In Vitro Activity of Cefepime/AAI101 and Comparators against Cefepime Non-susceptible Enterobacteriaceae
Abstract
:1. Introduction
2. Results
| Antimicrobial Agent | % Susceptible | MIC50 (mg/L) | MIC90 (mg/L) | Range (mg/L) |
|---|---|---|---|---|
| Cefepime/AAI101 | ND | 0.125 | 64 | ≤0.06 to >64 |
| Cefepime | 0 * | >64 | >64 | 4 to >64 |
| Ceftazidime | 5 | >64 | >64 | 0.5 to >64 |
| Ciprofloxacin | 9 | >16 | >16 | ≤0.015 to >16 |
| Ertapenem | 51 | 0.5 | 64 | ≤0.015 to >16 |
| Meropenem | 70 | 0.125 | 64 | ≤0.06 to >64 |
| Piperacillin/tazobactam | 43 | 32 | >256 | 1 to >256 |
| Tobramycin | 40 | 16 | 64 | ≤0.06 to >64 |
| Antimicrobial Agent | MIC50 (mg/L) | MIC90 (mg/L) | Range (mg/L) |
|---|---|---|---|
| Cefepime Resistant (n = 208) | 0.125 | 64 | ≤0.06 to >64 |
| Ciprofloxacin Resistant (n = 210) | 0.125 | 64 | ≤0.06 to >64 |
| Ertapenem Resistant (n = 110) | 1 | >64 | ≤0.06 to >64 |
| Piperacillin/tazobactam Resistant (n = 127) | 1 | >64 | ≤0.06 to >64 |
| Multi-drug Resistant (n = 200) | 0.125 | 64 | ≤0.06 to >64 |
| ESBL Producers (n = 150) | 0.125 | 0.5 | ≤0.06 to >64 |
| Carbapenemase Producers (n = 50) | 32 | >256 | ≤0.06 to >64 |
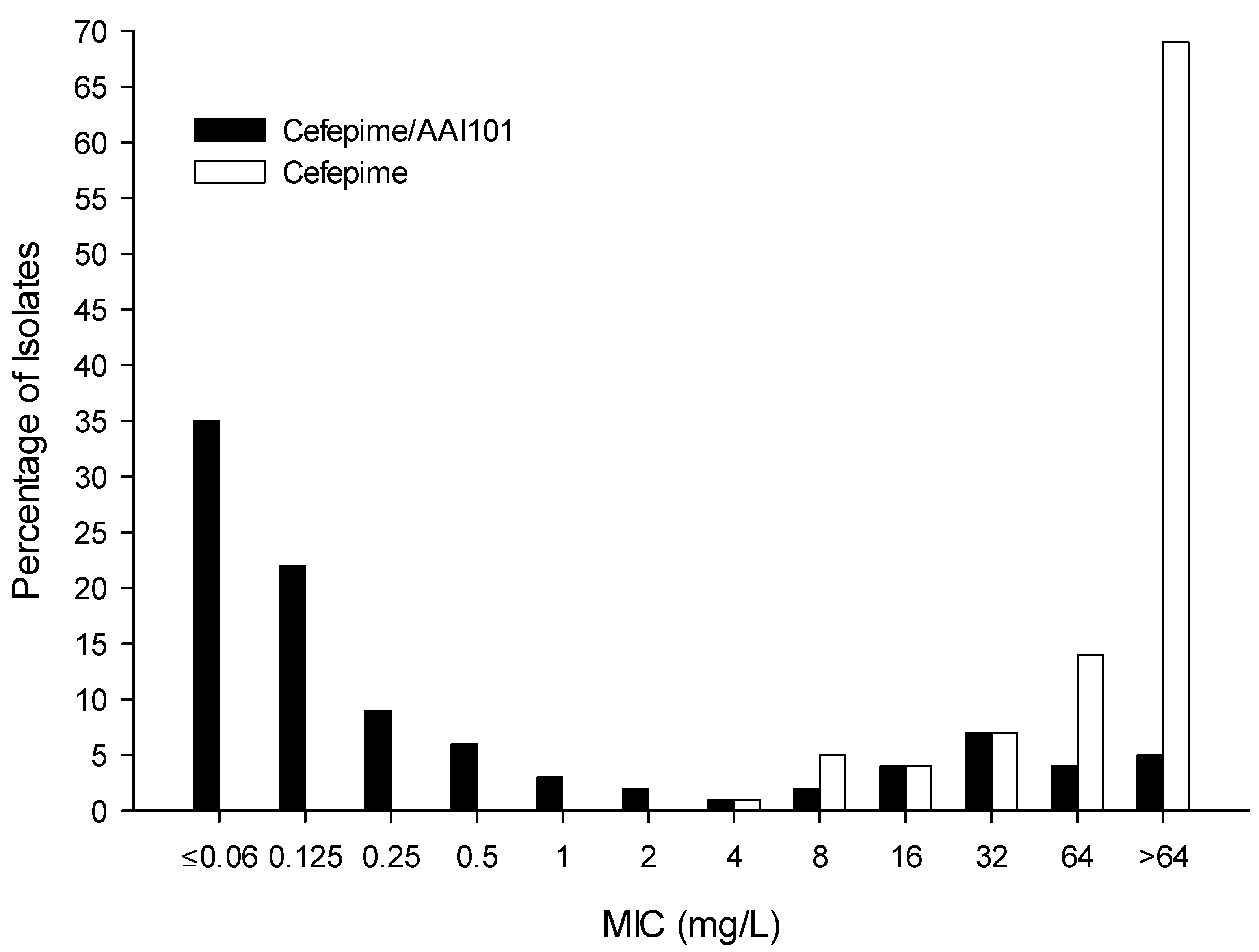
3. Discussion
4. Experimental Section
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupta, N.; Limbago, B.M.; Patel, J.B.; Kallen, A.J. Carbapenem-resistant Enterobacteriaceae: Epidemiology and prevention. Clin. Infect. Dis. 2011, 53, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P., III; Clark, N.M.; Zhanel, G.G. Evolution of antimicrobial resistance among Enterobacteriaceae (focus on extended spectrum beta-lactamases and carbapenemases). Expert Opin. Pharmacother. 2013, 14, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Chaudhry, A.; Adkin, R.; Woodford, N.; Benedict, N.; Pypstra, R.; Shapiro, S. In-vitro activity of diverse β-lactam/aai101 combinations vs. Multidrug-resistant gram-negative clinical strains. In Proceedings of the 24th European Congress of Clinical Microbiology and Infectious Diseases, Barcelona, Spain, 10–13 May 2014. Abstract eP452.
