Electrolyzed Oxidizing Water Modulates the Immune Response in BALB/c Mice Experimentally Infected with Trypanosoma cruzi
Abstract
1. Introduction
2. Results
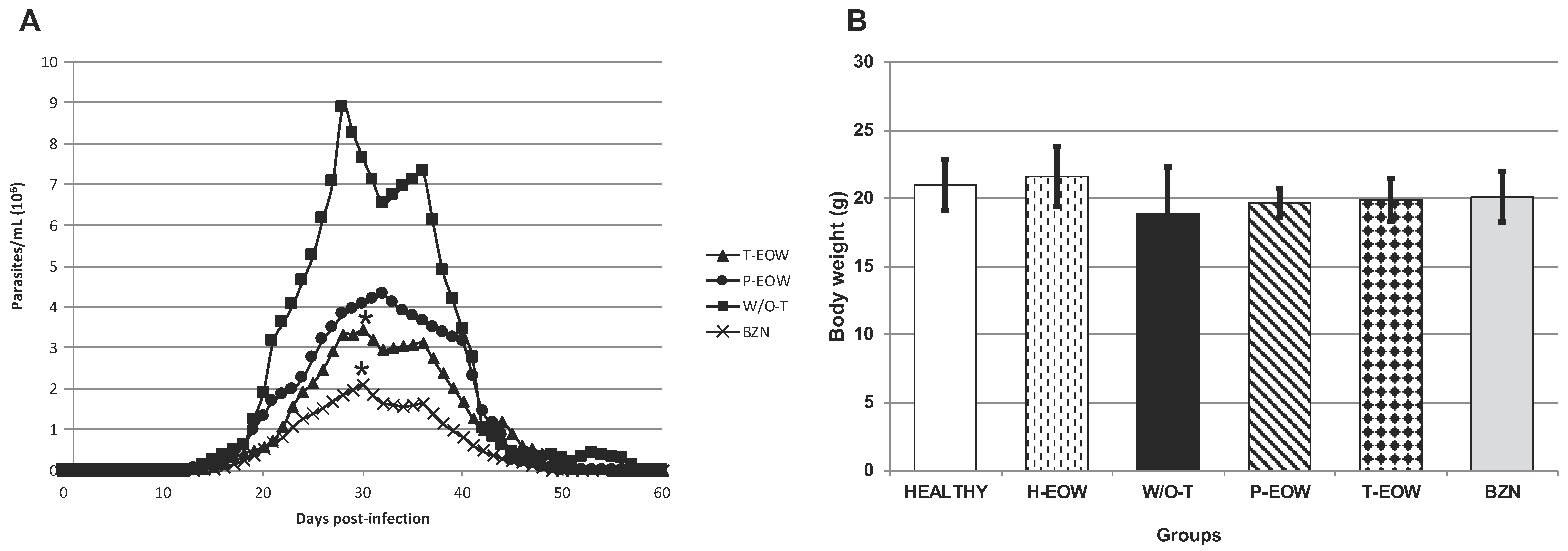
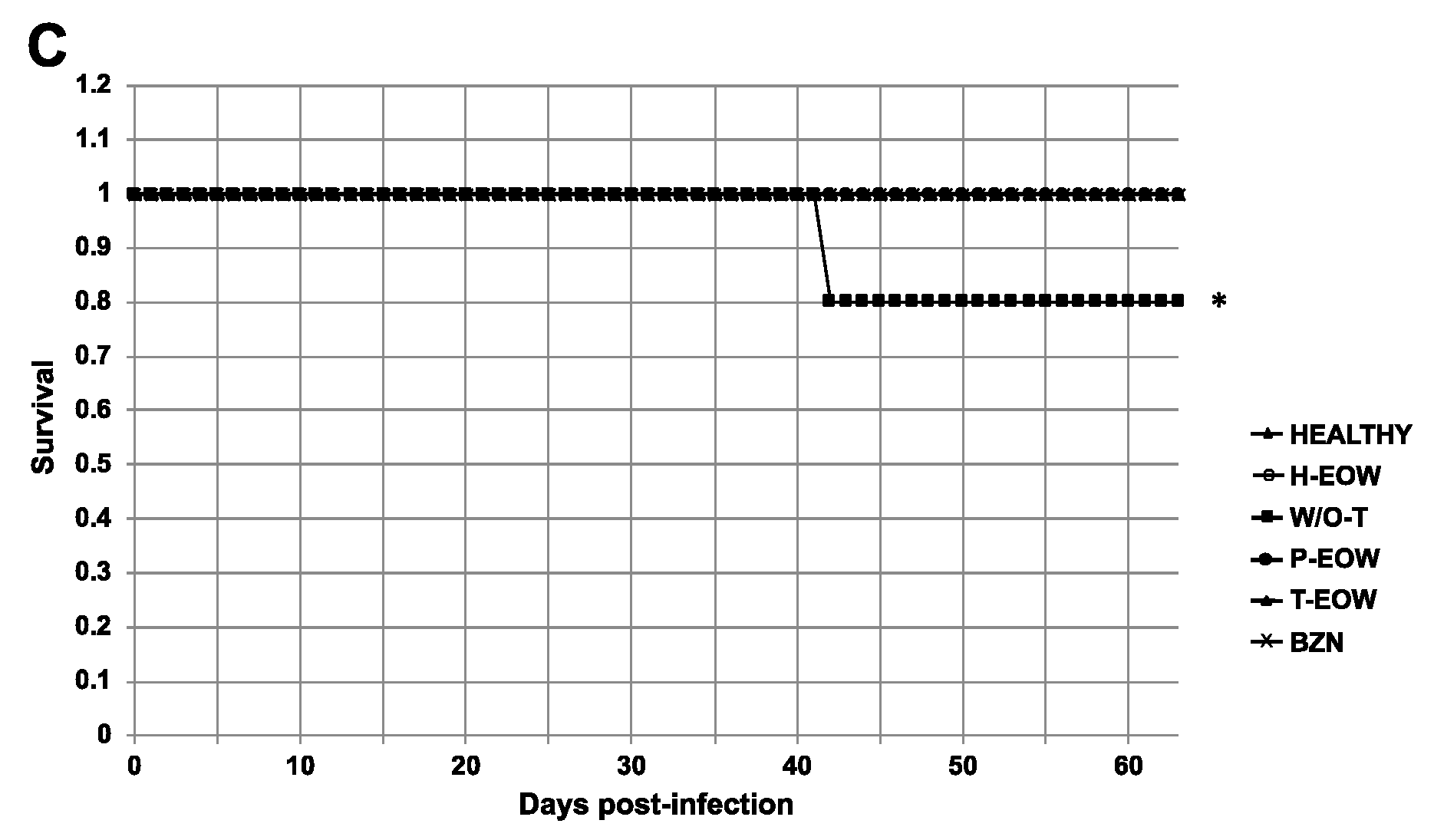
2.1. Beneficial Effects of EOW on Parasitaemia, Health Condition and Survival Rate
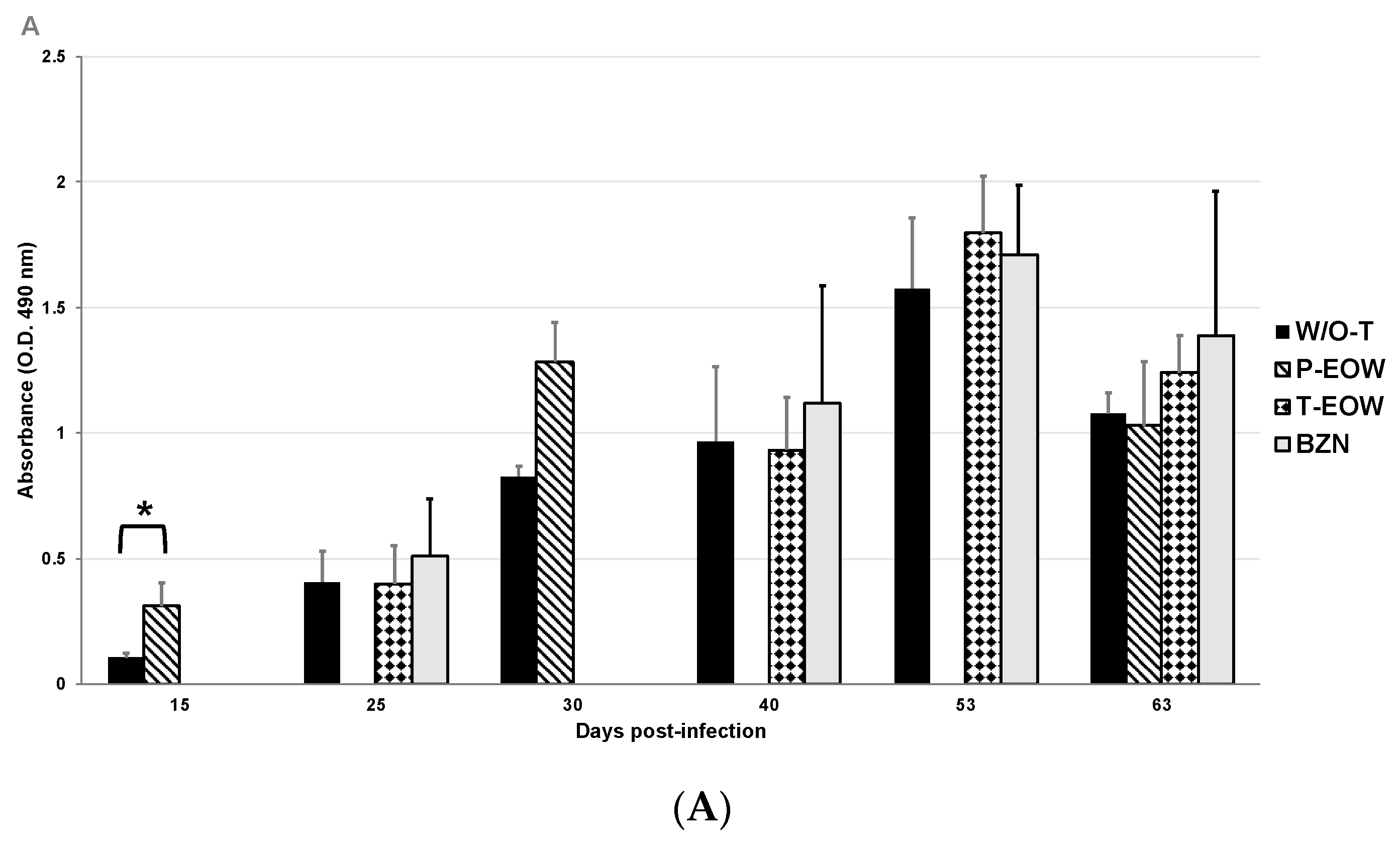
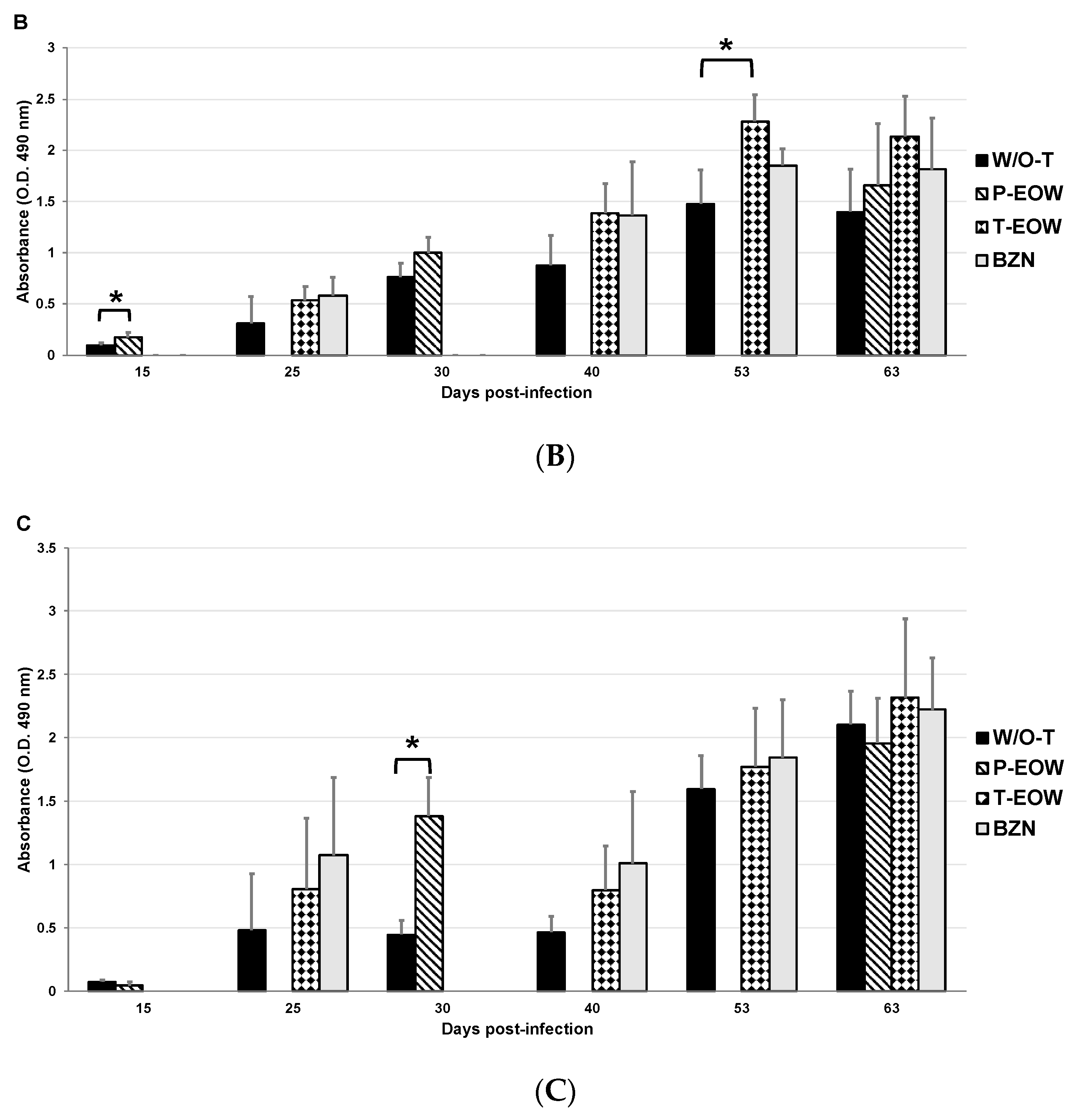
2.2. EOW Treatment Stimulates a Th2 Immune Response in the Early Acute Phase and Th1 in the Late One
2.3. Prophylactic EOW Administration Induces a 10-fold Increase of IFN-γ Production in the Acute Phase
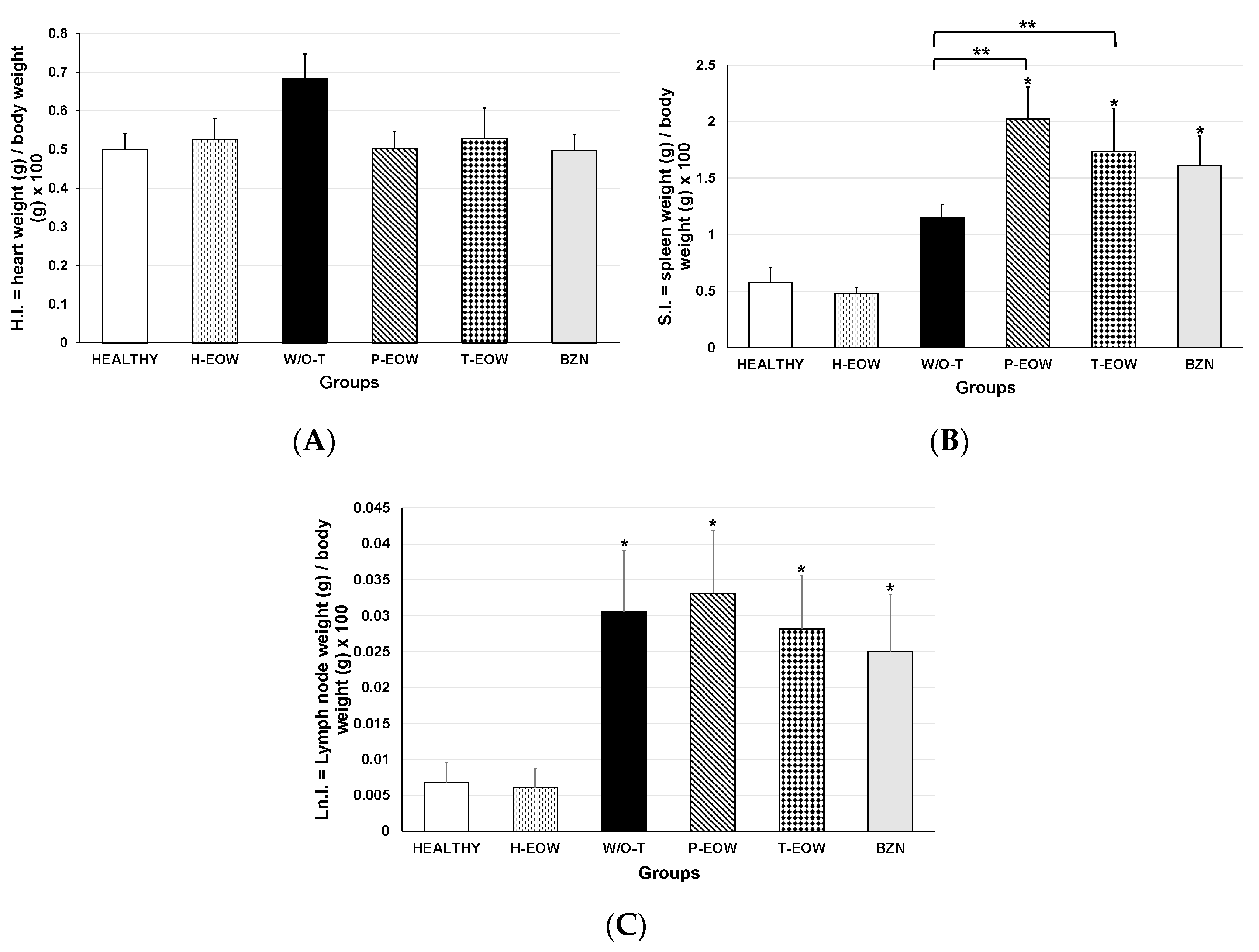
2.4. Effects of EOW on Cardiomegaly, Splenomegaly and Lymphadenopathy
2.5. EOW Administration Ameliorated the Inflammation Degree and Prevented Tissue Parasitism
3. Discussion
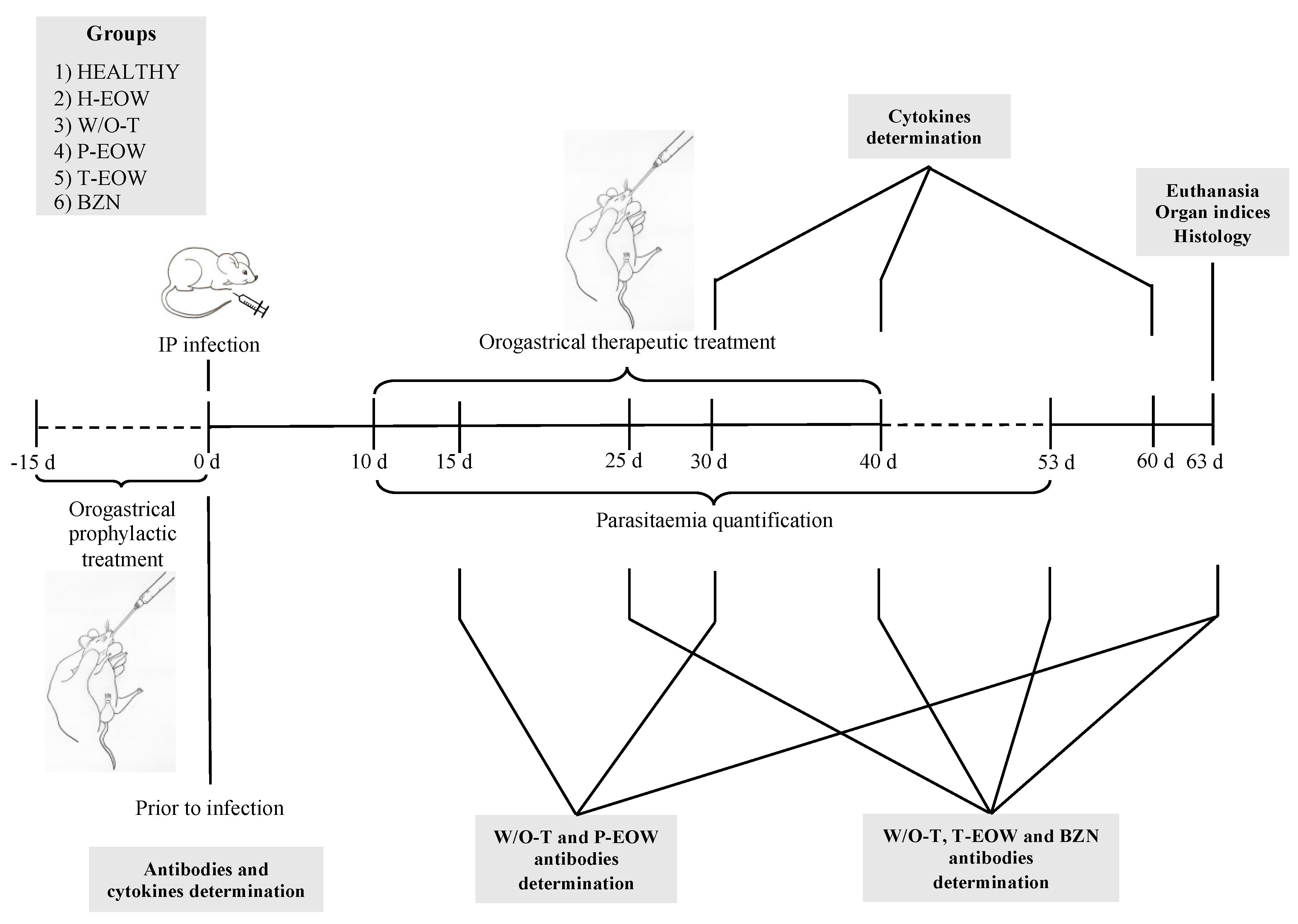
4. Materials and Methods
4.1. Animals and Experimental Groups
4.2. Infection
4.3. Parasitaemia and Survival
4.4. Prophylactic EOW and Therapeutic EOW Treatments by Orogastric Route
4.5. Collection, Handling and Storage of Blood Samples
4.6. Immunoglobulins Determination
4.7. Cytokine Determination
4.8. Splenomegaly, Cardiomegaly and Lymphadenopathy
4.9. Histology
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization (WHO). Chagas Disease (American Trypanosomiasis). 2018. Available online: http://www.who.int/mediacentre/factsheets/fs340/en/ (accessed on 15 June 2018).
- Ramsey, J.M.; Elizondo-Cano, M.; Sanchez-González, G.; Peña-Nieves, A.; Figueroa-Lara, A. Opportunity Cost for Early Treatment of Chagas Disease in Mexico. PLoS Negl. Trop. Dis. 2014, 8, e2776. [Google Scholar]
- Molina, I.; Salvador, F.; Sánchez-Montalvá, A. Actualización en enfermedad de Chagas. Enferm. Infecc. Microbiol. Clin. 2016, 34, 132–138. [Google Scholar]
- Salvador, F.; Treviño, B.; Sulleiro, E.; Sánchez-Montalva, A.; Cabezos, J.; Soriano, A.; Serre, N.; Gómez i Prat, J.; Pahissa, A.; Molina, I. Trypanosoma cruzi infection in a non-endemic country: Epidemiological and clinical profile. Clin. Microbiol. Infect. 2014, 20, 706–712. [Google Scholar]
