Trypanocidal Activity of Dual Redox-Active Quinones: Trypanosoma cruzi Mitochondrion as a Target Organelle In Vitro and Anti-Inflammatory Properties In Vivo
Abstract
1. Introduction
2. Materials and Methods
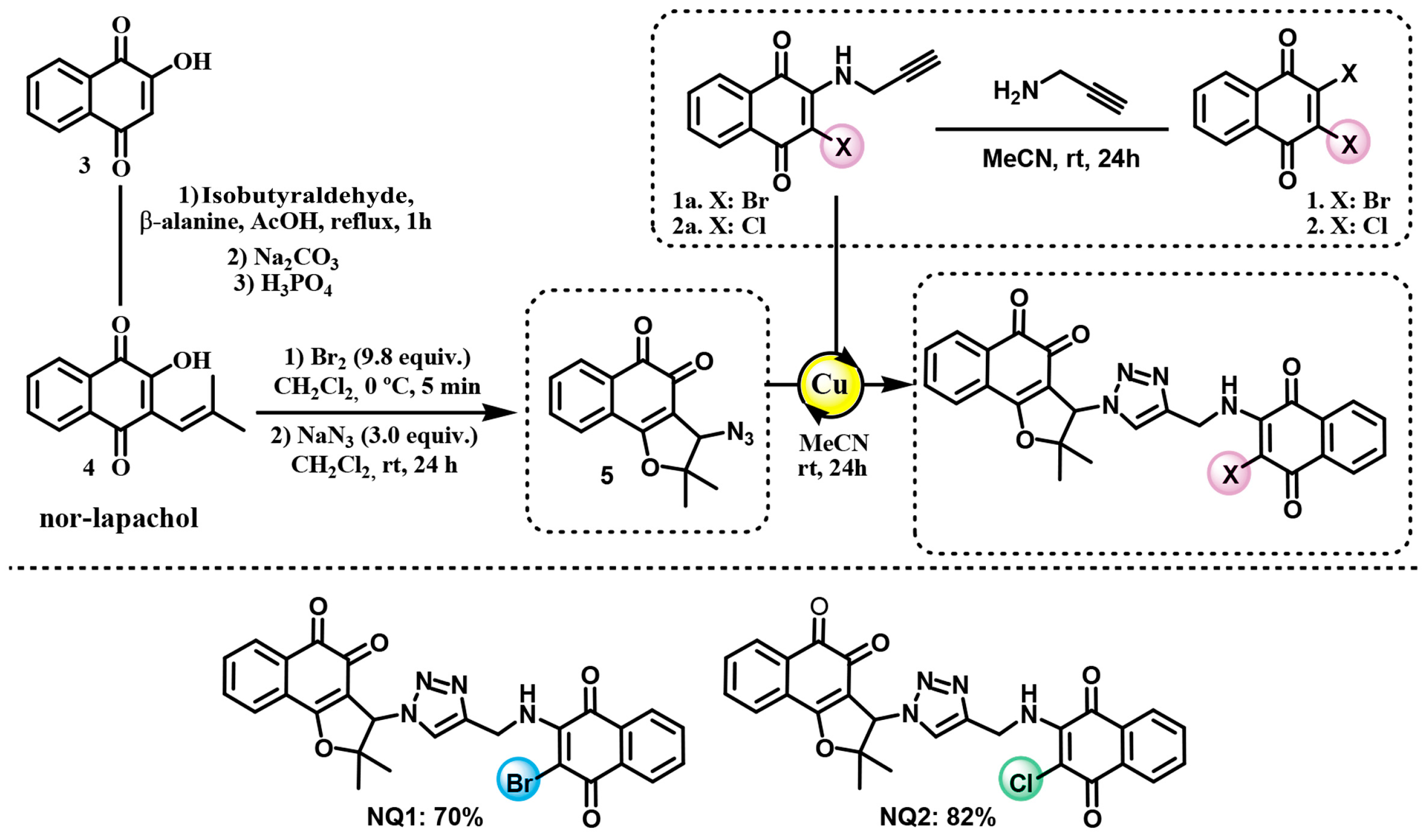
2.1. Naphthoquinones
2.2. Parasites and Host Cells
2.3. Trypanocidal Assays
2.4. Direct Effects of the Combination of Benznidazole and Naphthoquinones on Trypomastigotes
2.5. Ultrastructural Analysis
2.6. Flow Cytometry Analysis
2.7. In Vivo Analysis of Acute Toxicity
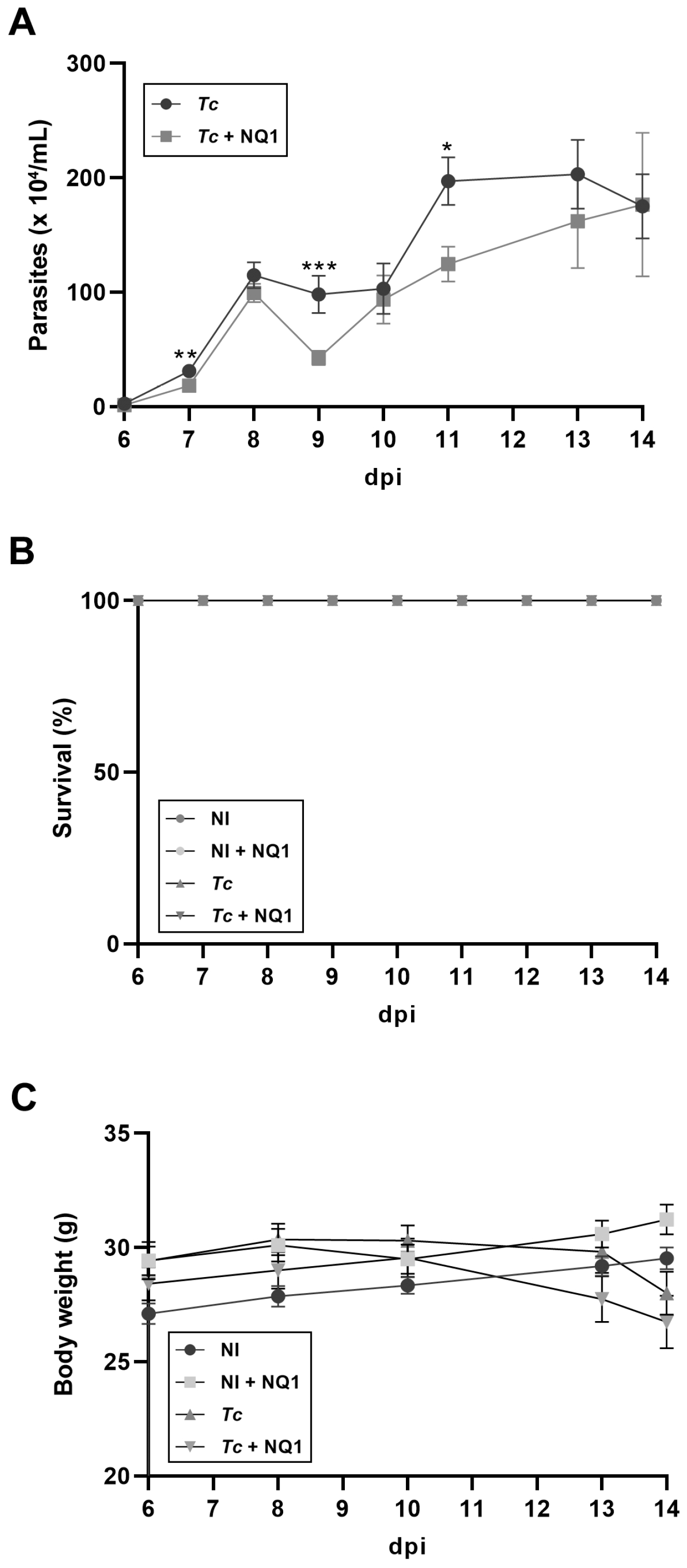
2.8. Acute Infection Model and Treatment In Vivo
2.9. Histopathological, Biochemical, and Cytokine Analyses
2.10. Electrocardiographic (ECG) Analysis
2.11. Ethics Statement
2.12. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bz | Benznidazole |
| NQ1 | Brominated nor-beta-lapachone-derived 1,2,3-triazole |
| NQ2 | Chlorinated nor-beta-lapachone-derived 1,2,3-triazole |
| ROS | Reactive oxygen species |
| ETS | Electron transport system |
| FBS | Fetal bovine serum |
| IC50 | Concentration that led to 50% lysis/proliferation inhibition of parasite |
| LC50 | Concentration that led to 50% lysis/proliferation inhibition of host cells |
| SI | Selectivity index |
| FIC | Fractional inhibitory concentration |
| TMRE | Tetramethylrhodamine |
| ΔΨm | Mitochondrial membrane potential |
| FCCP | Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone |
| DHE | Dihydroethidium |
| AA | Antimycin A |
| NOAEL | No-observed-adverse-effect level |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| dpi | Days post-infection |
| CK-MB | Cardiac isoform of creatine kinase |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| IFNγ | Interferon-γ |
| IL-12p70 | Interleukin-12p70 |
| TNF | Tumor necrosis factor |
| CBA | Cytometric bead array |
| ECG | Electrocardiograph |
References
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S.; Galimberti, L.; Bianco, R.; Grande, R.; Galli, M.; Corbellino, M. Chagas disease in Europe: A review for the internist in the globalized world. Eur. J. Intern. Med. 2017, 43, 6–15. [Google Scholar] [CrossRef]
- Tyler, K.M.; Engman, D.M. The life cycle of Trypanosoma cruzi revisited. Int. J. Parasitol. 2001, 31, 472–481. [Google Scholar] [CrossRef]