- Nordmann, P.; Girlich, D.; Benedict, N.; Pypstra, R.; Shapiro, S. Characterization of b-lactamase inhibition by aai101. In Proceedings of the 24th European Congress of Clinical Microbiology and Infectious Diseases, Barcelona, Spain, 10–13 May 2014. Abstract eP451.
- Clinical Laboratory Standard Institute. Performance Standards for Antimicrobial Suceptibility Testing; Twenty-Fourth Informational Supplement; M100-S24; CLSI publication: Wayne, PA, USA, 2014. [Google Scholar]
- Crandon, J.L.; Nicolau, D.P. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging gram-negative organisms, including metallo-beta-lactamase producers. Antimicrob. Agents Chemother. 2014, 57, 3299–3306. [Google Scholar] [CrossRef] [PubMed]
- Crandon, J.L.; Schuck, V.J.; Banevicius, M.A.; Beaudoin, M.E.; Nichols, W.W.; Tanudra, M.A.; Nicolau, D.P. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 56, 6137–6146. [Google Scholar] [CrossRef] [PubMed]
- VanScoy, B.; Mendes, R.E.; Nicasio, A.M.; Castanheira, M.; Bulik, C.C.; Okusanya, O.O.; Bhavnani, S.M.; Forrest, A.; Jones, R.N.; Friedrich, L.V.; et al. Pharmacokinetics-pharmacodynamics of tazobactam in combination with ceftolozane in an in vitro infection model. Antimicrob. Agents Chemother. 2013, 57, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Dudley, M.N. Combination beta-lactam and beta-lactamase-inhibitor therapy: Pharmacokinetic and pharmacodynamic considerations. Am. J. Health Syst. Pharm. 1995, 52, S23–S28. [Google Scholar] [PubMed]
- Sutherland, C.; Nicolau, D. Susceptibility profile of commonly utilized parenteral antimicrobials against E. coli, K. pneumoniae and P. aeruginosa from us hospitals. In Proceedings of the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, USA, 5–9 September 2014. Abstract C119.
- Sader, H.S.; Farrell, D.J.; Flamm, R.K.; Jones, R.N. Antimicrobial susceptibility of gram-negative organisms isolated from patients hospitalised with pneumonia in us and european hospitals: Results from the sentry antimicrobial surveillance program, 2009–2012. Int. J. Antimicrob. Agents 2014, 43, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Crandon, J.L.; Nicolau, D.P. In vivo activities of simulated human doses of cefepime and cefepime-aai101 against multidrug-resistant gram-negative Enterobacteriaceae. Antimicrob. Agents Chemother. 2015, 59, 2688–2694. [Google Scholar] [CrossRef] [PubMed]
- Dortet, L.; Poirel, L.; Nordmann, P. Rapid identification of carbapenemase types in Enterobacteriaceae and Pseudomonas spp. By using a biochemical test. Antimicrob. Agents Chemother. 2012, 56, 6437–6440. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crandon, J.L.; Nicolau, D.P. In Vitro Activity of Cefepime/AAI101 and Comparators against Cefepime Non-susceptible Enterobacteriaceae. Pathogens 2015, 4, 620-625. https://doi.org/10.3390/pathogens4030620
Crandon JL, Nicolau DP. In Vitro Activity of Cefepime/AAI101 and Comparators against Cefepime Non-susceptible Enterobacteriaceae. Pathogens. 2015; 4(3):620-625. https://doi.org/10.3390/pathogens4030620
Chicago/Turabian StyleCrandon, Jared L., and David P. Nicolau. 2015. "In Vitro Activity of Cefepime/AAI101 and Comparators against Cefepime Non-susceptible Enterobacteriaceae" Pathogens 4, no. 3: 620-625. https://doi.org/10.3390/pathogens4030620
APA StyleCrandon, J. L., & Nicolau, D. P. (2015). In Vitro Activity of Cefepime/AAI101 and Comparators against Cefepime Non-susceptible Enterobacteriaceae. Pathogens, 4(3), 620-625. https://doi.org/10.3390/pathogens4030620





