Application and Optimization of relE as a Negative Selection Marker for Making Definitive Genetic Constructs in Uropathogenic Escherichia coli
Abstract
:1. Introduction
2. Results and Discussion
2.1. Creation of an Isogenic Series of Definitive (Unmarked, Scarless) FimH Mutants
2.2. Definitive FimH Constructs Have No Artifactual Phenotypes
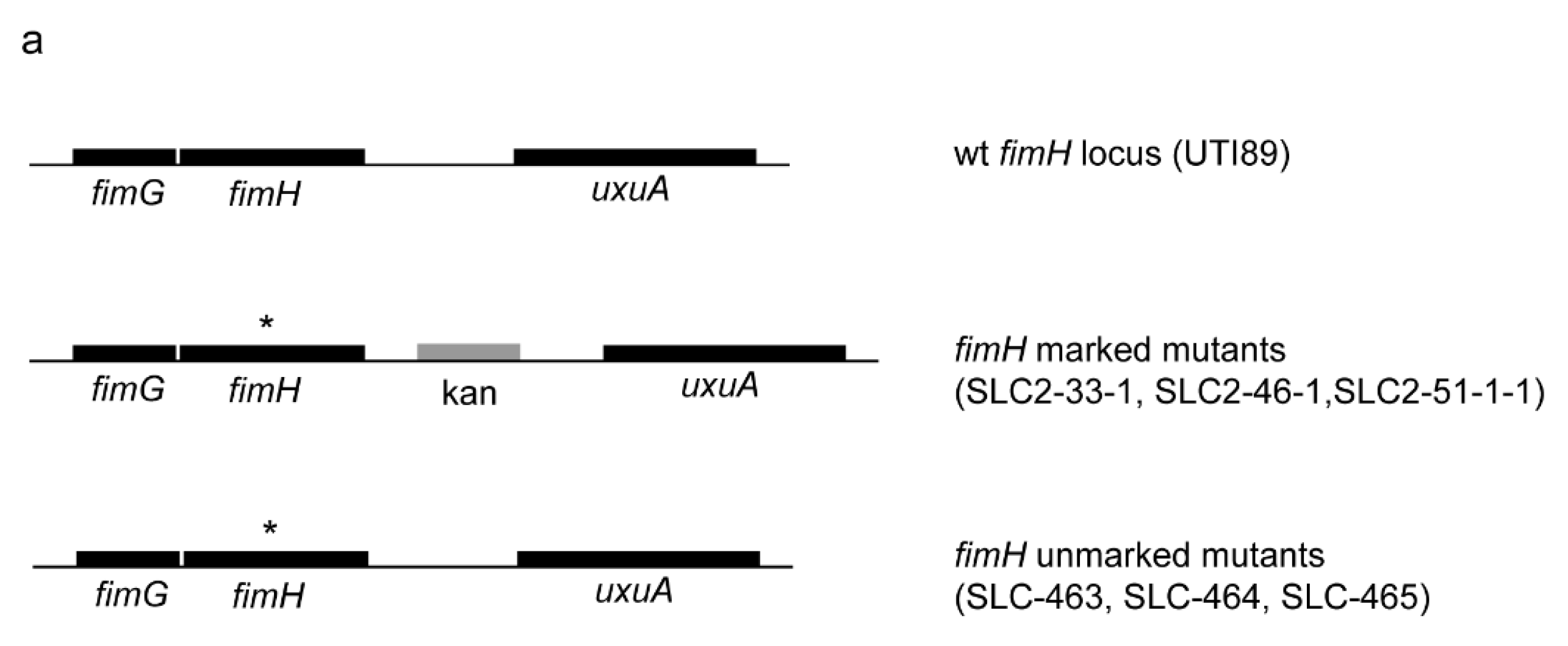
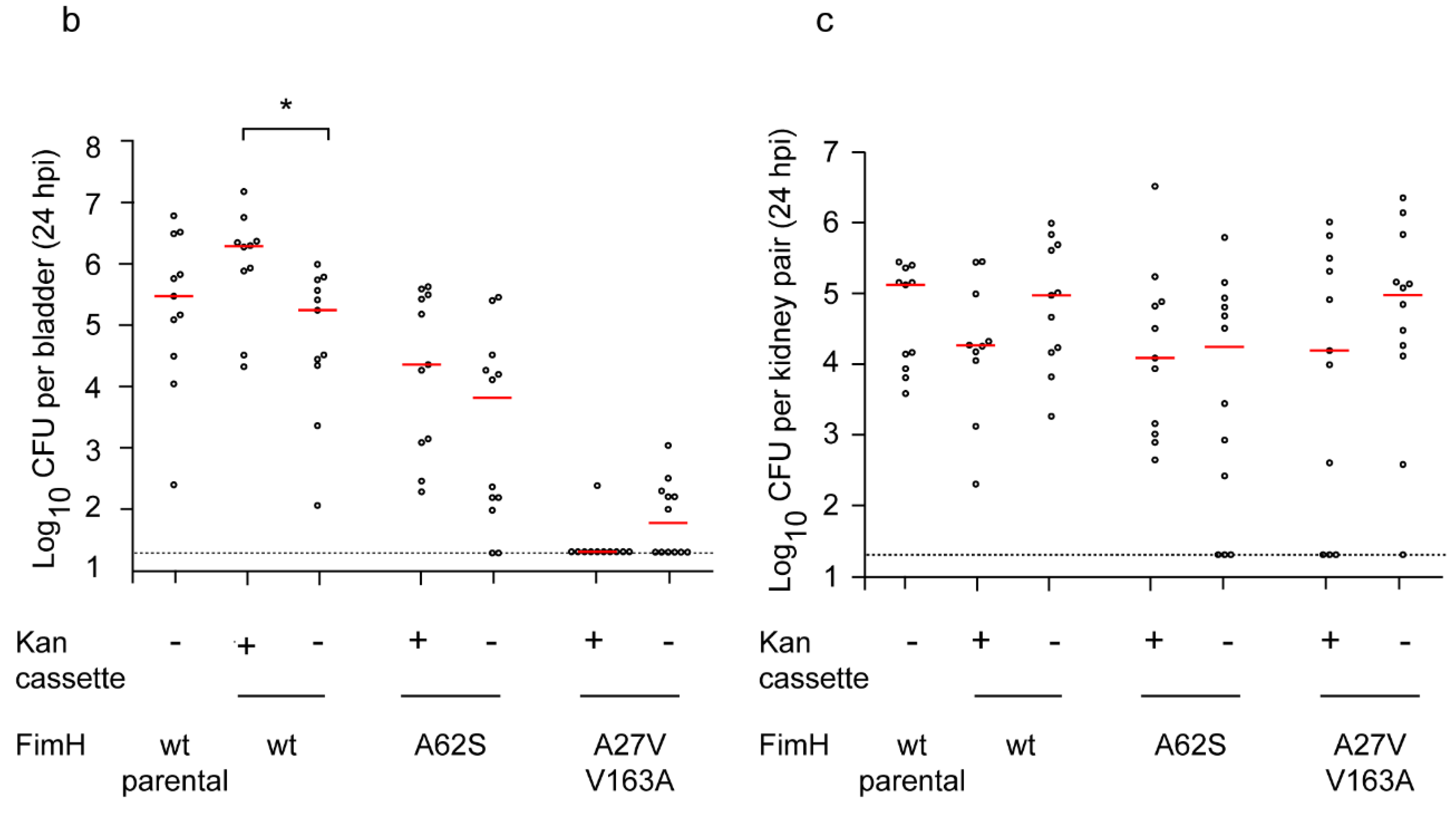
2.3. Redesigned Priming Sites Eliminate the Major Class of False Positive Colonies during Negative Selection-Mediated Recombineering
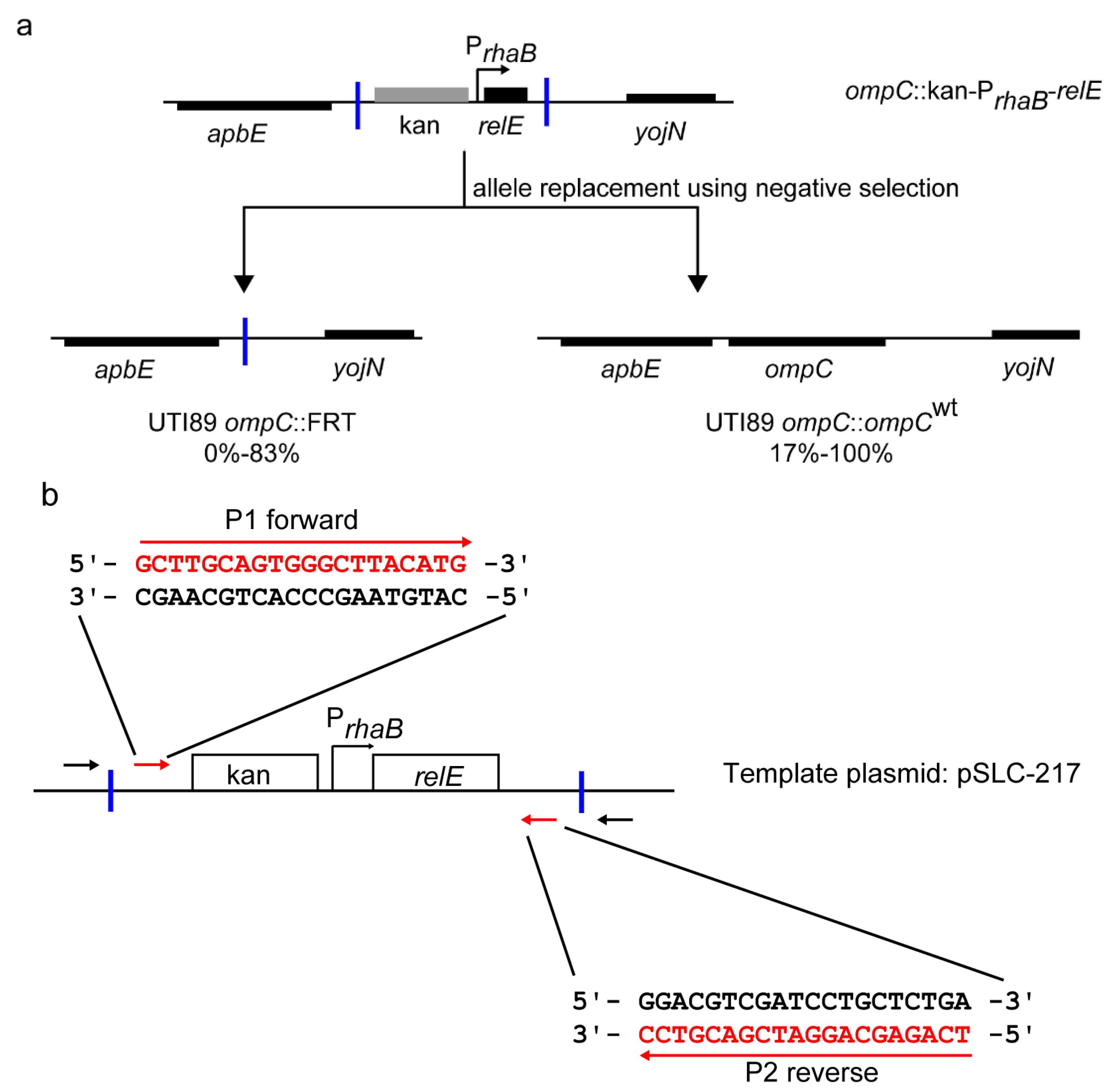
3. Experimental Section
3.1. Bacterial Strains and Plasmids
3.2. Chromosomal FimH Mutations and Recombineering
3.3. PCR and Sequencing
3.4. Hemagglutination Assay
3.5. In Vivo Mouse Infections
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.A.; Hooton, T.M.; Stamm, W.E.; Humphrey, P.A.; Hultgren, S.J. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007, 4, e329. [Google Scholar] [CrossRef] [PubMed]
- Robino, L.; Scavone, P.; Araujo, L.; Algorta, G.; Zunino, P.; Vignoli, R. Detection of intracellular bacterial communities in a child with Escherichia coli recurrent urinary tract infections. Pathog. Dis. 2013, 68, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Robino, L.; Scavone, P.; Araujo, L.; Algorta, G.; Zunino, P.; Pirez, M.C.; Vignoli, R. Intracellular bacteria in the pathogenesis of Escherichia coli urinary tract infection in children. Clin. Infect. Dis. 2014, 59, e158–e164. [Google Scholar] [CrossRef] [PubMed]