- Carabarin-Lima, A.; González-Vázquez, M.C.; Rodríguez-Morales, O.; Baylón-Pacheco, L.; Rosales-Encina, J.L.; Reyes-López, P.A.; Arce-Fonseca, M. Chagas disease (American tripanosomiasis) in Mexico: An update. Acta Trop. 2013, 127, 126–135. [Google Scholar] [PubMed]
- Arce-Fonseca, M.; Ballinas-Verdugo, M.A.; Abreu Zenteno, E.R.; Suárez-Flores, D.; Carrillo-Sánchez, S.C.; Alejandre-Aguilar, R.; Rosales-Encina, J.L.; Reyes, P.A.; Rodríguez-Morales, O. Specific humoral and cellular immunity induced by Trypanosoma cruzi DNA immunization in a canine model. Vet. Res. 2013, 44, 1–9. [Google Scholar]
- Teixeira, A.R.L.; Hecht, M.M.; Guimaro, M.C.; Sousa, A.O.; Nitz, N. Pathogenesis of Chagas’ Disease: Parasite Persistence and Autoinmunity. Clin. Microbiol. Rev. 2011, 24, 592–630. [Google Scholar]
- Cardillo, F.; Teixeira, P.R.; Zuquim, A.P.R.; Mengel, J. Immunity and immune modulation in Trypanosoma cruzi infection. Pathog. Dis. 2015, 73, ftv082. [Google Scholar]
- Basso, B. Modulation of immune response in experimental Chagas disease. World J. Exp. Med. 2013, 3, 1–10. [Google Scholar] [PubMed]
- Geiger, A.; Bossard, G.; Sereno, D.; Pissarra, J.; Lemesre, J.-P.; Vincendeau, P.; Holzmuller, P. Escaping Deleterious Immune Response in Their Hosts: Lessons from Trypanosomatids. Front. Immunol. 2016, 7, 1–21. [Google Scholar]
- Cardoso, M.S.; Reis-Cunha, J.L.; Bartholomeu, D.C. Evasion of the Immune Response by Trypanosoma cruzi during Acute Infection. Front. Immunol. 2016, 6, 1–15. [Google Scholar]
- Rodríguez-Morales, O.; Monteón-Padilla, V.; Carrillo-Sánchez, S.C.; Rios-Castro, M.; Martínez-Cruz, M.; Carabarin-Lima, A.; Arce-Fonseca, M. Experimental Vaccines against Chagas Disease: A Journey through History. J. Immunol. Res. 2015, 2015, e489758. [Google Scholar]
- Bern, C. Chagas’ Disease. N. Engl. J. Med. 2015, 373, 456–466. [Google Scholar] [PubMed]
