Revisiting Brucellosis in Small Ruminants of Western Border Areas in Pakistan
Abstract
1. Introduction
2. Materials and Methods
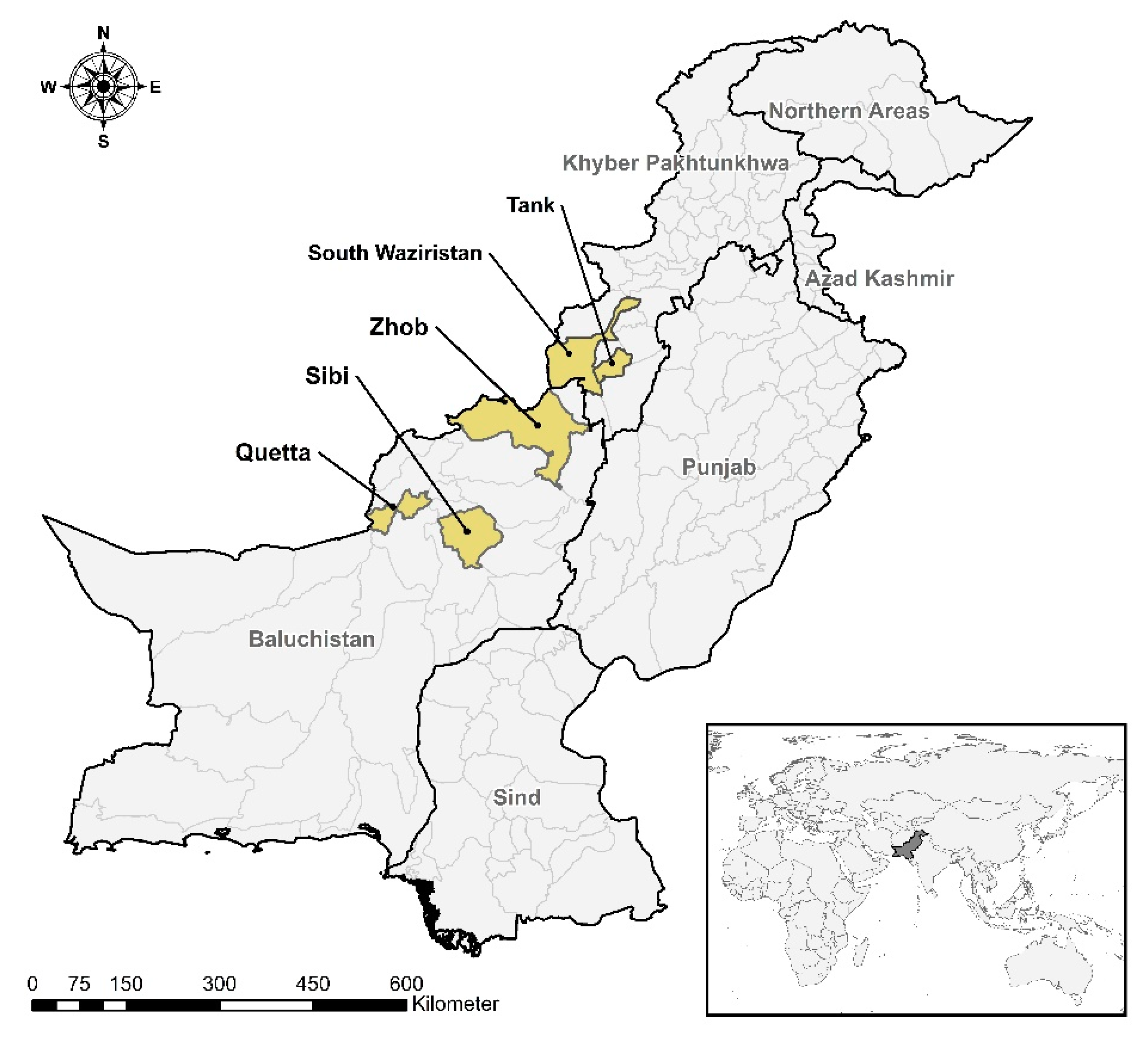
2.1. Study Area and Sampling Strategies
2.2. Statement of Ethics
2.3. Serological Analysis
2.4. Molecular Analyses
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diaz Aparicio, E. Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus. Rev. Sci. Tech. 2013, 32, 53–60. [Google Scholar] [CrossRef]
- Jamil, T.; Melzer, F.; Khan, I.; Iqbal, M.; Saqib, M.; Hammad Hussain, M.; Schwarz, S.; Neubauer, H. Serological and molecular investigation of Brucella species in dogs in Pakistan. Pathogens 2019, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.Z.; Akhtar, R.; Aslam, A.; Rashid, M.I.; Chaudhry, Z.I.; Manzoor, M.A.; Shah, B.A.; Ahmed, R.; Yasin, M. Evidence of Brucella abortus in non-preferred caprine and ovine hosts by real-time PCR assay. Pak. J. Zool. 2019, 51, 1187–1189. [Google Scholar] [CrossRef]
- Pérez-Sancho, M.; García-Seco, T.; Domínguez, L.; Álvarez, J. Control of animal brucellosis, The most effective tool to prevent human brucellosis. Updates Brucell. 2015, 10, 61222. [Google Scholar]
- Dadar, M.; Fakhri, Y.; Shahali, Y.; Mousavi Khaneghah, A. Contamination of milk and dairy products by Brucella species: A global systematic review and meta-analysis. Food Res. Int. 2020, 128, 108775. [Google Scholar] [CrossRef]
- Ali, S.; Ali, Q.; Neubauer, H.; Melzer, F.; Elschner, M.; Khan, I.; Abatih, E.; Ullah, N.; Irfan, M.; Akhter, S. Seroprevalence and risk factors associated with brucellosis as a professional hazard in Pakistan. J. Foodborne Pathog. Dis. 2013, 10, 500. [Google Scholar] [CrossRef]
