Intracellular | [44] |
| Ov-GST-1 | Potentially chemotherapeutic; Potentially protective | Glutathione S-transferase | ESP | [45] |
| M3, M4 | Potentially Chemotherapeutic | n-acetylcholine receptor subunit | Transmembrane | [46] |
| OvRAL-1 | Potentially protective | Calreticulin | ESP | [47] |
| Ov-ALT-1 | Potentially protective | Secreted Larval acid proteins | ESP | [48] |
| Ov-ASP-1 | Protective | Activation-associated secreted protein 1 | ESP | [49,50] |
| Ov-CHI-1, Ov-CHI-2 | Protective | Chitinase | ESP | [51,52] |
| Ov-B8 | Protective | Remodeling and spacing factor 1 | Intracellular | [39] |
| OvMSP-2 | Diagnostic | Major Sperm Protein 2 | Intracellular | [53] |
| Ov58GPCR | Potentially Diagnostic | GPCR * (Intimal thickness-related receptor) | Transmembrane | [54] |
| Ov-MIF-1, Ov-MIF-2 | Immunomodulatory | Macrophage Migration Inhibitory Factor | ESP | [55] |
| OvGM2AP | Diagnostic/Potentially Immunomodulatory | GM2 activator protein | ESP | [56] |
| OvMANE-1 | Diagnostic | Chimeric antigen | Not defined | [57] |
| Cyclophilin | Therapeutic | Peptidyl-prolyl cis-trans isomerases | Intracellular | [58] |
2. Excretory Secretory Products (ESPs)
2.1. Immunomodulation: Helminths Excretory Secretory Products and Interaction with the Immune System
2.2. Molecular Pathways Involved in ESP Actions
2.3. ESPs and Parasitic Evolution
2.4. ESPs in Neurologic Diseases
2.5. ESPs and Wound Healing
2.6. ESPs and Worm Cuticle
2.7. Hijacking ESPs for Research and a Cure
2.8. Available Tools for ESP Analysis
2.8.1. Genome Sequencing, Transcriptomics and Proteomics
2.8.2. Animal Models
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lafferty, K.D. Parasites. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780444641304. [Google Scholar]
- King, C.H. Health metrics for helminth infections. Acta Trop. 2015, 141, 150–160. [Google Scholar] [CrossRef] [PubMed]
- WHO. Progress Report 2000–2009 and Strategic Plan 2010–2020 of the Global Programme to Eliminate Lymphatic Filariasis: Halfway Towards Eliminating Lymphatic Filariasis; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Kiontke, K.; Fitch, D.H.A. Nematodes. Curr. Biol. 2013, 23, R862–R864. [Google Scholar] [PubMed]
- Blaxter, M.; Koutsovoulos, G. The evolution of parasitism in Nematoda. Parasitology 2015, 142, S26–S39. [Google Scholar] [CrossRef] [PubMed]
- Viney, M. The genomic basis of nematode parasitism. Brief. Funct. Genom. 2018, 17, 8–14. [Google Scholar]
- Hughes, A.L. Adaptive amino acid composition in collagens of parasitic nematodes. Infect. Genet. Evol. 2015. [Google Scholar] [CrossRef]
- Giribet, G.; Edgecombe, G.D. Current understanding of Ecdysozoa and its internal phylogenetic relationships. In Proceedings of the Integrative and Comparative Biology, New Orleans, LA, USA, 4–8 January 2017. [Google Scholar]
- WHO. Lymphatic Filariasis Epidemiology. Available online: https://www.who.int/lymphatic_filariasis/epidemiology/en/ (accessed on 19 November 2020).
- Duke, B.O.L. The population dynamics of Onchocerca volvulus in the human host. Trop. Med. Parasitol. 1993, 44, 61–68. [Google Scholar]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Lobos, E.; Altmann, M.; Mengod, G.; Weiss, N.; Rudin, W.; Karam, M. Identification of an Onchocerca volvulus cDNA encoding a low-molecular-weight antigen uniquely recognized by onchocerciasis patient sera. Mol. Biochem. Parasitol. 1990, 39, 135–145. [Google Scholar] [CrossRef]
- Nde, P.N.; Pogonka, T.; Bradley, J.E.; Titanji, V.P.K.; Lucius, R. Sensitive and specific serodiagnosis of onchocerciasis with recombinant hybrid proteins. Am. J. Trop. Med. Hyg. 2002, 66, 566–571. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Leahy, H.P.; Iadarola, M.J.; Nutman, T.B. A Four-Antigen Mixture for Rapid Assessment of Onchocerca volvulus Infection. PLoS Negl. Trop. Dis. 2009, 3, e438. [Google Scholar] [CrossRef]
- Lucius, R.; Schulz-Key, H.; Büttner, D.W.; Kern, A.; Kaltmann, B.; Prod’Hon, J.; Seeber, F.; Walter, R.D.; Saxena, K.C.; Diesfeld, H.J. Characterization of an immunodominant Onchocerca volvulus antigen with patient sera and a monoclonal antibody. J. Exp. Med. 1988. [Google Scholar] [CrossRef]
- Cabrera, Z.; Parkhouse, R.M.E.; Forsyth, K.; Gomez Priego, A.; Pabon, R.; Yarzabal, L. Specific detection of human antibodies to Onchocerca volvulus. Trop. Med. Parasitol. 1989, 40, 454. [Google Scholar] [PubMed]
- Mbacham, W.F.; Titanji, V.P.K.; Thunberg, L.; Holmdahl, R.; Rubin, K. A monoclonal antibody-based immunodiagnostic assay for onchocerciasis. Trop. Med. Parasitol. 1992, 43, 83–90. [Google Scholar] [PubMed]
- Ayong, L.S.; Tume, C.B.; Wembe, F.E.; Simo, G.; Asonganyi, T.; Lando, G.; Ngu, J.L. Development and evaluation of an antigen detection dipstick assay for the diagnosis of human onchocerciasis. Trop. Med. Int. Health 2005. [Google Scholar] [CrossRef]
- Zimmerman, P.A.; Guderian, R.H.; Aruajo, E.; Elson, L.; Phadke, P.; Kubofcik, J.; Nutman, T.B. Polymerase chain reaction-based diagnosis of Onchocerca volvulus infection: Improved detection of patients with onchocerciasis. J. Infect. Dis. 1994, 169, 686–689. [Google Scholar] [CrossRef]
- Denery, J.R.; Nunes, A.A.K.; Hixon, M.S.; Dickerson, T.J.; Janda, K.D. Metabolomics-based discovery of diagnostic biomarkers for onchocerciasis. PLoS Negl. Trop. Dis. 2010, 4, e834. [Google Scholar] [CrossRef]
- Lagatie, O.; Ediage, E.N.; Debrah, L.B.; Diels, L.; Nolten, C.; Vinken, P.; Debrah, A.; Dillen, L.; Silber, S.; Stuyver, L.J. Evaluation of the diagnostic potential of urinary N-Acetyltyramine-O,β-glucuronide (NATOG) as diagnostic biomarker for Onchocerca volvulus infection. Parasites Vectors 2016, 9. [Google Scholar] [CrossRef]
- Weil, G.J.; Steel, C.; Liftis, F.; Li, B.W.; Mearns, G.; Lobos, E.; Nutman, T.B. A rapid-format antibody card test for diagnosis of onchocerciasis. J. Infect. Dis. 2000, 182, 1796–1799. [Google Scholar] [CrossRef]
- Lobos, E.; Weiss, N.; Karam, M.; Taylort, H.R.; Otresen, E.A.; Nutman, T.B. An Immunogenic Onchocerca volvulus Antigen: A Specific and Early Marker of Infection. Science 1991, 251, 1603–1605. [Google Scholar] [CrossRef]
- Tekle, A.H.; Elhassan, E.; Isiyaku, S.; Amazigo, U.V.; Bush, S.; Noma, M.; Cousens, S.; Abiose, A.; Remme, J.H. Impact of long-term treatment of onchocerciasis with ivermectin in Kaduna State, Nigeria: First evidence of the potential for elimination in the operational area of the African Programme for Onchocerciasis Control. Parasites Vectors 2012, 5, 28. [Google Scholar] [CrossRef]
- Osei-Atweneboana, M.Y.; Awadzi, K.; Attah, S.K.; Boakye, D.A.; Gyapong, J.O.; Prichard, R.K. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Negl. Trop. Dis. 2011, 5, e998. [Google Scholar] [CrossRef]
- Bourguinat, C.; Pion, S.D.S.; Kamgno, J.; Gardon, J.; Gardon-Wendel, N.; Duke, B.O.L.; Prichard, R.K.; Boussinesq, M. Genetic polymorphism of the β-tubulin gene of Onchocerca volvulus in ivermectin naïve patients from Cameroon, and its relationship with fertility of the worms. Parasitology 2006, 132, 255. [Google Scholar] [CrossRef] [PubMed]