- Schilling, J.D.; Lorenz, R.G.; Hultgren, S.J. Effect of Trimethoprim-Sulfamethoxazole on Recurrent Bacteriuria and Bacterial Persistence in Mice Infected with Uropathogenic Escherichia coli. Infect. Immun. 2002, 70, 7042–7049. [Google Scholar] [CrossRef] [PubMed]
- Connell, I.; Agace, W.; Klemm, P.; Schembri, M.; Mărild, S.; Svanborg, C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA 1996, 93, 9827–9832. [Google Scholar] [CrossRef] [PubMed]
- Hultgren, S.J.; Porter, T.N.; Schaeffer, A.J.; Duncan, J.L. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect. Immun. 1985, 50, 370–377. [Google Scholar] [PubMed]
- Wu, X.R.; Sun, T.T.A.; Medina, J.J. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: Relation to urinary tract infections. Proc. Natl. Acad. Sci. USA 1996, 93, 9630–9635. [Google Scholar] [CrossRef] [PubMed]
- Eto, D.S.; Jones, T.A.; Sundsbak, J.L.; Mulvey, M.A. Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog. 2007, 3, e100. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.S.; Bouckaert, J.; Hung, D.; Pinkner, J.; Widberg, C.; DeFusco, A.; Auguste, C.G.; Strouse, R.; Langermann, S.; Waksman, G.; et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 2002, 44, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, R.; Gao, Z.; Thankavel, K.; van der Merwe, P.A.; Abraham, S.N. The mast cell tumor necrosis factor alpha response to FimH-expressing Escherichia coli is mediated by the glycosylphosphatidylinositol-anchored molecule CD48. Proc. Natl. Acad. Sci. USA 1999, 96, 8110–8115. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.J.; Mulvey, M.A.; Schilling, J.D.; Pinkner, J.S.; Hultgren, S.J. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000, 19, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.J.; Mann, E.L.; Cohen, M.S.; Ofek, I.; Sharon, N.; Abraham, S.N. The distinct binding specificities exhibited by enterobacterial type 1 fimbriae are determined by their fimbrial shafts. J. Biol. Chem. 2005, 280, 37707–37716. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Hung, C.S.; Pinkner, J.S.; Walker, J.N.; Cusumano, C.K.; Li, Z.; Bouckaert, J.; Gordon, J.I.; Hultgren, S.J. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc. Natl. Acad. Sci. USA 2009, 106, 22439–22444. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.J.; Seed, P.C.; Hultgren, S.J. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell. Microbiol. 2007, 9, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.J.; Kalas, V.; Pinkner, J.S.; Chen, S.L.; Spaulding, C.N.; Dodson, K.W.; Hultgren, S.J. Positively selected FimH residues enhance virulence during urinary tract infection by altering FimH conformation. Proc. Natl. Acad. Sci. USA 2013, 110, 15530–15537. [Google Scholar] [CrossRef] [PubMed]
- Sokurenko, E.V.; Chesnokova, V.; Dykhuizen, D.E.; Ofek, I.; Wu, X.R.; Krogfelt, K.A.; Struve, C.; Schembri, M.A.; Hasty, D.L. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc. Natl. Acad. Sci. USA 1998, 95, 8922–8926. [Google Scholar] [CrossRef] [PubMed]