- Traxler, R.M.; Guerra, M.A.; Morrow, M.G.; Haupt, T.; Morrison, J.; Saah, J.R.; Smith, C.G.; Williams, C.; Fleischauer, A.T.; Lee, P.A.; et al. Review of brucellosis cases from laboratory exposures in the United States in 2008 to 2011 and improved strategies for disease prevention. J. Clin. Microbiol. 2013, 51, 3132–3136. [Google Scholar] [CrossRef]
- Aragón-Aranda, B.; de Miguel, M.J.; Martínez-Gómez, E.; Zúñiga-Ripa, A.; Salvador-Bescós, M.; Moriyón, I.; Iriarte, M.; Muñoz, P.M.; Conde-Álvarez, R. Rev1 wbdR tagged vaccines against Brucella ovis. Vet. Res. 2019, 50, 95. [Google Scholar] [CrossRef]
- Jamil, T.; Melzer, F.; Njeru, J.; El-Adawy, H.; Neubauer, H.; Wareth, G. Brucella abortus: Current research and future trends. Curr. Clin. Microbiol. Rep. 2017, 4, 1–10. [Google Scholar] [CrossRef]
- Al-Sherida, Y.; El-Gohary, A.H.; Mohamed, A.; El-Diasty, M.; Wareth, G.; Neubauer, H.; Abdelkhalek, A. Sheep Brucellosis in Kuwait: A Large-Scale serosurvey, identification of Brucella species and zoonotic significance. Vet. Sci. 2020, 7, 132. [Google Scholar] [CrossRef]
- Farooq, U.; Fatima, Z.; Afzal, M.; Anwar, Z.; Jahangir, M. Sero-prevalence of brucellosis in bovines at farms under different management conditions. Br. J. Dairy Sci. 2011, 2, 35–39. [Google Scholar]
- Anonymous. Livestock Population; Pakistan Bureau of Statistics: Islamabad, Pakistan, 2006.
- Fazl-e-Haider, S. Issues in Balochistan’s livestock development. DAWN, 24 March 2008. [Google Scholar]
- Khan, A.Q.; Haleem, S.K.; Shafiq, M.; Khan, N.A.; Rahman, S.U. Seropositivity of brucellosis in human and livestock in tribal-Kurram agency of Pakistan indicates cross circulation. Thai J. Vet. Med. 2017, 47, 349–355. [Google Scholar]
- Cannon, R.; Roe, R. Livestock Disease Surveys. A Field Manual for Veterinarians; Bureau of Resource Science, Department of Primary Industry: Canberra, Australia, 1982.