- Mounchili, S.C.; Ghogomu, M.A.; Ghogomu, S.M.; Njume, F.N.; Robert, A. Analysis of Onchocerca volvulus β-tubulin gene polymorphism in the Mbonge sub-division of Cameroon: Evidence of gene selection by ivermectin. J. Genet. Mol. Biol. 2018, 2, 21–26. [Google Scholar]
- Bradley, J.E.; Tuan, R.S.; Shepley, K.J.; Tree, T.I.M.; Maizels, R.M.; Helm, R.; Gregory, W.F.; Unnasch, T.R. Onchocerca volvulus: Characterization of an immunodominant hypodermal antigen present in adult and larval parasites. Exp. Parasitol. 1993, 77, 414–424. [Google Scholar] [CrossRef]
- Lustigman, S.; Brotman, B.; Huima, T.; Prince, A.M.; McKerrow, J.H. Molecular cloning and characterization of onchocystatin, a cysteine proteinase inhibitor of Onchocerca volvulus. J. Biol. Chem. 1992, 267, 17339–17364. [Google Scholar]
- Cho-Ngwa, F.; Liu, J.; Lustigman, S. The Onchocerca volvulus cysteine proteinase inhibitor, Ov-CPI-2, is a target of protective antibody response that increases with age. PLoS Negl. Trop. Dis. 2010, 4, e800. [Google Scholar] [CrossRef]
- Chandrashekar, R.; Curtis, K.C.; Weil, G.J. Molecular characterization of a parasite antigen in sera from onchocerciasis patients that is immunologically cross-reactive with human keratin. J. Infect. Dis. 1995, 171, 1586–1592. [Google Scholar] [CrossRef]
- Cho-Ngwa, F.; Zhu, X.; Metuge, J.A.; Daggfeldt, A.; Grönvik, K.O.; Orlando, R.; Atwood, J.A.; Titanji, V.P. Identification of in vivo released products of Onchocerca with diagnostic potential, and characterization of a dominant member, the OV1CF intermediate filament. Infect. Genet. Evol. 2011, 11, 778–783. [Google Scholar] [CrossRef]
- Brattig, N.W.; Henkle-Dührsen, K.; Hounkpatin, S.; Liebau, E.; Kruppa, T.F.; Zipfel, P.F. Characterization of human immune responses to the cytosolic superoxide dismutase and glutathione S-transferase from Onchocerca volvulus. Trop. Med. Int. Health 1997, 2, 788–798. [Google Scholar] [CrossRef]
- Ajonina-Ekoti, I.; Ndjonka, D.; Tanyi, M.K.; Wilbertz, M.; Younis, A.E.; Boursou, D.; Kurosinski, M.A.; Eberle, R.; Lüersen, K.; Perbandt, M.; et al. Functional characterization and immune recognition of the extracellular superoxide dismutase from the human pathogenic parasite Onchocerca volvulus (OvEC-SOD). Acta Trop. 2012, 124, 15–26. [Google Scholar] [CrossRef]
- Kennedy, M.W.; Garside, L.H.; Goodrick, L.E.; McDermott, L.; Brass, A.; Price, N.C.; Kelly, S.M.; Cooper, A.; Bradley, J.E. The Ov20 protein of the parasitic nematode Onchocerca volvulus. A structurally novel class of small helix-rich retinol-binding proteins. J. Biol. Chem. 1997, 272, 29442–29448. [Google Scholar] [CrossRef] [PubMed]
- Lustigman, S.; Brotman, B.; Johnson, E.H.; Smith, A.B.; Huima, T.; Prince, A.M. Identification and characterization of an Onchocerca volvulus cDNA clone encoding a microfilarial surface-associated antigen. Mol. Biochem. Parasitol. 1992, 50, 79–93. [Google Scholar] [CrossRef] [PubMed]
- George, P.J.; Hess, J.A.; Jain, S.; Patton, J.B.; Zhan, T.; Tricoche, N.; Zhan, B.; Bottazzi, M.E.; Hotez, P.J.; Abraham, D.; et al. Antibody responses against the vaccine antigens Ov-103 and Ov-RAL-2 are associated with protective immunity to Onchocerca volvulus infection in both mice and humans. PLoS Negl. Trop. Dis. 2019, 13, e7730. [Google Scholar] [CrossRef]
- Irvine, M.; Huima, T.; Prince, A.M.; Lustigman, S. Identification and characterization of an Onchocerca volvulus cDNA clone encoding a highly immunogenic calponin-like protein. Mol. Biochem. Parasitol. 1994, 65, 135–146. [Google Scholar] [CrossRef]
- Abraham, D.; Leon, O.; Leon, S.; Lustigman, S. Development of a recombinant antigen vaccine against infection with the filarial worm Onchocerca volvulus. Infect. Immun. 2001, 69, 262–270. [Google Scholar] [CrossRef]
- McCarthy, J.S.; Wieseman, M.; Tropea, J.; Kaslow, D.; Abraham, D.; Lustigman, S.; Tuan, R.; Guderian, R.H.; Nutman, T.B. Onchocerca volvulus glycolytic enzyme fructose-1,6-bisphosphate aldolase as a target for a protective immune response in humans. Infect. Immun. 2002, 70, 851–858. [Google Scholar] [CrossRef]
- Jolodar, A.; Fischer, P.; Bergmann, S.; Büttner, D.W.; Hammerschmidt, S.; Brattig, N.W. Molecular cloning of an α-enolase from the human filarial parasite Onchocerca volvulus that binds human plasminogen. Biochim. Biophys. Acta Gene Struct. Expr. 2003, 1627, 111–120. [Google Scholar] [CrossRef]
- Lucius, R.; Kern, A.; Seeber, F.; Pogonka, T.; Willenbucher, J.; Taylor, H.R.; Pinder, M.; Ghalib, H.W.; Schulz-Key, H.; Soboslay, P. Specific and sensitive IgG4 immunodiagnosis of onchocerciasis with a recombinant 33 kD Onchocerca volvulus protein (Ov33). Trop. Med. Parasitol. 1992, 43, 139. [Google Scholar]
- Abdel-Wahab, N.; Kuo, Y.M.; Wu, Y.; Tuan, R.S.; Bianco, A.E. OvB20, an Onchocerca volvulus -cloned antigen selected by differential immunoscreening with vaccination serum in a cattle model of onchocerciasis. Mol. Biochem. Parasitol. 1996, 76, 187–199. [Google Scholar] [CrossRef]
- Jenkins, R.E.; Taylor, M.J.; Gilvary, N.J.; Bianco, A.E. Tropomyosin implicated in host protective responses to microfilariae in onchocerciasis. Proc. Natl. Acad. Sci. USA 1998, 95, 7550–7555. [Google Scholar] [CrossRef]
- Perbandt, M.; Höppner, J.; Burmeister, C.; Lüersen, K.; Betzel, C.; Liebau, E. Structure of the Extracellular Glutathione S-Transferase OvGST1 from the Human Pathogenic Parasite Onchocerca volvulus. J. Mol. Biol. 2008, 377, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Triteeraprapab, S.; Richie, T.L.; Tuan, R.S.; Shepley, K.J.; Dinman, J.D.; Neubert, T.A.; Scott, A.L. Molecular cloning of a gene expressed during early embryonic development in Onchocerca volvulus. Mol. Biochem. Parasitol. 1995, 69, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Rokeach, L.A.; Zimmerman, P.A.; Unnasch, T.R. Epitopes of the Onchocerca volvulus RAL1 antigen, a member of the calreticulin family of proteins, recognized by sera from patients with onchocerciasis. Infect. Immun. 1994, 62, 3696–3704. [Google Scholar] [CrossRef] [PubMed]
- Joseph, G.T.; Huima, T.; Lustigman, S. Characterization of an Onchocerca volvulus L3-specific larval antigen, Ov-ALT-1. Mol. Biochem. Parasitol. 1998, 96, 177–183. [Google Scholar] [CrossRef]
- He, Y.; Barker, S.J.; MacDonald, A.J.; Yu, Y.; Cao, L.; Li, J.; Parhar, R.; Heck, S.; Hartmann, S.; Golenbock, D.T.; et al. Recombinant Ov -ASP-1, a Th1-Biased Protein Adjuvant Derived from the Helminth Onchocerca volvulus, Can Directly Bind and Activate Antigen-Presenting Cells. J. Immunol. 2009, 182, 4005–4016. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.J.; Tawe, W.; Leon, O.; Cao, L.; Liu, J.; Oksov, Y.; Abraham, D.; Lustigman, S. Ov-ASP-1, the Onchocerca volvulus homologue of the activation associated secreted protein family is immunostimulatory and can induce protective anti-larval immunity. Parasite Immunol. 2004, 26, 53–62. [Google Scholar] [CrossRef]