- Silva-Dos-Santos, D.; Barreto-de-Albuquerque, J.; Guerra, B.; Moreira, O.C.; Berbert, L.R.; Ramos, M.T.; Mascarenhas, B.A.S.; Britto, C.; Morrot, A.; Serra Villa-Verde, D.M.; et al. Unraveling Chagas disease transmission through the oral route: Gateways to Trypanosoma cruzi infection and target tissues. PLoS Negl. Trop. Dis. 2017, 11, e0005507. [Google Scholar] [CrossRef]
- Bern, C. Chagas’ Disease. N. Engl. J. Med. 2015, 373, 1882. [Google Scholar] [CrossRef]
- Prata, A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect. Dis. 2001, 1, 92–100. [Google Scholar] [CrossRef]
- Urbina, J.A. Specific chemotherapy of Chagas disease: Relevance, current limitations and new approaches. Acta Trop. 2010, 115, 55–68. [Google Scholar] [CrossRef]
- Soeiro, M.N.; de Castro, S.L. Trypanosoma cruzi targets for new chemotherapeutic approaches. Expert. Opin. Ther. Targets 2009, 13, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Coura, J.R.; de Castro, S.L. A critical review on Chagas’ disease chemotherapy. Mem. Inst. Oswaldo Cruz 2002, 97, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.A.; Rosa, M.E.; Boisselier, E.; Albuquerque, V.; Tempone, A.G.; Caseli, L.; Lago, J.H.G. Anti-Trypanosoma cruzi Effect of Fatty Acids from Porcelia macrocarpa Is Related to Interactions of Cell Membranes at Different Microdomains as Assessed Using Langmuir Monolayers. ACS Omega 2025, 10, 21747–21754. [Google Scholar] [CrossRef]
- Navarrete-Carriola, D.V.; Rivera, G.; Ortiz-Pérez, E.; Paz-González, A.D.; Martínez-Vázquez, A.V.; Aquino-González, L.V.; Argueta-Figueroa, L.; Doyle, M.P.; Moreno-Rodríguez, A. Antiparasitic Effect of Polyphenols and Terpenes from Natural Products Against Trypanosoma cruzi and Leishmania mexicana. Metabolites 2025, 15, 560. [Google Scholar] [CrossRef]
- de Bilbao, N.V.; Giebelhaus, R.T.; Dias, R.P.; Ferreira, M.E.; Martínez, M.; Velasco-Carneros, L.; Nam, S.L.; de la Mata, A.; Maréchal, J.D.; Adou, A.I.; et al. Exploring the Anti-Chagas Activity of Zanthoxylum chiloperone Seedlings Through Metabolomics and Protein-Ligand Docking. Plants 2025, 14, 954. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, R.P.B.; de Assis, E.B.; de Sousa, N.F.; de Souza, J.M.S.; da França Rodrigues, K.A.; Scotti, L.; Tavares, J.F.; da Silva, M.S.; Scotti, M.T. Exploring Lamiaceae diterpenoids as potential multitarget therapeutics for leishmaniasis and chagas disease. Mol. Divers. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.; Teixeira, C.P.; Lima Júnior, J.P.; Almeida, M.P.O.; Paschoalino, M.; Luz, L.C.; Dos Santos, N.C.L.; de Oliveira, R.M.; Damasceno, I.S.; Barbosa, M.C.; et al. Trypanosoma cruzi Growth Is Impaired by Oleoresin and Leaf Hydroalcoholic Extract from Copaiferamultijuga in Human Trophoblast and Placental Explants. Pathogens 2025, 14, 736. [Google Scholar] [CrossRef]
- Arenas, P. Medicine and magic among the Maka Indians of the Paraguayan Chaco. J. Ethnopharmacol. 1987, 21, 279–295. [Google Scholar] [CrossRef]
- Hazra, B.; das Sarma, M.; Sanyal, U. Separation methods of quinonoid constituents of plants used in Oriental traditional medicines. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 812, 259–275. [Google Scholar] [CrossRef]
- Garavaglia, P.A.; Rubio, M.F.; Laverrière, M.; Tasso, L.M.; Fichera, L.E.; Cannata, J.J.B.; García, G.A. Trypanosoma cruzi: Death phenotypes induced by ortho-naphthoquinone substrates of the aldo-keto reductase (TcAKR). Role of this enzyme in the mechanism of action of β-lapachone. Parasitology 2018, 145, 1251–1259. [Google Scholar] [CrossRef]
- Guiraud, P.; Steiman, R.; Campos-Takaki, G.M.; Seigle-Murandi, F.; Simeon de Buochberg, M. Comparison of antibacterial and antifungal activities of lapachol and beta-lapachone. Planta Med. 1994, 60, 373–374. [Google Scholar] [CrossRef]
- Dantas-Pereira, L.; Cunha-Junior, E.F.; Andrade-Neto, V.V.; Bower, J.F.; Jardim, G.A.M.; da Silva Júnior, E.N.; Torres-Santos, E.C.; Menna-Barreto, R.F.S. Naphthoquinones and Derivatives for Chemotherapy: Perspectives and Limitations of their Anti-trypanosomatids Activities. Curr. Pharm. Des. 2021, 27, 1807–1824. [Google Scholar] [CrossRef]
- Xiang, S.; Li, Y.; Khan, S.N.; Zhang, W.; Yuan, G.; Cui, J. Exploiting the Anticancer, Antimicrobial and Antiviral Potential of Naphthoquinone Derivatives: Recent Advances and Future Prospects. Pharmaceuticals 2025, 18, 350. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R.; De Souza, W.; Cruz, F.S.; Roitman, I.; Cover, B.; Gutteridge, W.E. Ultrastructural alterations and peroxide formation induced by naphthoquinones in different stages of Trypanosoma cruzi. Zeitschrift für Parasitenkunde 1978, 57, 189–198. [Google Scholar] [CrossRef]