- Caldas, S.; Caldas, I.S.; Cecílio, A.B.; Diniz, L.D.; Talvani, A.; Ribeiro, I.; Bahia, M.T. Therapeutic responses to different anti-Trypanosoma cruzi drugs in experimental infection by benznidazole-resistant parasite stock. Parasitology 2014, 141, 1628–1637. [Google Scholar]
- Manne, J.M.; Snively, C.S.; Ramsey, J.M.; Salgado, M.O.; Bärnighausen, T.; Reich, M.R. Barriers to Treatment Access for Chagas Disease in Mexico. PLoS Negl. Trop. Dis. 2013, 7, e2488. [Google Scholar]
- Nachón-García, F.J.; Díaz-Tellez, J.; Nachón-García, M.G. Tolerancia peritoneal a la solución de alta selectividad iónica con pH neutro en ratas macho Wistar. Rev. Med. Univ. Veracruzana 2005, 5, 15–20. [Google Scholar]
- Cabello, G.C.; Rosete, O.D.P.; Manjarrez, Z.M.E. Efecto de una solución electrolizada de superoxidación con pH neutro sobre la infección del virus de influenza A en células MDCK. Rev. Inst. Nal. Enf. Resp. 2009, 22, 280–287. [Google Scholar]
- García, F.J.N.; Téllez, J.D.; Espinoza, V.R.; González, J.S.; García, M.N.; García, F.G.; García, J.S. Esterilización por inmersión. Estudio comparativo entre glutaraldehído al 2%, agua electrolizada superoxidada con pH neutro y solución electrolizada por selectividad iónica con pH neutro. Rev. Med. Univ. Veracruzana 2008, 8, 5–10. [Google Scholar]
- Rassi, A., Jr.; Rassi, A.; de Rezende, J.M. American trypanosomiasis (Chagas disease). Infect. Dis. Clin. 2012, 26, 275–291. [Google Scholar]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar]
- Jelicks, L.A.; Tanowitz, H.B. Advances in Imaging of Animal Models of Chagas Disease. Adv. Parasitol. 2011, 75, 193–208. [Google Scholar]
- Morita, C.; Nishida, T.; Ito, K. Biological toxicity of acid electrolyzed functional water: Effect of oral administration on mouse digestive tract and changes in body weight. Arch. Oral. Biol. 2011, 56, 359–366. [Google Scholar]
- Franceschelli, S.; Gatta, D.M.; Pesce, M.; Ferrone, A.; Patruno, A.; de Lutiis, M.A.; Grilli, A.; Felaco, M.; Croce, F.; Speranza, L. New Approach in Translational Medicine: Effects of Electrolyzed Reduced Water (ERW) on NF-κB/iNOS Pathway in U937 Cell Line under Altered Redox State. Int. J. Mol. Sci. 2016, 17, 1–14. [Google Scholar]