- OIE. Brucellosis (Brucella abortus, B. melitensis and B. suis) (infection with B. abortus, B. melitensis and B. suis). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; World Organization for Animal Health: Paris, France, 2018; pp. 355–398. [Google Scholar]
- Probert, W.S.; Schrader, K.N.; Khuong, N.Y.; Bystrom, S.L.; Graves, M.H. Real-time multiplex PCR assay for detection of Brucella spp., B. abortus, and B. melitensis. J. Clin. Microbiol. 2004, 42, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Gwida, M.M.; El-Gohary, A.H.; Melzer, F.; Tomaso, H.; Rosler, U.; Wernery, U.; Wernery, R.; Elschner, M.C.; Khan, I.; Eickhoff, M.; et al. Comparison of diagnostic tests for the detection of Brucella spp. in camel sera. BMC Res. Notes 2011, 4, 525. [Google Scholar] [CrossRef]
- Nielsen, K. Diagnosis of brucellosis by serology. Vet. Microbiol. 2002, 90, 447–459. [Google Scholar] [CrossRef]
- Ducrotoy, M.J.; Munoz, P.M.; Conde-Alvarez, R.; Blasco, J.M.; Moriyon, I. A systematic review of current immunological tests for the diagnosis of cattle brucellosis. Prev. Vet. Med. 2018, 151, 57–72. [Google Scholar] [CrossRef]
- Gusi, A.M.; Bertu, W.J.; de Miguel, M.J.; Dieste-Perez, L.; Smits, H.L.; Ocholi, R.A.; Blasco, J.M.; Moriyon, I.; Munoz, P.M. Comparative performance of lateral flow immunochromatography, iELISA and Rose Bengal tests for the diagnosis of cattle, sheep, goat and swine brucellosis. PLoS Negl. Trop. Dis. 2019, 13, e0007509. [Google Scholar] [CrossRef]
- Navarro, E.; Casao, M.A.; Solera, J. Diagnosis of human brucellosis using PCR. Expert Rev. Mol. Diagn. 2004, 4, 115–123. [Google Scholar] [CrossRef]
- Dal, T.; Kara, S.S.; Cikman, A.; Balkan, C.E.; Acikgoz, Z.C.; Zeybek, H.; Uslu, H.; Durmaz, R. Comparison of multiplex real-time polymerase chain reaction with serological tests and culture for diagnosing human brucellosis. J. Infect. Public Health 2019, 12, 337–342. [Google Scholar] [CrossRef]
- Batool, B.T.; Tareen, A.; Ahmed, S.S.; Ejaz, H.; Kakar, M.A.; Rehman, S.A.; Saeed, M.; Ahmad, Z.; Tariq, M.M.; Awan, M.A.; et al. Prevalence of zoonotic tuberculosis and brucellosis in animals of Quetta and Pishin districts, Balochistan. Pak. J. Zool. 2017, 49, 363–365. [Google Scholar] [CrossRef]
- Shafee, M.; Ahmed, N.; Razzaq, A.; ur Rehman, F.; Yakoob, M. Seroprevalence of brucellosis in small ruminants in Turbat (Kech), Balochistan. Lasbela Univ. J. Sci. Technol. 2016, V, 86–89. [Google Scholar]
- Achakzai, M. A Study on the Seroprevalence of Brucellosis in Caprine in Balochistan; Sindh Agriculture University: Tandojam, Pakistan, 2008. [Google Scholar]
- Naeem, K.; Kamram, J.; Ullah, A. Seroprevalence and risk factors associated with Crimean-Congo haemorrhagic fever and brucellosis in people and livestock in Baluchistan and Khyber Pakhtunkhwa Provinces, Pakistan. In Proceedings of the South Asia Regional One Health Symposium, Paro, Bhutan, 2–6 December 2013. [Google Scholar]