- Wu, Y.; Egerton, G.; McCarthy, J.S.; Nutman, T.B.; Bianco, A.E. Human immune responses to infective stage larval-specific chitinase of filarial parasite, Onchocerca volvulus, Ov-CHI-1. Filaria J. 2003. [Google Scholar] [CrossRef]
- Wu, Y.; Adam, R.; Williams, S.A.; Bianco, A.E. Chitinase genes expressed by infective larvae of the filarial nematodes, Acanthocheilonema viteae and Onchocerca volvulus. Mol. Biochem. Parasitol. 1996, 75, 207–219. [Google Scholar] [CrossRef]
- Park, J.; Dickerson, T.J.; Janda, K.D. Major sperm protein as a diagnostic antigen for onchocerciasis. Bioorganic Med. Chem. 2008, 16, 7206–7209. [Google Scholar] [CrossRef]
- Shey, R.A.; Ghogomu, S.M.; Njume, F.N.; Gainkam, L.O.T.; Poelvoorde, P.; Mutesa, L.; Robert, A.; Humblet, P.; Munyampundu, J.P.; Kamgno, J.; et al. Prediction and validation of the structural features of Ov58GPCR, an immunogenic determinant of Onchocerca volvulus. PLoS ONE 2018, 13, e0202915. [Google Scholar] [CrossRef]
- Ajonina-Ekoti, I.; Kurosinski, M.A.; Younis, A.E.; Ndjonka, D.; Tanyi, M.K.; Achukwi, M.; Eisenbarth, A.; Ajonina, C.; Lüersen, K.; Breloer, M.; et al. Comparative analysis of macrophage migration inhibitory factors (MIFs) from the parasitic nematode Onchocerca volvulus and the free-living nematode Caenorhabditis elegans. Parasitol. Res. 2013, 112, 3335–3346. [Google Scholar] [CrossRef] [PubMed]
- Njume, F.N.; Ghogomu, S.M.; Shey, R.A.; Gainkam, L.O.T.; Poelvoorde, P.; Humblet, P.; Kamgno, J.; Robert, A.; Mutesa, L.; Lelubre, C.; et al. Identification and characterization of the Onchocerca volvulus Excretory Secretory Product Ov28CRP, a putative GM2 activator protein. PLoS Negl. Trop. Dis. 2019, 13, e0007591. [Google Scholar] [CrossRef]
- Shintouo, C.M.; Shey, R.A.; Nebangwa, D.N.; Esoh, K.K.; Nongley, N.F.; Nguve, J.E.; Giron, P.; Mutesa, L.; Vanhamme, L.; Souopgui, J.; et al. In Silico Design and Validation of OvMANE1, a Chimeric Antigen for Human Onchocerciasis Diagnosis. Pathogens 2020, 9, 495. [Google Scholar]
- Dong, M.; Nelson, L.S.; LeCoz, K.; Poole, C.; Carlow, C.K.S. A novel cyclophilin from parasitic and free-living nematodes with a unique substrate- and drug-binding domain. J. Biol. Chem. 2002, 277, 14925–14932. [Google Scholar] [CrossRef]
- Marcilla, A.; Trelis, M.; Cortés, A.; Sotillo, J.; Cantalapiedra, F.; Minguez, M.T.; Valero, M.L.; Sánchez del Pino, M.M.; Muñoz-Antoli, C.; Toledo, R.; et al. Extracellular Vesicles from Parasitic Helminths Contain Specific Excretory/Secretory Proteins and Are Internalized in Intestinal Host Cells. PLoS ONE 2012, 7, e45974. [Google Scholar] [CrossRef]
- Buck, A.H.; Coakley, G.; Simbari, F.; McSorley, H.J.; Quintana, J.F.; Le Bihan, T.; Kumar, S.; Abreu-Goodger, C.; Lear, M.; Harcus, Y.; et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat. Commun. 2014, 5, 5488. [Google Scholar] [CrossRef] [PubMed]
- Trelis, M.; Galiano, A.; Bolado, A.; Toledo, R.; Marcilla, A.; Bernal, D. Subcutaneous injection of exosomes reduces symptom severity and mortality induced by Echinostoma caproni infection in BALB/c mice. Int. J. Parasitol. 2016, 46, 799–808. [Google Scholar] [CrossRef]
- Samoil, V.; Dagenais, M.; Ganapathy, V.; Aldridge, J.; Glebov, A.; Jardim, A.; Ribeiro, P. Vesicle-based secretion in schistosomes: Analysis of protein and microRNA (miRNA) content of exosome-like vesicles derived from Schistosoma mansoni. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Eichenberger, R.M.; Talukder, M.H.; Field, M.A.; Wangchuk, P.; Giacomin, P.; Loukas, A.; Sotillo, J. Characterization of Trichuris muris secreted proteins and extracellular vesicles provides new insights into host–parasite communication. J. Extracell. Vesicles 2018, 7, 1428004. [Google Scholar] [CrossRef]
- Zakeri, A.; Hansen, E.P.; Andersen, S.D.; Williams, A.R.; Nejsum, P. Immunomodulation by helminths: Intracellular pathways and extracellular vesicles. Front. Immunol. 2018, 9, 2349. [Google Scholar]
- Johnston, C.J.C.; Smyth, D.J.; Kodali, R.B.; White, M.P.J.; Harcus, Y.; Filbey, K.J.; Hewitson, J.P.; Hinck, C.S.; Ivens, A.; Kemter, A.M.; et al. A structurally distinct TGF-β mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Osborne, J.; Devaney, E. The L3 of Brugia induces a T(h)2-polarized response following activation of an IL-4-producing CD4-CD8- αβ T cell population. Int. Immunol. 1998, 10, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; Yazdanbakhsh, M. Immune regulation by helminth parasites: Cellular and molecular mechanisms. Nat. Rev. Immunol. 2003, 3, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.J.; MacDonald, A.S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002, 2, 499–511. [Google Scholar] [PubMed]
- Lawrence, R.A.; Allen, J.E.; Osborne, J.; Maizels, R.M. Adult and microfilarial stages of the filarial parasite Brugia malayi stimulate contrasting cytokine and Ig isotype responses in BALB/c mice. J. Immunol. 1994, 153, 1216–1224. [Google Scholar]
- Zaccone, P.; Burton, O.; Miller, N.; Jones, F.M.; Dunne, D.W.; Cooke, A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur. J. Immunol. 2009, 39, 1098–1107. [Google Scholar] [CrossRef]
- Navarro, S.; Pickering, D.A.; Ferreira, I.B.; Jones, L.; Ryan, S.; Troy, S.; Leech, A.; Hotez, P.J.; Zhan, B.; Laha, T.; et al. Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci. Transl. Med. 2016, 8, 362ra143. [Google Scholar] [CrossRef]
- Dubremetz, J.F.; Achbarou, A.; Bermudes, D.; Joiner, K.A. Kinetics and pattern of organelle exocytosis during Toxoplasma gondii/host-cell interaction. Parasitol. Res. 1993, 79, 402–408. [Google Scholar] [CrossRef]
- Carruthers, V.B.; Sibley, L.D. Sequential protein secretion front three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 1997, 73, 114–123. [Google Scholar]
- Saeij, J.P.J.; Coller, S.; Boyle, J.P.; Jerome, M.E.; White, M.W.; Boothroyd, J.C. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 2007, 445, 324–327. [Google Scholar] [CrossRef]
- Etheridge, R.D.; Alaganan, A.; Tang, K.; Lou, H.J.; Turk, B.E.; Sibley, L.D. The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe 2014, 15, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Sibley, L.D. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 2012, 10, 766–778. [Google Scholar] [PubMed]
- Moser, M.; Murphy, K.M. Dendritic cell regulation of Th 1-Th 2 development. Nat. Immunol. 2000, 1, 199–205. [Google Scholar] [PubMed]
- Yamazaki, S.; Inaba, K.; Tarbell, K.V.; Steinman, R.M. Dendritic cells expand antigen-specific Foxp3+CD25+CD4+ regulatory T cells including suppressors of alloreactivity. Immunol. Rev. 2006, 212, 314–329. [Google Scholar] [PubMed]
- MacDonald, A.S.; Maizels, R.M. Alarming dendritic cells for Th2 induction. J. Exp. Med. 2008, 205, 13–17. [Google Scholar]
- Gaze, S.; McSorley, H.J.; Daveson, J.; Jones, D.; Bethony, J.M.; Oliveira, L.M.; Speare, R.; McCarthy, J.S.; Engwerda, C.R.; Croese, J.; et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012, 8, e1002520. [Google Scholar] [CrossRef]