- Shirahata, S.; Kabayama, S.; Nakano, M.; Miura, T.; Kusumoto, K.; Gotoh, M.; Hayashi, H.; Otsuo, K.; Morisawa, S.; Katakura, Y. Electrolyzed-reduced water scavenges active oxygen species and protects DNA from oxidative damage. Biochem. Biophys. Res. Commun. 1997, 234, 269–274. [Google Scholar]
- Vorobjeva, N.V. Selective stimulation of the growth of anaerobic microflora in the human intestinal tract by electrolyzed reducing water. Med. Hypotheses 2005, 64, 543–546. [Google Scholar]
- Jin, D.; Ryu, S.H.; Kim, H.W.; Yang, E.J.; Lim, S.J.; Ryang, Y.S.; Chung, C.H.; Park, S.K.; Lee, K.J. Anti-diabetic effect of alkaline-reduced water on OLETF rats. Biosci. Biotechnol. Biochem. 2006, 70, 31–37. [Google Scholar] [PubMed]
- Kim, M.J.; Kim, H.K. Anti-diabetic effects of electrolyzed reduced water in streptozotocin-induced and genetic diabetic mice. Life Sci. 2006, 79, 2288–2292. [Google Scholar]
- Hanaoka, K.; Sun, D.; Lawrence, R.; Kamitani, Y.; Fernandes, G. The mechanism of the enhanced antioxidant effects against superoxide anion radicals of reduced water produced by electrolysis. Biophys. Chem. 2004, 107, 71–82. [Google Scholar]
- Watanabe, T. Effect of alkaline ionized water on reproduction in gestational and lactational rats. J. Toxicol. Sci. 1995, 20, 135–142. [Google Scholar]
- Huang, K.C.; Yang, C.C.; Lee, K.T.; Chien, C.T. Reduced hemodialysis-induced oxidative stress in end-stage renal disease patients by electrolyzed reduced water. Kidney Int. 2003, 64, 704–714. [Google Scholar]
- Weidman, J.; Holsworth, R.E., Jr.; Brossman, B.; Cho, D.J.; Cyr, J.S.; Fridman, G. Effect of electrolyzed high-pH alkaline water on blood viscosity in healthy adults. J. Int. Soc. Sports Nutr. 2016, 13, 1–13. [Google Scholar]
- Machado, F.S.; Dutra, W.O.; Esper, L.; Gollob, K.J.; Teixeira, M.M.; Factor, S.M.; Weiss, L.M.; Nagajyouthi, F.; Tanowithz, H.B.; Garg, N.J. Current Understanding of Immunity to Trypanosoma cruzi infection and Pathogenesis of Chagas Disease. Semin. Immunopathol. 2012, 34, 753–770. [Google Scholar] [PubMed]