- Ali, S.; Akbar, A.; Shafee, M.; Tahira, B.; Muhammed, A.; Ullah, N. Sero-epidemiological study of brucellosis in small ruminants and associated human beings in district Quetta, Balochistan. Pure Appl. Biol. 2017, 6, 797–804. [Google Scholar] [CrossRef]
- El-Diasty, M.; Wareth, G.; Melzer, F.; Mustafa, S.; Sprague, L.D.; Neubauer, H. Isolation of Brucella abortus and Brucella melitensis from seronegative cows is a serious impediment in brucellosis control. Vet. Sci. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Islam, M.A.; Khatun, M.M.; Saha, S.; Basir, M.S.; Hasan, M.M. Molecular detection of Brucella spp. from milk of seronegative cows from some selected area in Bangladesh. J. Pathog. 2018, 2018, 9378976. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Rind, R.; Adil, M.; Khan, M.; Farmanullah, S.A.; Waheed, U.; Salim, M. Seroprevalence of brucellosis in sheep and humans in district Kohat, Pakistan. Adv. Anim. Vet. Sci. 2014, 2, 516–523. [Google Scholar] [CrossRef]
- Khan, A.; Shafee, M.; Khan, N.; Rahman, A.; Rafiullah; Ali, I.; Khan, I.; Rahman, S.U. Incidence of brucellosis in aborted animals and occupationally exposed veterinary professionals of Bannu, Khyber Pakhtunkhwa, Pakistan. Thai J. Vet. Med. 2018, 48, 47–54. [Google Scholar]
- Khan, S.I.; Muti-ur-Rehmana, S.M.; Khanc, A.; Khand, A.; Shakeeld, M.; Shah, M.A.; Naseerd, Z. Patho-epidemiology of bovine brucellosis in Aza-kheli buffalo in Pakistan. Veterinaria 2017, 4, 21–26. [Google Scholar]
- Khan, M.Z.; Usman, T.; Sadique, U.; Qureshi, M.S.; Hassan, M.F.; Shahid, M.; Khan, A. Molecular characterization of Brucella abortus and Brucella melitensis in cattle and humans at the north west of Pakistan. Pak. Vet. J. 2017, 37, 427–430. [Google Scholar]
- Mahmood, R.; Ali, T.; Waheed, U.; Asif, M.; Khan, Q.M. Application of serum based PCR and fluorescence polarization assay for diagnosis of brucellosis among people occupationally at risk to disease. Int. J. Agric. Biol. 2016, 18, 311–318. [Google Scholar] [CrossRef]
- Ullah, Q.; Jamil, T.; Melzer, F.; Saqib, M.; Hussain, M.H.; Aslam, M.A.; Jamil, H.; Iqbal, M.A.; Tahir, U.; Ullah, S.; et al. Epidemiology and Associated Risk Factors for Brucellosis in Small Ruminants Kept at Institutional Livestock Farms in Punjab, Pakistan. Front. Vet. Sci. 2020, 7, 526. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.T.; Khan, A.; Rizvi, F.; Hussain, I. Sero-prevalence of brucellosis in food animals in the Punjab, Pakistan. Pak. Vet. J. 2014, 34, 454–458. [Google Scholar]
- Iqbal, Z.; Jamil, H.; Qureshi, Z.I.; Saqib, M.; Lodhi, L.A.; Waqas, M.S.; Safdar, M. Seroprevalence of ovine brucellosis by modified Rose Bengal test and ELISA in Southern Punjab, Pakistan. Pak. Vet. J. 2013, 1, 2–5. [Google Scholar]
- Din, A.M.; Khan, S.A.; Ahmad, I.; Rind, R.; Hussain, T.; Shahid, M.; Ahmed, S. A study on the seroprevalence of brucellosis in human and goat populations of district Bhimber, Azad Jammu and Kashmir. J. Anim. Plant Sci. 2013, 23, 113–118. [Google Scholar]