- Donnelly, S.; Stack, C.M.; O’Neill, S.M.; Sayed, A.A.; Williams, D.L.; Dalton, J.P. Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB J. 2008, 22, 4022–4032. [Google Scholar] [CrossRef]
- Terrazas, C.A.; Sánchez-Muñoz, F.; Mejía-Domínguez, A.M.; Amezcua-Guerra, L.M.; Terrazas, L.I.; Bojalil, R.; Gómez-García, L. Cestode antigens induce a tolerogenic-like phenotype and inhibit LPS in-flammatory responses in human dendritic cells. Int. J. Biol. Sci. 2011, 7, 1391–1400. [Google Scholar] [CrossRef][Green Version]
- Riganò, R.; Buttari, B.; Profumo, E.; Ortona, E.; Delunardo, F.; Margutti, P.; Mattei, V.; Teggi, A.; Sorice, M.; Siracusano, A. Echinococcus granulosus antigen B impairs human dendritic cell differentiation and polarizes immature dendritic cell maturation towards a Th2 cell response. Infect. Immun. 2007, 75, 1667–1678. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.S.; Fang, B.B.; Li, Z.D.; Li, L.; Bi, X.J.; Li, W.D.; Zhang, N.; Lin, R.Y.; Wen, H. Thioredoxin peroxidase secreted by Echinococcus granulosus (sensu stricto) promotes the alternative activation of macrophages via PI3K/AKT/mTOR pathway. Parasites Vectors 2019, 12, 542. [Google Scholar] [CrossRef]
- Holland, M.J.; Harcus, Y.M.; Riches, P.L.; Maizels, R.M. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur. J. Immunol. 2000, 30, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.S.; Straw, A.D.; Bauman, B.; Pearce, E.J. CD8—Dendritic Cell Activation Status Plays an Integral Role in Influencing Th2 Response Development. J. Immunol. 2001, 167, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Perona-Wright, G.; Lundie, R.J.; Jenkins, S.J.; Webb, L.M.; Grencis, R.K.; MacDonald, A.S. Concurrent Bacterial Stimulation Alters the Function of Helminth-Activated Dendritic Cells, Resulting in IL-17 Induction. J. Immunol. 2012, 188, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
- Aranzamendi, C.; Fransen, F.; Langelaar, M.; Franssen, F.; Van Der Ley, P.; Van Putten, J.P.M.; Rutten, V.; Pinelli, E. Trichinella spiralis-secreted products modulate DC functionality and expand regulatory T cells in vitro. Parasite Immunol. 2012, 34, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Nono, J.K.; Pletinckx, K.; Lutz, M.B.; Brehm, K. Excretory/secretory-products of echinococcus multilocularis larvae induce apoptosis and tolerogenic properties in dendritic cells in vitro. PLoS Negl. Trop. Dis. 2012, 6, e1516. [Google Scholar] [CrossRef]
- Semnani, R.T.; Liu, A.Y.; Sabzevari, H.; Kubofcik, J.; Zhou, J.; Gilden, J.K.; Nutman, T.B. Brugia malayi Microfilariae Induce Cell Death in Human Dendritic Cells, Inhibit Their Ability to Make IL-12 and IL-10, and Reduce Their Capacity to Activate CD4+ T Cells. J. Immunol. 2003, 171, 1950–1960. [Google Scholar] [CrossRef]
- Gruden-Movsesijan, A.; Ilic, N.; Colic, M.; Majstorovic, I.; Vasilev, S.; Radovic, I.; Sofronic-Milosavljevic, L.J. The impact of Trichinella spiralis excretory-secretory products on dendritic cells. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 429–439. [Google Scholar] [CrossRef]
- Van der Kleij, D.; Latz, E.; Brouwers, J.F.H.M.; Kruize, Y.C.M.; Schmitz, M.; Kurt-Jones, E.A.; Espevik, T.; De Jong, E.C.; Kapsenberg, M.L.; Golenbock, D.T.; et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 2002, 277, 48122–48129. [Google Scholar] [CrossRef]
- Li, Z.; Liu, G.; Chen, Y.; Liu, Y.; Liu, B.; Su, Z. The phenotype and function of naturally existing regulatory dendritic cells in nematode-infected mice. Int. J. Parasitol. 2011, 41, 1129–1137. [Google Scholar] [CrossRef]
- Correale, J.; Farez, M. Helminth Antigens Modulate Immune Responses in Cells from Multiple Sclerosis Patients through TLR2-Dependent Mechanisms. J. Immunol. 2009, 183, 5999–6012. [Google Scholar] [CrossRef]
- Everts, B.; Hussaarts, L.; Driessen, N.N.; Meevissen, M.H.J.; Schramm, G.; van der Ham, A.J.; van der Hoeven, B.; Scholzen, T.; Burgdorf, S.; Mohrs, M.; et al. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J. Exp. Med. 2012, 209, 1753–1767. [Google Scholar] [CrossRef] [PubMed]
- Pineda, M.A.; Lumb, F.; Harnett, M.M.; Harnett, W. ES-62, a therapeutic anti-inflammatory agent evolved by the filarial nematode Acanthocheilonema viteae. Mol. Biochem. Parasitol. 2014, 194, 1–8. [Google Scholar] [PubMed]
- Harnett, W.; Al-Riyami, L.; Rzepecka, J.; Harnett, M.M. Modulation of autoimmune and allergic responses by defined nematode molecules. In Parasitic Nematodes: Molecular Biology, Biochemistry and Immunology; CABI: Boston, MA, USA, 2013. [Google Scholar]
- Tundup, S.; Srivastava, L.; Harn, D.A., Jr. Polarization of host immune responses by helminth-expressed glycans. Ann. N. Y. Acad. Sci. 2012, 1253, E1–E13. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Fujita, K. Molecules of Parasites as Immunomodulatory Drugs. Curr. Top. Med. Chem. 2005, 4, 539–552. [Google Scholar] [CrossRef]
- Prieto-Lafuente, L.; Gregory, W.F.; Allen, J.E.; Maizels, R.M. MIF homologues from a filarial nematode parasite synergize with IL-4 to induce alternative activation of host macrophages. J. Leukoc. Biol. 2009, 85, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Jones, B.F.; Vermeire, J.J.; Leng, L.; DiFedele, L.; Harrison, L.M.; Xiong, H.; Kwong, Y.K.A.; Chen, Y.; Bucala, R.; et al. Structural and functional characterization of a secreted hookworm macrophage Migration Inhibitory Factor (MIF) that interacts with the human MIF receptor CD74. J. Biol. Chem. 2007, 282, 23447–23456. [Google Scholar] [CrossRef]
- Chauhan, N.; Sharma, R.; Hoti, S.L. Identification and biochemical characterization of macrophage migration inhibitory factor-2 (MIF-2) homologue of human lymphatic filarial parasite, Wuchereria bancrofti. Acta Trop. 2015, 142, 71–78. [Google Scholar] [CrossRef]
- Younis, A.E.; Soblik, H.; Ajonina-Ekoti, I.; Erttmann, K.D.; Luersen, K.; Liebau, E.; Brattig, N.W. Characterization of a secreted macrophage migration inhibitory factor homologue of the parasitic nematode Strongyloides acting at the parasite-host cell interface. Microbes Infect. 2012, 14, 279–289. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, M.M.; Wang, S.; Ehsan, M.; Yan, R.F.; Song, X.K.; Xu, L.X.; Li, X.R. Characterization of a secreted macrophage migration inhibitory factor homologue of the parasitic nematode Haemonchus Contortus acting at the parasite-host cell interface. Oncotarget 2017, 8, 40052–40064. [Google Scholar] [CrossRef]
- Qu, G.; Fetterer, R.; Leng, L.; Du, X.; Zarlenga, D.; Shen, Z.; Han, W.; Bucala, R.; Tuo, W. Ostertagia ostertagi macrophage migration inhibitory factor is present in all developmental stages and may cross-regulate host functions through interaction with the host receptor. Int. J. Parasitol. 2014, 44, 355–367. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Li, L.; Liu, Q.; Liu, P.; Li, S.; Yang, D.; Chen, Y.; Pagnotta, S.; Favery, B.; Abad, P.; et al. A MIF-like effector suppresses plant immunity and facilitates nematode parasitism by interacting with plant annexins. J. Exp. Bot. 2019, 70, 5943–5958. [Google Scholar] [CrossRef] [PubMed]