- Gomes, J.A.S.; Bahia-Oliveira, L.M.G.; Rocha, M.O.C.; Martins-Filho, O.A.; Gazzinelli, G.; Correa-Oliveira, R. Evidence that Development of Severe Cardiomyopathy in Human Chagas’ Disease Is Due to a Th1-Specific Immune Response. Infect. Immun. 2003, 71, 1185–1193. [Google Scholar] [PubMed]
- Hoft, D.F.; Eickhoff, C.S. Type 1 immunity provides optimal protection against both mucosal and systemic Trypanosoma cruzi challenges. Infect. Immun. 2002, 70, 6715–6725. [Google Scholar] [PubMed]
- Paiva, C.N.; Castelo-Branco, M.T.; Lannes-Vieira, J.; Gattass, C.R. Trypanosoma cruzi: Protective response of vaccinated mice is mediated by CD8+ cells, prevents signs of polycolonal T lymphocyte activation, and allows restoration of a resting immune state after challenge. Exp. Parasitol. 1999, 91, 7–19. [Google Scholar] [PubMed]
- Lee, K.J.; Jin, D.; Chang, B.S.; Teng, Y.C.; Kim, D.H. The immunological effects of electrolyzed reduced water on the Echinostoma hortense infection in C57BL/6 mice. Biol. Pharm. Bull. 2009, 32, 456–462. [Google Scholar]
- You, H.S.; Fadriquela, A.; Sajo, M.E.J.; Bajgai, J.; Ara, J.; Kim, C.S.; Kim, S.K.; Oh, J.R.; Shim, K.Y.; Lim, H.K.; et al. Wound Healing Effect of Slightly Acidic Electrolyzed Water on Cutaneous Wounds in Hairless Mice via Immune-Redox Modulation. Biol. Pharm. Bull. 2017, 40, 1423–1431. [Google Scholar]
- Michailowsky, V.; Mursta, S.M.F.; Carvalho-Oliveira, L.; Pereira, M.E.; Ferreira, L.R.; Brener, Z.; Romanha, A.J.; Gazzinelli, R.T. Interleukin-12 Enhances In Vivo Parasiticidal Effect of Benznidazole during Acute Experimental Infection with a Naturally Drug-Resistant Strain of Trypanosoma cruzi. Antimicrob. Agents Chemother. 1998, 42, 2549–2556. [Google Scholar]
- Cutrullis, R.A.; Moscatelli, G.F.; Moroni, S.; Volta, B.J.; Cardoni, R.L.; Altcheh, J.M.; Corral, R.S.; Freilij, H.L.; Petray, P.B. Benznidazole Therapy Modulates Interferon-γ and M2 Muscarinic Receptor Autoantibody Responses in Trypanosoma cruzi-Infected Children. PLoS ONE 2011, 6, e27133. [Google Scholar]