- Ghani, M.; Siraj, M.; Zeb, A.; Naeem, M. Sero-epidemiological study of brucellosis among goats and sheep at Pshawar district. Asian-Australas. J. Anim. Sci. 1995, 8, 489–494. [Google Scholar] [CrossRef]
- Arshad, M.; Munir, M.; Iqbal, K.; Abbas, R.; Rasool, M.; Khalil, N. Sero-prevalence of brucellosis in goats from public and private livestock farms in Pakistan. Online J. Vet. Res. 2011, 15, 297–304. [Google Scholar]
- Addis, S.A.; Desalegn, A.Y. Comparative sero-epidemiological study of brucellosis in sheep under smallholder farming and governmental breeding ranches of central and north east Ethiopia. J. Vet. Med. 2018, 4. [Google Scholar] [CrossRef]
- Coelho, A.C.; Díez, J.G.; Coelho, A.M. Risk factors for Brucella spp. in domestic and wild animals. In Updates on Brucellosis; Intech Open: London, UK, 2015. [Google Scholar] [CrossRef]
- Al-Majali, A.M.; Majok, A.A.; Amarin, N.M.; Al-Rawashdeh, O.F. Prevalence of, and risk factors for, brucellosis in Awassi sheep in southern Jordan. Small Rumin. Res 2007, 73, 300–303. [Google Scholar] [CrossRef]
- Mikolon, A.B.; Gardner, I.A.; Hietala, S.K.; de Anda, J.H.; Chamizo Pestana, E.; Hennager, S.G.; Edmondson, A.J. Evaluation of North American antibody detection tests for diagnosis of brucellosis in goats. J. Clin. Microbiol. 1998, 36, 1716–1722. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Abbas, S.A.; Khan, M. Health complications associated with self-medication. J. Phys. Fit. Treat. Sports 2018, 1, 555566. [Google Scholar] [CrossRef]
- Khurshid, A.; Hassan, M.; Alam, M.M.; Aamir, U.B.; Rehman, L.; Sharif, S.; Shaukat, S.; Rana, M.S.; Angez, M.; Zaidi, S.S.Z. CCHF virus variants in Pakistan and Afghanistan: Emerging diversity and epidemiology. J. Clin. Virol. 2015, 67, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.M.; Masood, I.; Yousaf, M.; Saleem, H.; Ye, D.; Fang, Y. Pattern of medication selling and self-medication practices: A study from Punjab, Pakistan. PLoS ONE 2018, 13, e0194240. [Google Scholar] [CrossRef] [PubMed]
- Zia, U.-E. Analysis of milk marketing chain-Pakistan. Ital. J. Anim. Sci. 2007, 6, 1384–1386. [Google Scholar] [CrossRef]
- Zia, U.-E.; Mahmood, T.; Ali, M. Dairy Development in Pakistan; FAO: Rome, Italy, 2011. [Google Scholar]
- Ali, S.; Nawaz, Z.; Akhtar, A.; Aslam, R.; Zahoor, M.A.; Ashraf, M. Epidemiological investigation of human brucellosis in Pakistan. Jundishapur J. Microbiol. 2018, 11, e61764. [Google Scholar] [CrossRef]
- Ali, S.; Akhter, S.; Neubauer, H.; Scherag, A.; Kesselmeier, M.; Melzer, F.; Khan, I.; El-Adawy, H.; Azam, A.; Qadeer, S.; et al. Brucellosis in pregnant women from Pakistan: An observational study. BMC Infect. Dis. 2016, 2, 468. [Google Scholar] [CrossRef]
- Mukhtar, F. Brucellosis in a high risk occupational group: Seroprevalence and analysis of risk factors. J. Pak. Med. Assoc. 2010, 60, 1031–1034. [Google Scholar]
- Ahmad, H.; Ali, I.; Ahmad, T.; Tufail, M.; Ahmad, K.; Murtaza, B.N. Prevalence of brucellosis in human population of district Swat, Pakistan. Pak. J. Zool. 2017, 49, 415–418. [Google Scholar] [CrossRef]