- Price, D.R.G.; Nisbet, A.J.; Frew, D.; Bartley, Y.; Oliver, E.M.; McLean, K.; Inglis, N.F.; Watson, E.; Corripio-Miyar, Y.; McNeilly, T.N. Characterisation of a niche-specific excretory-secretory peroxiredoxin from the parasitic nematode Teladorsagia circumcincta. Parasites Vectors 2019, 12, 1–14. [Google Scholar] [CrossRef]
- Kron, M.A.; Metwali, A.; Vodanovic-Jankovic, S.; Elliott, D. Nematode asparaginyl-tRNA synthetase resolves intestinal inflammation in mice with T-cell transfer colitis. Clin. Vaccine Immunol. 2013, 20, 276–281. [Google Scholar] [CrossRef][Green Version]
- Eichenberger, R.M.; Sotillo, J.; Loukas, A. Immunobiology of parasitic worm extracellular vesicles. Immunol. Cell Biol. 2018, 96, 704–713. [Google Scholar]
- Eichenberger, R.M.; Ryan, S.; Jones, L.; Buitrago, G.; Polster, R.; de Oca, M.M.; Zuvelek, J.; Giacomin, P.R.; Dent, L.A.; Engwerda, C.R.; et al. Hookworm secreted extracellular vesicles interact with host cells and prevent inducible colitis in mice. Front. Immunol. 2018, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Schabussova, I.; Amer, H.; van Die, I.; Kosma, P.; Maizels, R.M. O-Methylated glycans from Toxocara are specific targets for antibody binding in human and animal infections. Int. J. Parasitol. 2007, 37, 97–109. [Google Scholar] [CrossRef]
- Tawill, S.; Le Goff, L.; Ali, F.; Blaxter, M.; Allen, J.E. Both Free-Living and Parasitic Nematodes Induce a Characteristic Th2 Response That Is Dependent on the Presence of Intact Glycans. Infect. Immun. 2004, 72, 398–407. [Google Scholar] [CrossRef]
- Rodríguez, E.; Kalay, H.; Noya, V.; Brossard, N.; Giacomini, C.; Van Kooyk, Y.; García-Vallejo, J.J.; Freire, T. Fasciola hepatica glycoconjugates immuneregulate dendritic cells through the dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin inducing T cell anergy. Sci. Rep. 2017, 7, srep46748. [Google Scholar] [CrossRef]
- Klaver, E.J.; Van Der Pouw Kraan, T.C.T.M.; Laan, L.C.; Kringel, H.; Cummings, R.D.; Bouma, G.; Kraal, G.; Van Die, I. Trichuris suis soluble products induce Rab7b expression and limit TLR4 responses in human dendritic cells. Genes Immun. 2015, 16, 378–387. [Google Scholar] [CrossRef]
- Laan, L.C.; Williams, A.R.; Stavenhagen, K.; Giera, M.; Kooij, G.; Vlasakov, I.; Kalay, H.; Kringel, H.; Nejsum, P.; Thamsborg, S.M.; et al. The whipworm (Trichuris suis) secretes prostaglandin E2 to suppress proinflammatory properties in human dendritic cells. FASEB J. 2017, 31, 719–731. [Google Scholar] [CrossRef]
- Haslam, S.M.; Khoo, K.H.; Houston, K.M.; Harnett, W.; Morris, H.R.; Dell, A. Characterisation of the phosphorylcholine-containing N-linked oligosaccharides in the excretory-secretory 62 kDa glycoprotein of Acanthocheilonema viteae. Mol. Biochem. Parasitol. 1997, 85, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, H.S.; McGuiness, S.; Houston, K.M.; Egan, C.A.; Al-Riyami, L.; Alcocer, M.J.C.; Harnett, M.M.; Harnett, W. Phosphorylcholine mimics the effects of ES-62 on macrophages and dendritic cells. Parasite Immunol. 2007, 29, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Al-Riyami, L.; Harnett, W. Immunomodulatory Properties of ES-62, a Phosphorylcholine—Containing Glycoprotein Secreted by Acanthocheilonema viteae. Endocr. Metab. Immune Disord. Targets 2012, 12, 45–52. [Google Scholar] [CrossRef]
- Narasimhan, P.B.; Bennuru, S.; Meng, Z.; Cotton, R.N.; Elliott, K.R.; Ganesan, S.; McDonald-Fleming, R.; Veenstra, T.D.; Nutman, T.B.; Tolouei Semnani, R. Microfilariae of Brugia malayi Inhibit the mTOR pathway and induce autophagy in human dendritic cells. Infect. Immun. 2016, 84, 2463–2472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, P.G.; Carter, M.R.; Atochina, O.; Da’Dara, A.A.; Piskorska, D.; McGuire, E.; Harn, D.A. Maturation of Dendritic Cell 2 Phenotype by a Helminth Glycan Uses a Toll-Like Receptor 4-Dependent Mechanism. J. Immunol. 2003, 171, 5837–5841. [Google Scholar] [CrossRef] [PubMed]
- Harnett, W.; Harnett, M.M. Helminth-derived immunomodulators: Can understanding the worm produce the pill? Nat. Rev. Immunol. 2010, 10, 278–284. [Google Scholar]
- Klotz, C.; Ziegler, T.; Figueiredo, A.S.; Rausch, S.; Hepworth, M.R.; Obsivac, N.; Sers, C.; Lang, R.; Hammerstein, P.; Lucius, R.; et al. A helminth immunomodulator exploits host signaling events to regulate cytokine production in macrophages. PLoS Pathog. 2011, 7, e1001248. [Google Scholar] [CrossRef]
- Manoury, B.; Gregory, W.F.; Maizels, R.M.; Watts, C. Bm-CPI-2, a cystatin homolog secreted by the filarial parasite Brugia malayi, inhibits class II MHC-restricted antigen processing. Curr. Biol. 2001, 11, 447–451. [Google Scholar] [CrossRef]
- Kobpornchai, P.; Flynn, R.J.; Reamtong, O.; Rittisoonthorn, N.; Kosoltanapiwat, N.; Boonnak, K.; Boonyuen, U.; Ampawong, S.; Jiratanh, M.; Tattiyapong, M.; et al. A novel cystatin derived from Trichinella spiralis suppresses macrophage-mediated inflammatory responses. PLoS Negl. Trop. Dis. 2020, 14, e0008192. [Google Scholar] [CrossRef]
- Schönemeyer, A.; Lucius, R.; Sonnenburg, B.; Brattig, N.; Sabat, R.; Schilling, K.; Bradley, J.; Hartmann, S. Modulation of human T cell responses and macrophage functions by onchocystatin, a secreted protein of the filarial nematode Onchocerca volvulus. J. Immunol. 2001, 167, 3207–3215. [Google Scholar] [CrossRef]
- Schnoeller, C.; Rausch, S.; Pillai, S.; Avagyan, A.; Wittig, B.M.; Loddenkemper, C.; Hamann, A.; Hamelmann, E.; Lucius, R.; Hartmann, S. A Helminth Immunomodulator Reduces Allergic and Inflammatory Responses by Induction of IL-10-Producing Macrophages. J. Immunol. 2008, 180, 4265–4272. [Google Scholar] [CrossRef] [PubMed]
- Dainichi, T.; Maekawa, Y.; Ishii, K.; Zhang, T.; Fawzy Nashed, B.; Sakai, T.; Takashima, M.; Himeno, K. Nippocystatin, a cysteine protease inhibitor from Nippostrongylus brasiliensis, inhibits antigen processing and modulates antigen-specific immune response. Infect. Immun. 2001, 69, 7380–7386. [Google Scholar] [CrossRef] [PubMed]
- Hosken, N.A.; Shibuya, K.; Heath, A.W.; Murphy, K.M.; O’Garra, A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-αβ-transgenic model. J. Exp. Med. 1995, 182, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Harnett, W.; Harnett, M.M. What causes lymphocyte hyporesponsiveness during filarial nematode infection? Trends Parasitol. 2006, 22, 105–110. [Google Scholar] [CrossRef]
- Deehan, M.R.; Harnett, W.; Harnett, M.M. A filarial nematode-secreted phosphorylcholine-containing glycoprotein uncouples the B cell antigen receptor from extracellular signal-regulated kinase-mitogen-activated protein kinase by promoting the surface Ig-mediated recruitment of Src homology 2 domain-containing tyrosine phosphatase-1 and Pac-1 mitogen-activated kinase-phosphatase. J. Immunol. 2001, 166, 7462–7468. [Google Scholar] [CrossRef]