- Espinoza, B.; Rico, T.; Sosa, S.; Oaxaca, E.; Vizcaino-Castillo, A.; Caballero, M.L.; Martínez, I. Mexican Trypanosoma cruzi T. cruzi I Strains with Different Degrees of Virulence Induce Diverse Humoral and Cellular Immune Responses in a Murine. J. Biomed. Biotechnol. 2010, 2010, 1–10. [Google Scholar]
- Guedes, P.M.M.; Veloso, V.M.; Afonso, L.C.C.; Caliari, M.V.; Carneiro, C.M.; Diniz, L.F.; Marques-da-Silva, E.A.; Caldas, I.S.; Do Valle, M.M.A.; Souza, S.M.; et al. Development of chronic cardiomyopathy in canine Chagas disease correlates with high IFN-γ, TNF-α, and low IL-10 production during the acute infection phase. Vet. Immunol. Immunopathol. 2009, 130, 43–52. [Google Scholar]
- Parada, H.; Carrasco, H.A.; Añez, N.; Fuenmayor, C.; Inglessis, I. Cardiac involvement is a constant finding in acute Chagas’ disease: A clinical, parasitological and histopathological study. Int. J. Cardiol. 1997, 60, 49–54. [Google Scholar] [PubMed]
- Gupta, S.; Garg, N.J. Prophylactic Efficacy of TcVac2 against Trypanosoma cruzi in Mice. PloS Negl. Trop. Dis. 2010, 4, e797. [Google Scholar]
- Arce-Fonseca, M.; Ramos-Ligonio, A.; López-Monteón, A.; Salgado-Jiménez, B.; Talamás-Rohana, P.; Rosales-Encina, J.L. A DNA Vaccine Encoding for TcSSP4 Induces Protection against Acute and Chronic Infection in Experimental Chagas Disease. Int. J. Biol. Sci. 2011, 7, 1230–1238. [Google Scholar]
- Henry, M.; Chambron, J. Physico-Chemical, Biological and Therapeutic Characteristics of Electrolyzed Reduced Alkaline Water (ERAW). Water 2013, 5, 2094–2115. [Google Scholar]
- Kapur, V.; Marwaha, A.K. Evaluation of effect and comparison of superoxidised solution (oxum) v/s povidone iodine (betadine). Indian J. Surg. 2011, 73, 48–53. [Google Scholar]
- Ezz-Eldin, Y.M.; Aboseif, A.A.; Khalaf, M.M. Potential anti-inflammatory and immunomodulatory effects of carvacrol against ovalbumin-induced asthma in rats. Life Sci. 2020, 242, 117222. [Google Scholar]