- Hannan, T.J.; Mysorekar, I.U.; Chen, S.L.; Walker, J.N.; Jones, J.M.; Pinkner, J.S.; Hultgren, S.J.; Seed, P.C. LeuX tRNA-dependent and -independent mechanisms of Escherichia coli pathogenesis in acute cystitis. Mol. Microbiol. 2008, 67, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Khetrapal, V.; Mehershahi, K.; Rafee, S.; Chen, S.; Lim, C.L.; Chen, S.L. A set of powerful negative selection systems for unmodified Enterobacteriaceae. Nucl. Acids Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.S.; Dodson, K.W.; Hultgren, S.J. A murine model of urinary tract infection. Nat. Protoc. 2009, 4, 1230–1243. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khetrapal, V.; Mehershahi, K.S.; Chen, S.; Chen, S.L. Application and Optimization of relE as a Negative Selection Marker for Making Definitive Genetic Constructs in Uropathogenic Escherichia coli. Pathogens 2016, 5, 9. https://doi.org/10.3390/pathogens5010009
Khetrapal V, Mehershahi KS, Chen S, Chen SL. Application and Optimization of relE as a Negative Selection Marker for Making Definitive Genetic Constructs in Uropathogenic Escherichia coli. Pathogens. 2016; 5(1):9. https://doi.org/10.3390/pathogens5010009
Chicago/Turabian StyleKhetrapal, Varnica, Kurosh S. Mehershahi, Siyi Chen, and Swaine L. Chen. 2016. "Application and Optimization of relE as a Negative Selection Marker for Making Definitive Genetic Constructs in Uropathogenic Escherichia coli" Pathogens 5, no. 1: 9. https://doi.org/10.3390/pathogens5010009
APA StyleKhetrapal, V., Mehershahi, K. S., Chen, S., & Chen, S. L. (2016). Application and Optimization of relE as a Negative Selection Marker for Making Definitive Genetic Constructs in Uropathogenic Escherichia coli. Pathogens, 5(1), 9. https://doi.org/10.3390/pathogens5010009