- Melendez, A.J.; Harnett, M.M.; Pushparaj, P.N.; Wong, W.F.; Tay, H.K.; McSharry, C.P.; Harnett, W. Inhibition of FcεRI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nat. Med. 2007, 13, 1375–1381. [Google Scholar] [CrossRef]
- Furuhashi, Y.; Imai, S.; Tezuka, H.; Fujita, K. Recombinant Dirofilaria immitis-derived antigen can suppress passive cutaneous anaphylaxis reactions. Int. Arch. Allergy Immunol. 2001, 125, 144–151. [Google Scholar] [CrossRef]
- Correale, J.; Farez, M.F. Parasite Infections in Multiple Sclerosis Modulate Immune Responses through a Retinoic Acid–Dependent Pathway. J. Immunol. 2013, 191, 3827–3837. [Google Scholar] [CrossRef]
- Vukman, K.V.; Adams, P.N.; O’Neill, S.M. Fasciola hepatica tegumental coat antigen suppresses MAPK signalling in dendritic cells and up-regulates the expression of SOCS3. Parasite Immunol. 2013, 35, 234–238. [Google Scholar] [CrossRef]
- Gomez-Escobar, N.; Bennett, C.; Prieto-Lafuente, L.; Aebischer, T.; Blackburn, C.C.; Maizels, R.M. Heterologous expression of the filarial nematode alt gene products reveals their potential to inhibit immune function. BMC Biol. 2005, 3, 8. [Google Scholar] [CrossRef]
- Schierack, P.; Lucius, R.; Sonnenburg, B.; Schilling, K.; Hartmann, S. Parasite-specific immunomodulatory functions of filarial cystatin. Infect. Immun. 2003, 71, 2422–2429. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, T.; Rausch, S.; Steinfelder, S.; Klotz, C.; Hepworth, M.R.; Kühl, A.A.; Burda, P.-C.; Lucius, R.; Hartmann, S. A Novel Regulatory Macrophage Induced by a Helminth Molecule Instructs IL-10 in CD4+ T Cells and Protects against Mucosal Inflammation. J. Immunol. 2015, 194, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Tanabe, M.; Terauchi, Y.; Ota, T.; Matsuda, S.; Asano, T.; Kadowaki, T.; Takeuchi, T.; Koyasu, S. P13K-mediated negative feedback regulation of IL-12 production in DCs. Nat. Immunol. 2002, 3, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.J.C.; Smyth, D.J.; Dresser, D.W.; Maizels, R.M. TGF-β in tolerance, development and regulation of immunity. Cell. Immunol. 2016, 299, 14–22. [Google Scholar] [CrossRef]
- Szkudliński, J. Occurrence of prostaglandins and other eicosanoids in helminths and their role in host-parasite interaction—Review article. Helminthologia 2000, 46, 439–446. [Google Scholar]
- Fusco, A.C.; Salafsky, B.; Kevin, M.B. Schistosoma mansoni: Eicosanoid production by cercariae. Exp. Parasitol. 1985, 59, 44–50. [Google Scholar] [CrossRef]
- Leid, R.W.; McConnell, L.A. PGE2 generation and release by the larval stage of the cestode, Taenia Taeniaeformis. Prostaglandins Leukot. Med. 1983, 11, 317–323. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of Immune Responses by Prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef]
- Viney, M.E. How did parasitic worms evolve? BioEssays 2009, 31, 496–499. [Google Scholar] [CrossRef]
- Hunt, V.L.; Tsai, I.J.; Coghlan, A.; Reid, A.J.; Holroyd, N.; Foth, B.J.; Tracey, A.; Cotton, J.A.; Stanley, E.J.; Beasley, H.; et al. The genomic basis of parasitism in the Strongyloides clade of nematodes. Nat. Genet. 2016, 48, 299–307. [Google Scholar] [CrossRef]
- Xu, L.; Xu, M.; Sun, X.; Xu, J.; Zeng, X.; Shan, D.; Yuan, D.; He, P.; He, W.; Yang, Y.; et al. The genetic basis of adaptive evolution in parasitic environment from the Angiostrongylus cantonensis genome. PLoS Negl. Trop. Dis. 2019, 13, e0007846. [Google Scholar] [CrossRef]
- Ham, P.J.; Smail, A.J.; Groeger, B.K. Surface carbohydrate changes on onchocerca lienalis larvae as they develop from microfilariae to the infective third-stage in simulium ornatum. J. Helminthol. 1988. [Google Scholar] [CrossRef]
- Hagen, H.E.; Grunewald, J.; Ham, P.J. Differential lectin binding of Onchocerca lienalis and Onchocerca ochengi infective larvae following their development in Simulium ornatum s.l. Trop. Med. Parasitol. 1994, 45, 13–16. [Google Scholar] [PubMed]
- Riddle, D.L. C Elegans II, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997. [Google Scholar]
- Politz, S.M.; Chin, K.J.; Herman, D.L. Genetic analysis of adult-specific surface antigenic differences between varieties of the nematode Caenorhabditis elegans. Genetics 1987, 117, 467–476. [Google Scholar] [PubMed]
- Hussey, R.S.; Grundler, F.M.W. The Physiology and Biochemistry of free-living and plant-parasitic nematodes. In Nematode Parasitism of Plants; CABI: Wallingford, UK, 1998; ISBN 085199-231-5. [Google Scholar]
- Fetterer, R.H.; Rhoads, M.L. Biochemistry of the nematode cuticle: Relevance to parasitic nematodes of livestock. Vet. Parasitol. 1993, 46, 103–111. [Google Scholar] [CrossRef]
- Sotillo, J.; Sanchez-Flores, A.; Cantacessi, C.; Harcus, Y.; Pickering, D.; Bouchery, T.; Camberis, M.; Tang, S.C.; Giacomin, P.; Mulvenna, J.; et al. Secreted proteomes of different developmental stages of the gastrointestinal nematode Nippostrongylus brasiliensis. Mol. Cell. Proteom. 2014, 13, 2736–2751. [Google Scholar] [CrossRef]
- Logan, J.; Manda, S.S.; Choi, Y.-J.; Field, M.; Eichenberger, R.; Mulvenna, J.; Nagaraj, S.; Fujiwara, R.; Gazzinelli-Guimaraes, P.; Bueno, L.; et al. Comprehensive analysis of human hookworm secreted proteins using a proteogenomic approach. bioRxiv 2018. [Google Scholar] [CrossRef]
- Mulvenna, J.; Hamilton, B.; Nagaraj, S.H.; Smyth, D.; Loukas, A.; Gorman, J.J. Proteomics analysis of the excretory/secretory component of the blood-feeding stage of the hookworm, Ancylostoma caninum. Mol. Cell. Proteom. 2009, 8, 109–121. [Google Scholar] [CrossRef]
- Chang, D.Z.; Serra, L.; Lu, D.; Mortazavi, A.; Dillman, A.R. A core set of venom proteins is released by entomopathogenic nematodes in the genus Steinernema. PLoS Pathog. 2019, 15, e1007626. [Google Scholar] [CrossRef]
- Lee, J.D.; Tsai, L.Y.; Chen, C.H.; Wang, J.J.; Hsiao, J.K.; Yen, C.M. Blood-brain barrier dysfunction occurring in mice infected with Angiostrongylus cantonensis. Acta Trop. 2006, 97, 204–211. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.H.; Lan, L.; Wu, F.F.; Zhang, E.P.; Song, Z.M.; Huang, H.C.; Luo, F.J.; Pan, C.W.; Tan, F. In vitro study of the effects of Angiostrongylus cantonensis larvae extracts on apoptosis and dysfunction in the blood-brain barrier (bbb). PLoS ONE 2012, 7, e32161. [Google Scholar] [CrossRef]
- Chen, K.M.; Liu, J.Y.; Lai, S.C.; Hsu, L.S.; Lee, H.H. Association of plasminogen activators and matrix metalloproteinase-9 proteolytic cascade with blood-CNS barrier damage of angiostrongyliasis. Int. J. Exp. Pathol. 2006, 87, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Hambrook, J.R.; Kaboré, A.L.; Pila, E.A.; Hanington, P.C. A metalloprotease produced by larval Schistosoma mansoni facilitates infection establishment and maintenance in the snail host by interfering with immune cell function. PLoS Pathog. 2018, 14, e1007393. [Google Scholar] [CrossRef]
- Naqvi, M.A.U.H.; Li, H.; Gao, W.; Naqvi, S.Z.; Jamil, T.; Aimulajiang, K.; Xu, L.; Song, X.; Li, X.; Yan, R. Haemonchus contortus: SiRNA mediated knockdown of matrix metalloproteinase 12A (MMP-12) results in reduction of infectivity. Parasites Vectors 2020, 13, 1–11. [Google Scholar] [CrossRef]