- Bombeiro, A.L.; Pereira, B.T.N.; Bonfanti, A.P.; Oliveira, A.L.R. Immunomodulation by dimethyl fumarate treatment improves mouse sciatic nerve regeneration. Brain Res. Bull. 2020, 160, 24–32. [Google Scholar]
- Hegazy-Hassan, W.; Zepeda-Escobar, J.A.; Ochoa-García, L.; Contreras-Ortiz, J.M.E.; Tenorio-Borroto, E.; Barbabosa-Pliego, A.; Aparicio-Burgos, J.E.; Oros-Pantoja, R.; Rivas-Santiago, B.; Díaz-Albiter, H.; et al. TcVac1 vaccine delivery by intradermal electroporation enhances vaccine induced immune protection against Trypanosoma cruzi infection in mice. Vaccine 2019, 37, 248–257. [Google Scholar]
- Pereira, I.R.; Vilar-Pereira, G.; Marques, V.; da Silva, A.A.; Caetano, B.; Moreira, O.C.; Machado, A.V.; Bruna-Romero, O.; Rodrigues, M.M.; Gazzinelli, R.T.; et al. A human type 5 adenovirus-based Trypanosoma cruzi therapeutic vaccine re-programs immune response and reverses chronic cardiomyopathy. PloS Pathog. 2015, 11, e1004594. [Google Scholar]
- da Silva, T.A.; Oliveira-Brito, P.K.M.; de Thomaz, S.M.O.; Roque-Barreira, M.C. Artin M: Purification and Evaluation of Biological Activities. Methods Mol. Biol. 2020, 2132, 349–358. [Google Scholar]
- Garber, J.C.; Wayne Barbee, R.; Bielitzki, J.T.; Clayton, L.A.; Donovan, J.C.; Hendriksen, C.F.M.; Kohn, D.F.; Lipman, N.S.; Locke, P.A.; Melcher, J.; et al. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Norma Oficial Mexicana NOM-062-ZOO-1999: Especificaciones para el Cuidado y uso de Animales de Laboratorio; Diario Oficial de la Federación: Mexico City, Mexico. 1999. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 16 December 2019).
- Urribarrí, R.S. Estudio comparativo de dos Métodos para valoración Cuantitativa de la Parasitemia por Tripanosomas. KASMERA 1974, 5, 103–110. [Google Scholar]
- Rodríguez-Morales, O.; Carrillo-Sánchez, S.C.; García-Mendoza, H.; Aranda-Fraustro, A.; Ballinas-Verdugo, M.A.; Alejandre-Aguilar, R.; Rosales-Encina, J.L.; Vallejo, M.; Arce-Fonseca, M. Effect of the Plasmid-DNA Vaccination on Macroscopic and Microscopic Damage Caused by the Experimental Chronic Trypanosoma cruzi Infection in the Canine Model. Biomed. Res. Int. 2013, 2013, e826570. [Google Scholar]