- Bombaça, A.C.S.; Silva, L.A.; Chaves, O.A.; da Silva, L.S.; Barbosa, J.M.C.; da Silva, A.M.; Ferreira, A.B.B.; Menna-Barreto, R.F.S. Novel N,N-di-alkylnaphthoimidazolium derivative of β-lapachone impaired Trypanosoma cruzi mitochondrial electron transport system. Biomed. Pharmacother. 2021, 135, 111186. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Da Silva, E.N.; Pinto, A.V.; De Castro, S.L.; Menna-Barreto, R.F. A novel triazolicnaphthofuranquinone induces autophagy in reservosomes and impairment of mitosis in Trypanosoma cruzi. Parasitology 2012, 139, 26–36. [Google Scholar] [CrossRef]
- Cascabulho, C.M.; Meuser-Batista, M.; Moura, K.C.G.; Pinto, M.D.C.; Duque, T.L.A.; Demarque, K.C.; Guimarães, A.C.R.; Manso, P.P.A.; Pelajo-Machado, M.; Oliveira, G.M.; et al. Antiparasitic and anti-inflammatory activities of beta-lapachone-derived naphthoimidazoles in experimental acute Trypanosoma cruzi infection. Mem. Inst. Oswaldo Cruz. 2020, 115, e190389. [Google Scholar] [CrossRef] [PubMed]
- Lizzi, F.; Veronesi, G.; Belluti, F.; Bergamini, C.; López-Sánchez, A.; Kaiser, M.; Brun, R.; Krauth-Siegel, R.L.; Hall, D.G.; Rivas, L.; et al. Conjugation of quinones with natural polyamines: Toward an expanded antitrypanosomatid profile. J. Med. Chem. 2012, 55, 10490–10500. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Bergamini, C.; Molina, M.T.; Falchi, F.; Cavalli, A.; Kaiser, M.; Brun, R.; Fato, R.; Bolognesi, M.L. 2-Phenoxy-1,4-naphthoquinones: From a Multitarget Antitrypanosomal to a Potential Antitumor Profile. J. Med. Chem. 2015, 58, 6422–6434. [Google Scholar] [CrossRef]
- Martín-Escolano, R.; Guardia, J.J.; Martín-Escolano, J.; Cirauqui, N.; Fernández, A.; Rosales, M.J.; Chahboun, R.; Sánchez-Moreno, M.; Alvarez-Manzaneda, E.; Marín, C. In Vivo Biological Evaluation of a Synthetic Royleanone Derivative as a Promising Fast-Acting Trypanocidal Agent by Inducing Mitochondrial-Dependent Necrosis. J. Nat. Prod. 2020, 83, 3571–3583. [Google Scholar] [CrossRef] [PubMed]
- Fivelman, Q.L.; Adagu, I.S.; Warhurst, D.C. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 2004, 48, 4097–4102. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Timm, B.L.; da Silva, P.B.; Batista, M.M.; da Silva, F.H.; da Silva, C.F.; Tidwell, R.R.; Patrick, D.A.; Jones, S.K.; Bakunov, S.A.; Bakunova, S.M.; et al. In vitro and in vivo biological effects of novel arylimidamide derivatives against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2014, 58, 3720–3726. [Google Scholar] [CrossRef]