- König, R.; Nassri, A.; Meindl, M.; Matuja, W.; Kidunda, A.R.; Siegmund, V.; Bretzel, G.; Löscher, T.; Jilek-Aall, L.; Schmutzhard, E.; et al. The role of Onchocerca volvulus in the development of epilepsy in a rural area of Tanzania. Parasitology 2010, 137, 1559–1568. [Google Scholar] [CrossRef]
- Kaiser, C.; Rubaale, T.; Tukesiga, E.; Kipp, W.; Kabagambe, G.; Ojony, J.O.; Asaba, G. Association between onchocerciasis and epilepsy in the Itwara hyperendemic focus, West Uganda: Controlling for time and intensity of exposure. Am. J. Trop. Med. Hyg. 2011, 85, 225–228. [Google Scholar] [CrossRef]
- Vezzani, A.; Fujinami, R.S.; White, H.S.; Preux, P.M.; Blümcke, I.; Sander, J.W.; Löscher, W. Infections, inflammation and epilepsy. Acta Neuropathol. 2016, 131, 211–234. [Google Scholar]
- Chesnais, C.B.; Nana-Djeunga, H.C.; Njamnshi, A.K.; Lenou-Nanga, C.G.; Boullé, C.; Bissek, A.C.Z.K.; Kamgno, J.; Colebunders, R.; Boussinesq, M. The temporal relationship between onchocerciasis and epilepsy: A population-based cohort study. Lancet Infect. Dis. 2018, 18, 1278–1286. [Google Scholar] [CrossRef]
- Vogel, G. Parasitic worm may trigger mystery nodding syndrome. Science 2017, 355, 678. [Google Scholar]
- Galán-Puchades, M.T. Onchocerciasis-associated epilepsy. Lancet Infect. Dis. 2019, 19, 21–22. [Google Scholar]
- Johnson, T.P.; Tyagi, R.; Lee, P.R.; Lee, M.H.; Johnson, K.R.; Kowalak, J.; Elkahloun, A.; Medynets, M.; Hategan, A.; Kubofcik, J.; et al. Nodding syndrome may be an autoimmune reaction to the parasitic worm Onchocerca volvulus. Sci. Transl. Med. 2017, 9, eaaf6953. [Google Scholar] [CrossRef]
- Chen, K.Y.; Wang, L.C. Stimulation of IL-1β and IL-6 through NF-κB and sonic hedgehog-dependent pathways in mouse astrocytes by excretory/secretory products of fifth-stage larval Angiostrongylus cantonensis. Parasites Vectors 2017, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Cheng, C.; Cheng, C.; Jhan, K.; Chen, Y.; Id, L.W. The excretory/secretory products of fifth-stage larval Angiostrongylus cantonensis induces autophagy via the Sonic hedgehog pathway in mouse brain astrocytes. PLoS Negl. Trop. Dis. 2020, 14, e8290. [Google Scholar] [CrossRef]
- Steinfelder, S.; Andersen, J.F.; Cannons, J.L.; Feng, C.G.; Joshi, M.; Dwyer, D.; Caspar, P.; Schwartzberg, P.L.; Sher, A.; Jankovic, D. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1). J. Exp. Med. 2009, 206, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Smout, M.J.; Sotillo, J.; Laha, T.; Papatpremsiri, A.; Rinaldi, G.; Pimenta, R.N.; Chan, L.Y.; Johnson, M.S.; Turnbull, L.; Whitchurch, C.B.; et al. Carcinogenic Parasite Secretes Growth Factor That Accelerates Wound Healing and Potentially Promotes Neoplasia. PLoS Pathog. 2015, 11, e1005209. [Google Scholar] [CrossRef]
- Strachan, D.P. Hay fever, hygiene, and household size. Br. Med. J. 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Yazdanbakhsh, M.; Kremsner, P.G.; Van Ree, R. Immunology: Allergy, parasites, and the hygiene hypothesis. Science 2002, 296, 490–494. [Google Scholar] [PubMed]
- Bach, J.F. The hygiene hypothesis in autoimmunity: The role of pathogens and commensals. Nat. Rev. Immunol. 2018, 18, 105–120. [Google Scholar]
- Crowe, J.; Lumb, F.E.; Harnett, M.M.; Harnett, W. Parasite excretory-secretory products and their effects on metabolic syndrome. Parasite Immunol. 2017, 39, e12410. [Google Scholar] [CrossRef]
- Berbudi, A.; Surendar, J.; Ajendra, J.; Gondorf, F.; Schmidt, D.; Neumann, A.L.; Wardani, A.P.F.; Layland, L.E.; Hoffmann, L.S.; Pfeifer, A.; et al. Filarial Infection or Antigen Administration Improves Glucose Tolerance in Diet-Induced Obese Mice. J. Innate Immun. 2016, 8, 601–616. [Google Scholar] [CrossRef]
- Wolfs, I.M.J.; Stöger, J.L.; Goossens, P.; Pöttgens, C.; Gijbels, M.J.J.; Wijnands, E.; Van Der Vorst, E.P.C.; Van Gorp, P.; Beckers, L.; Engel, D.; et al. Reprogramming macrophages to an anti-inflammatory phenotype by helminth antigens reduces murine atherosclerosis. FASEB J. 2014, 28, 288–299. [Google Scholar] [CrossRef] [PubMed]
- La Flamme, A.C.; Harvie, M.; Kenwright, D.; Cameron, K.; Rawlence, N.; Low, Y.S.; Mckenzie, S. Chronic exposure to schistosome eggs reduces serum cholesterol but has no effect on atherosclerotic lesion development. Parasite Immunol. 2007, 29, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Aprahamian, T.R.; Zhong, X.; Amir, S.; Binder, C.J.; Chiang, L.-K.; Al-Riyami, L.; Gharakhanian, R.; Harnett, M.M.; Harnett, W.; Rifkin, I.R. The immunomodulatory parasitic worm product ES-62 reduces lupus-associated accelerated atherosclerosis in a mouse model. Int. J. Parasitol. 2015, 45, 203–207. [Google Scholar] [PubMed]
- Summers, R.W.; Elliott, D.E.; Urban, J.F.; Thompson, R.A.; Weinstock, J.V. Trichuris suis therapy for active ulcerative colitis: A randomized controlled trial. Gastroenterology 2005, 128, 825–832. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, L.R.; Chen, F.S.; Zhu, J.P.; Zhu, M.H. Trichuris suis ova therapy in inflammatory bowel disease: A meta-analysis. Medicine 2018, 97, e12087. [Google Scholar] [CrossRef]
- Melon, A.; Wang, A.; Phan, V.; McKay, D.M. Infection with hymenolepis diminuta is more effective than daily corticosteroids in blocking chemically induced colitis in mice. J. Biomed. Biotechnol. 2010. [Google Scholar] [CrossRef]
- Bodammer, P.; Waitz, G.; Loebermann, M.; Holtfreter, M.C.; Maletzki, C.; Krueger, M.R.; Nizze, H.; Emmrich, J.; Reisinger, E.C. Schistosoma mansoni infection but not egg antigen promotes recovery from colitis in outbred NMRI mice. Dig. Dis. Sci. 2011, 56, 70–78. [Google Scholar] [CrossRef]
- Smith, P.; Mangan, N.E.; Walsh, C.M.; Fallon, R.E.; McKenzie, A.N.J.; van Rooijen, N.; Fallon, P.G. Infection with a Helminth Parasite Prevents Experimental Colitis via a Macrophage-Mediated Mechanism. J. Immunol. 2007, 178, 4557–4566. [Google Scholar] [CrossRef]
- Summers, R.W.; Elliot, D.E.; Urban, J.F.; Thompson, R.; Weinstock, J.V. Trichuris suis therapy in Crohn’s disease. Gut 2005, 54, 87–90. [Google Scholar] [CrossRef]
- Rzepecka, J.; Siebeke, I.; Coltherd, J.C.; Kean, D.E.; Steiger, C.N.; Al-Riyami, L.; McSharry, C.; Harnett, M.M.; Harnett, W. The helminth product, ES-62, protects against airway inflammation by resetting the Th cell phenotype. Int. J. Parasitol. 2013, 43, 211–223. [Google Scholar] [CrossRef]