- Barr, S.C.; Schmidt, S.P.; Brown, C.C.; Klei, T.R. Pathologic features of dogs inoculated with North American Trypanosoma cruzi isolates. Am. J. Vet. Res. 1991, 52, 2033–2039. [Google Scholar] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Morales, O.; Cabrera-Mata, J.J.; Carrillo-Sánchez, S.d.C.; Gutiérrez-Ocejo, R.A.; Baylón-Pacheco, L.; Pérez-Reyes, O.L.; Rosales-Encina, J.L.; Aranda-Fraustro, A.; Hernández-García, S.; Arce-Fonseca, M. Electrolyzed Oxidizing Water Modulates the Immune Response in BALB/c Mice Experimentally Infected with Trypanosoma cruzi. Pathogens 2020, 9, 974. https://doi.org/10.3390/pathogens9110974
Rodríguez-Morales O, Cabrera-Mata JJ, Carrillo-Sánchez SdC, Gutiérrez-Ocejo RA, Baylón-Pacheco L, Pérez-Reyes OL, Rosales-Encina JL, Aranda-Fraustro A, Hernández-García S, Arce-Fonseca M. Electrolyzed Oxidizing Water Modulates the Immune Response in BALB/c Mice Experimentally Infected with Trypanosoma cruzi. Pathogens. 2020; 9(11):974. https://doi.org/10.3390/pathogens9110974
Chicago/Turabian StyleRodríguez-Morales, Olivia, Juan José Cabrera-Mata, Silvia del C. Carrillo-Sánchez, Rodolfo A. Gutiérrez-Ocejo, Lidia Baylón-Pacheco, Olga L. Pérez-Reyes, José Luis Rosales-Encina, Alberto Aranda-Fraustro, Sergio Hernández-García, and Minerva Arce-Fonseca. 2020. "Electrolyzed Oxidizing Water Modulates the Immune Response in BALB/c Mice Experimentally Infected with Trypanosoma cruzi" Pathogens 9, no. 11: 974. https://doi.org/10.3390/pathogens9110974
APA StyleRodríguez-Morales, O., Cabrera-Mata, J. J., Carrillo-Sánchez, S. d. C., Gutiérrez-Ocejo, R. A., Baylón-Pacheco, L., Pérez-Reyes, O. L., Rosales-Encina, J. L., Aranda-Fraustro, A., Hernández-García, S., & Arce-Fonseca, M. (2020). Electrolyzed Oxidizing Water Modulates the Immune Response in BALB/c Mice Experimentally Infected with Trypanosoma cruzi. Pathogens, 9(11), 974. https://doi.org/10.3390/pathogens9110974