- Brener, Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop. Sao Paulo 1962, 4, 389–396. [Google Scholar]
- Silverio, J.; Pereira, I.R.; Cipitelli, M.C.; Vinagre, N.F.; Rodrigues, M.M.; Gazzinelli, R.T. CD8+ T-cells expressing interferon gamma or perforin play antagonistic roles in heart injury in experimental Trypanosoma cruzi-elicited cardiomyopathy. PLoS Pathog. 2012, 8, e1002645. [Google Scholar] [CrossRef]
- Vilar-Pereira, G.; Castaño Barrios, L.; da Silva, A.A.; Martins Batista, A.; Resende Pereira, I.; Moreira, O.C.; Britto, C.; Mata Dos Santos, H.A.; Lannes-Vieira, J. Memory impairment in chronic experimental Chagas disease: Benznidazole therapy reversed cognitive deficit in association with reduction of parasite load and oxidative stress in the nervous tissue. PLoS ONE 2021, 16, e0244710. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Liu, S.W.; Chen, M.R.; Cho, K.H.; Chen, T.Y.; Chu, P.H.; Kao, Y.Y.; Hsu, C.H.; Lin, K.M. Neonatal Death and Heart Failure in Mouse with Transgenic HSP60 Expression. Biomed. Res. Int. 2015, 2015, 539805. [Google Scholar] [CrossRef]
- de Oliveira, J.C.; Abreu, B.U.; Paz, E.R.S.; Almeida, R.G.; Honorato, J.; Souza, C.P.; Fantuzzi, F.; Ramos, V.F.S.; Menna-Barreto, R.F.S.; Araujo, M.H.; et al. SuFEx-Functionalized Quinones via Ruthenium-Catalyzed C-H Alkenylation: A Potential Building Block for Bioactivity Valorization. Chem. Asian J. 2024, 19, e202400757. [Google Scholar] [CrossRef]
- Jardim, G.A.M.; Silva, T.L.; Goulart, M.O.F.; de Simone, C.A.; Barbosa, J.M.C.; Salomão, K.; de Castro, S.L.; Bower, J.F.; da Silva Júnior, E.N. Rhodium-catalyzed C-H bond activation for the synthesis of quinonoid compounds: Significant Anti-Trypanosoma cruzi activities and electrochemical studies of functionalized quinones. Eur. J. Med. Chem. 2017, 136, 406–419. [Google Scholar] [CrossRef]
- Pinto, A.V.; de Castro, S.L. The trypanocidal activity of naphthoquinones: A review. Molecules 2009, 14, 4570–4590. [Google Scholar] [CrossRef]