- Yousefi-Nooraie, R.; Mortaz-Hejri, S.; Mehrani, M.; Sadeghipour, P. Antibiotics for treating human brucellosis. Cochrane Database Syst. Rev. 2012, 10, CD007179. [Google Scholar] [CrossRef]
- Rasool, H.; Avais, M.; Rabbani, M.; Ahmed, R.; Muhammad, K.; Ali, M. An innovative approach to treat brucellosis in Buffaloes. Biomed. Lett. 2018, 4, 19–23. [Google Scholar]
- Nawaz, G.; Malik, M.N.; Mushtaq, M.H.; Ahmad, F.M.; Shah, A.A.; Iqbal, F.; Ali, S.W.; Fatima, Z.; Khan, A. Surveillance of brucellosis in livestock in rural communities of Punjab. J. Agric. Res. 2016, 29, 392–398. [Google Scholar]
| Variable | OR | 95% CI | df | * p-Value |
|---|---|---|---|---|
| Flock size | <0.001 | |||
| Small (≤20) (n = 41) | 0.88 | 0.19–3.95 | 1 | 1.00 |
| Medium (21–100) (n = 14) | Ref | |||
| Large (>100) (n = 51) | ||||
| Presence of cattle | ||||
| Yes (n = 77) | ─ | ─ | 1 | 0.282 |
| No (n = 29) | ||||
| Presence of Dogs | ||||
| Yes (n = 97) | ─ | ─ | 1 | 0.593 |
| No (n = 9) | ||||
| Presence of equine | ||||
| Yes (n = 22) | ─ | ─ | 1 | 0.115 |
| No (n = 84) | ||||
| Quarantine of newly introduced animals | ||||
| Yes (n = 7) | ─ | ─ | 1 | 1.00 |
| No (n = 99) | ||||
| Farm animal contact with other farm animals | ||||
| Yes (n = 82) | ─ | ─ | 1 | 0.265 |
| No (n = 24) | ||||
| Grazing area used by animals of other farms | ||||
| Yes (n = 83) | 0.28 | 0.07–1.02 | 1 | 0.058 |
| No (n = 23) | Ref | |||
| Farm hygiene | ||||
| Satisfactory (n = 39) | 3.44 | 0.94–12.64 | 1 | 0.094 |
| Poor (n = 67) | Ref | |||
| Proper disposal of aborted fetus | ||||
| Yes (n = 14) | ─ | ─ | 1 | 0.637 |
| No (n = 92) |
| RBPT | iELISA | Total | |
|---|---|---|---|
| Negative | Positive | ||
| Negative | 1803 | 18 | 1803 |
| Positive | 0 | 18 | 18 |
| Total | 1803 | 18 | 1821 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, T.; Kasi, K.K.; Melzer, F.; Saqib, M.; Ullah, Q.; Khan, M.R.; Dadar, M.; Tayyab, M.H.; Schwarz, S.; Neubauer, H. Revisiting Brucellosis in Small Ruminants of Western Border Areas in Pakistan. Pathogens 2020, 9, 929. https://doi.org/10.3390/pathogens9110929
Jamil T, Kasi KK, Melzer F, Saqib M, Ullah Q, Khan MR, Dadar M, Tayyab MH, Schwarz S, Neubauer H. Revisiting Brucellosis in Small Ruminants of Western Border Areas in Pakistan. Pathogens. 2020; 9(11):929. https://doi.org/10.3390/pathogens9110929
Chicago/Turabian StyleJamil, Tariq, Khushal Khan Kasi, Falk Melzer, Muhammad Saqib, Qudrat Ullah, Muhammad Roidar Khan, Maryam Dadar, Muhammad Haleem Tayyab, Stefan Schwarz, and Heinrich Neubauer. 2020. "Revisiting Brucellosis in Small Ruminants of Western Border Areas in Pakistan" Pathogens 9, no. 11: 929. https://doi.org/10.3390/pathogens9110929
APA StyleJamil, T., Kasi, K. K., Melzer, F., Saqib, M., Ullah, Q., Khan, M. R., Dadar, M., Tayyab, M. H., Schwarz, S., & Neubauer, H. (2020). Revisiting Brucellosis in Small Ruminants of Western Border Areas in Pakistan. Pathogens, 9(11), 929. https://doi.org/10.3390/pathogens9110929