- Park, S.K.; Cho, M.K.; Park, H.-K.; Lee, K.H.; Lee, S.J.; Choi, S.H.; Ock, M.S.; Jeong, H.J.; Lee, M.H.; Yu, H.S. Macrophage Migration Inhibitory Factor Homologs of Anisakis simplex Suppress Th2 Response in Allergic Airway Inflammation Model via CD4+ CD25+ Foxp3+ T Cell Recruitment. J. Immunol. 2009, 182, 6907–6914. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.K.; Lee, C.H.; Yu, H.S. Amelioration of intestinal colitis by macrophage migration inhibitory factor isolated from intestinal parasites through Toll-like receptor 2. Parasite Immunol. 2011, 33, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Daniłowicz-Luebert, E.; Steinfelder, S.; Kühl, A.A.; Drozdenko, G.; Lucius, R.; Worm, M.; Hamelmann, E.; Hartmann, S. A nematode immunomodulator suppresses grass pollen-specific allergic responses by controlling excessive Th2 inflammation. Int. J. Parasitol. 2013, 43, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Cotton, J.A.; Bennuru, S.; Grote, A.; Harsha, B.; Tracey, A.; Beech, R.; Doyle, S.R.; Dunn, M.; Hotopp, J.C.D.; Holroyd, N.; et al. The genome of Onchocerca volvulus, agent of river blindness. Nat. Microbiol. 2016, 2, 16216. [Google Scholar] [CrossRef] [PubMed]
- Bennuru, S.; Cotton, J.A.; Ribeiro, J.M.C.; Grote, A.; Harsha, B.; Holroyd, N.; Mhashilkar, A.; Molina, D.M.; Randall, A.Z.; Shandling, A.D.; et al. Stage-specific transcriptome and proteome analyses of the filarial parasite Onchocerca volvulus and its Wolbachia endosymbiont. MBio 2016, 7, e02028-16. [Google Scholar] [CrossRef] [PubMed]
- McNulty, S.N.; Rosa, B.A.; Fischer, P.U.; Rumsey, J.M.; Erdman-Gilmore, P.; Curtis, K.C.; Specht, S.; Townsend, R.R.; Weil, G.J.; Mitreva, M. An integrated multi-omics approach to identify candidate antigens for serodiagnosis of human onchocerciasis. Mol. Cell. Proteom. 2015, 14, 3224–3233. [Google Scholar] [CrossRef]
- Cooper, P.J.; Guderian, R.H.; Proaño, R.; Taylor, D.W. Absence of cellular responses to a putative autoantigen in onchocercal chorioretinopathy: Cellular autoimmunity in onchocercal chorioretinopathy. Investig. Ophthalmol. Vis. Sci. 1996, 37, 405–412. [Google Scholar]
- Dowell, S.F.; Sejvar, J.J.; Riek, L.; Vandemaele, K.A.H.; Lamunu, M.; Kuesel, A.C.; Schmutzhard, E.; Matuja, W.; Bunga, S.; Foltz, J.; et al. Nodding syndrome. Emerg. Infect. Dis. 2013, 19, 1374–1384. [Google Scholar] [CrossRef]
- Choi, Y.J.; Tyagi, R.; McNulty, S.N.; Rosa, B.A.; Ozersky, P.; Martin, J.; Hallsworth-Pepin, K.; Unnasch, T.R.; Norice, C.T.; Nutman, T.B.; et al. Genomic diversity in Onchocerca volvulus and its Wolbachia endosymbiont. Nat. Microbiol. 2016, 2, 1–10. [Google Scholar] [CrossRef]
- Ford, L.; Guiliano, D.B.; Oksov, Y.; Debnath, A.K.; Liu, J.; Williams, S.A.; Blaxter, M.L.; Lustigman, S. Characterization of a novel filarial serine protease inhibitor, Ov-SPI-1, from Onchocerca volvulus, with potential multifunctional roles during development of the parasite. J. Biol. Chem. 2005, 280, 40845–40856. [Google Scholar] [CrossRef]
- Lustigman, S.; Zhang, J.; Liu, J.; Oksov, Y.; Hashmi, S. RNA interference targeting cathepsin L and Z-like cysteine proteases of Onchocerca volvulus confirmed their essential function during L3 molting. Mol. Biochem. Parasitol. 2004, 138, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Aboobaker, A.A.; Blaxter, M.L. Use of RNA interference to investigate gene function in the human filarial nematode parasite Brugia malayi. Mol. Biochem. Parasitol. 2003, 129, 41–51. [Google Scholar] [CrossRef]
- Singh, M.; Singh, P.K.; Misra-Bhattacharya, S. RNAi mediated silencing of ATPase RNA helicase gene in adult filarial parasite Brugia malayi impairs in vitro microfilaria release and adult parasite viability. J. Biotechnol. 2012, 157, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Gallup, J.M.; Day, T.A.; Bartholomay, L.C.; Kimber, M.J. Development of an in Vivo RNAi protocol to investigate gene function in the filarial nematode, Brugia malayi. PLoS Pathog. 2010, 6, e1001239. [Google Scholar] [CrossRef]
- Landmann, F.; Foster, J.M.; Slatko, B.E.; Sullivan, W. Efficient in vitro RNA interference and immunofluorescence-based phenotype analysis in a human parasitic nematode, Brugia malayi. Parasites Vectors 2012, 5, 16. [Google Scholar] [CrossRef]
- Verma, S.; Kashyap, S.S.; Robertson, A.P.; Martin, R.J. Functional genomics in Brugia malayi reveal diverse muscle nAChRs and differences between cholinergic anthelmintics. Proc. Natl. Acad. Sci. USA 2017, 114, 5539–5544. [Google Scholar] [CrossRef]
- Misra, S.; Gupta, J.; Misra-Bhattacharya, S. RNA interference mediated knockdown of Brugia malayi UDP-Galactopyranose mutase severely affects parasite viability, embryogenesis and in vivo development of infective larvae. Parasites Vectors 2017, 10, 34. [Google Scholar] [CrossRef][Green Version]
- Ngwewondo, A.; Wang, M.; Manfo, F.P.T.; Samje, M.; Ganin’s, J.N.; Ndi, E.; Andersen, R.J.; Cho-Ngwa, F. Filaricidal properties of Lantana camara and Tamarindus indica extracts, and Lantadene A from L. camara against Onchocerca ochengi and Loa loa. PLoS Negl. Trop. Dis. 2018, 12, e0006565. [Google Scholar] [CrossRef]
- Cho-Ngwa, F.; Monya, E.; Azantsa, B.K.; Manfo, F.P.T.; Babiaka, S.B.; Mbah, J.A.; Samje, M. Filaricidal activities on Onchocerca ochengi and Loa loa, toxicity and phytochemical screening of extracts of Tragia benthami and Piper umbellatum. BMC Complement. Altern. Med. 2016, 16, 326. [Google Scholar] [CrossRef]
- Ngwewondo, A.; Tsague Manfo, F.P.; Samje, M.; Monya, E.; Cho-Ngwa, F. Macro and microfilaricidal activities of extracts of Annona senegalensis and Milletia comosa against Onchocerca ochengi and Loa loa. Exp. Parasitol. 2019, 198, 71–78. [Google Scholar] [CrossRef]
- Samje, M.; Metuge, J.; Mbah, J.; Nguesson, B.; Cho-Ngwa, F. In vitro anti-Onchocerca ochengi activities of extracts and chromatographic fractions of Craterispermum laurinum and Morinda lucida. BMC Complement. Altern. Med. 2014, 14, 325. [Google Scholar] [CrossRef]
- Metuge, J.A.; Babiaka, S.B.; Mbah, J.A.; Ntie-Kang, F.; Ayimele, G.A.; Cho-Ngwa, F. Anti-onchocerca Metabolites from Cyperus articulatus: Isolation, In Vitro Activity and In Silico ‘Drug-Likeness’. Nat. Products Bioprospect. 2014, 4, 243–249. [Google Scholar] [CrossRef]
- Gang, S.S.; Castelletto, M.L.; Bryant, A.S.; Yang, E.; Mancuso, N.; Lopez, J.B.; Pellegrini, M.; Hallem, E.A. Targeted mutagenesis in a human-parasitic nematode. PLoS Pathog. 2017, 13, e1006675. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanhamme, L.; Souopgui, J.; Ghogomu, S.; Ngale Njume, F. The Functional Parasitic Worm Secretome: Mapping the Place of Onchocerca volvulus Excretory Secretory Products. Pathogens 2020, 9, 975. https://doi.org/10.3390/pathogens9110975
Vanhamme L, Souopgui J, Ghogomu S, Ngale Njume F. The Functional Parasitic Worm Secretome: Mapping the Place of Onchocerca volvulus Excretory Secretory Products. Pathogens. 2020; 9(11):975. https://doi.org/10.3390/pathogens9110975
Chicago/Turabian StyleVanhamme, Luc, Jacob Souopgui, Stephen Ghogomu, and Ferdinand Ngale Njume. 2020. "The Functional Parasitic Worm Secretome: Mapping the Place of Onchocerca volvulus Excretory Secretory Products" Pathogens 9, no. 11: 975. https://doi.org/10.3390/pathogens9110975
APA StyleVanhamme, L., Souopgui, J., Ghogomu, S., & Ngale Njume, F. (2020). The Functional Parasitic Worm Secretome: Mapping the Place of Onchocerca volvulus Excretory Secretory Products. Pathogens, 9(11), 975. https://doi.org/10.3390/pathogens9110975