- Salas, C.O.; Faúndez, M.; Morello, A.; Maya, J.D.; Tapia, R.A. Natural and synthetic naphthoquinones active against Trypanosoma cruzi: An initial step towards new drugs for Chagas disease. Curr. Med. Chem. 2011, 18, 144–161. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.A.; Wada, T. Synthesis and antiviral evaluation of 5-(1,2,3-triazol-1-ylmethyl)uridine derivatives. Z. Naturforsch. C J. Biosci. 2009, 64, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.B.; Costa, M.S.; Boechat, N.; Bezerra, R.J.; Genestra, M.S.; Canto-Cavalheiro, M.M.; Kover, W.B.; Ferreira, V.F. Synthesis and evaluation of new difluoromethyl azoles as antileishmanial agents. Eur. J. Med. Chem. 2007, 42, 1388–1395. [Google Scholar] [CrossRef]
- Holla, B.S.; Mahalinga, M.; Karthikeyan, M.S.; Poojary, B.; Akberali, P.M.; Kumari, N.S. Synthesis, characterization and antimicrobial activity of some substituted 1,2,3-triazoles. Eur. J. Med. Chem. 2005, 40, 1173–1178. [Google Scholar] [CrossRef]
- Lopes, J.N.; Cruz, F.S.; Docampo, R.; Vasconcellos, M.E.; Vasconcellos, M.E.; Sampaio, M.C.; Pinto, A.V.; Gilbert, B. In vitro and in vivo evaluation of the toxicity of 1,4-naphthoquinone and 1,2-naphthoquinone derivatives against Trypanosoma cruzi. Ann. Trop. Med. Parasitol. 1978, 72, 523–531. [Google Scholar] [CrossRef]
- Boveris, A.; Docampo, R.; Turrens, J.F.; Stoppani, A.O. Effect of beta and alpha-lapachone on the production of H2O2 and on the growth of Trypanosoma cruzi. Rev. Asoc. Argent. Microbiol. 1977, 9, 54–61. [Google Scholar] [PubMed]
- Boveris, A.; Stoppani, A.O. Hydrogen peroxide generation in Trypanosoma cruzi. Experientia 1977, 33, 1306–1308. [Google Scholar] [CrossRef]
- Vilar-Pereira, G.; Carneiro, V.C.; Mata-Santos, H.; Vicentino, A.R.; Ramos, I.P.; Giarola, N.L.; Feijó, D.F.; Meyer-Fernandes, J.R.; Paula-Neto, H.A.; Medei, E.; et al. Resveratrol Reverses Functional Chagas Heart Disease in Mice. PLoS Pathog. 2016, 12, e1005947. [Google Scholar] [CrossRef]
- Pereira-Barretto, A.C.; Bacal, F.; de Albuquerque, D.C. Most Heart Failure Patients Die from Pump Failure: Implications for Therapy. Am. J. Cardiovasc. Drugs 2015, 15, 387–393. [Google Scholar] [CrossRef]
- Ramos, E.I.; Garza, K.M.; Krauth-Siegel, R.L.; Bader, J.; Martinez, L.E.; Maldonado, R.A. 2,3-diphenyl-1,4-naphthoquinone: A potential chemotherapeutic agent against Trypanosoma cruzi. J. Parasitol. 2009, 95, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, K.; Moreno-Rodríguez, A.; Domínguez-Díaz, L.R.; Bertrand, J.; Salas, C.O.; Rivera, G.; Cervera, Y.P.; Bocanegra-García, V. In vitro and In vivo Biological Activity of Two Aryloxy-naphthoquinones in Mice Infected with Trypanosoma cruzi Strains. Med. Chem. 2024, 20, 938–943. [Google Scholar] [CrossRef] [PubMed]







| Compound | Bloodstream Trypomastigote | Epimastigote | |
|---|---|---|---|
| 5% Blood, 4 °C | 0% Blood, 37 °C | ||
| NQ1 | 6.8 1 ± 0.7 | 0.8 3 ± 0.2 | 0.8 3 ± 0.2 |
| NQ2 | 8.2 1 ± 0.7 | 3.1 3 ± 0.7 | 1.8 3 ± 0.1 |
| Bz | 103.6 2 ± 0.6 | 9.7 4 ± 2.3 | 17.1 3 ± 0.5 |
| Bz–NQ1 | FIC Bz | FIC NQ1 | ∑FIC |
|---|---|---|---|
| 4:1 | 1.87 1,2 ± 0.57 | 2.37 ± 0.86 | 4.24 |
| 3:2 | 2.37 ± 0.93 | 3.02 ± 0.45 | 5.39 |
| 2:3 | 1.78 ± 0.48 | 1.76 ± 0.56 | 3.54 |
| 1:4 | 0.56 ± 0.32 | 0.52 ± 0.01 | 1.09 |
| Mean of ∑ in combination | 1.65 | 1.92 | 3.56 |
| Bz–NQ2 | FIC Bz | FIC NQ2 | ∑FIC |
|---|---|---|---|
| 4:1 | 2.76 1,2 ± 2.04 | 1.42 ± 0.53 | 4.18 |
| 3:2 | 1.7 ± 0.73 | 1.31 ± 0.45 | 3.00 |
| 2:3 | 1.17 ± 0.11 | 0.77 ± 0.17 | 1.94 |
| 1:4 | 0.48 ± 0.12 | 0.5 ± 0.43 | 0.98 |
| Mean of ∑ in combination | 1.53 | 1.00 | 2.53 |
| Dose (µM) | Median | IV 1 | |
|---|---|---|---|
| Control | - | 34,565.3 ± 4172.8 2,3 | 0.0 |
| NQ1 | 0.4 | 34,520.1 ± 2808.7 | 0.01 |
| 0.8 | 6778.1 ± 2082.5 * | −0.80 | |
| NQ2 | 0.9 | 35,367.2 ± 4865.6 | 0.02 |
| 1.8 | 37,430.2 ± 3744.2 | −0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Duarte, R.B.; Ramos, V.F.S.; Barbosa, J.M.C.; Oliveira, G.M.; Diogo, E.B.T.; Almeida, R.G.; Lennox, A.J.J.; da Silva Júnior, E.N.; Pedra-Rezende, Y.; Menna-Barreto, R.F.S. Trypanocidal Activity of Dual Redox-Active Quinones: Trypanosoma cruzi Mitochondrion as a Target Organelle In Vitro and Anti-Inflammatory Properties In Vivo. Pathogens 2026, 15, 17. https://doi.org/10.3390/pathogens15010017
Duarte RB, Ramos VFS, Barbosa JMC, Oliveira GM, Diogo EBT, Almeida RG, Lennox AJJ, da Silva Júnior EN, Pedra-Rezende Y, Menna-Barreto RFS. Trypanocidal Activity of Dual Redox-Active Quinones: Trypanosoma cruzi Mitochondrion as a Target Organelle In Vitro and Anti-Inflammatory Properties In Vivo. Pathogens. 2026; 15(1):17. https://doi.org/10.3390/pathogens15010017
Chicago/Turabian StyleDuarte, Raquel B., Victor F. S. Ramos, Juliana M. C. Barbosa, Gabriel M. Oliveira, Emilay B. T. Diogo, Renata G. Almeida, Alastair J. J. Lennox, Eufrânio N. da Silva Júnior, Yasmin Pedra-Rezende, and Rubem F. S. Menna-Barreto. 2026. "Trypanocidal Activity of Dual Redox-Active Quinones: Trypanosoma cruzi Mitochondrion as a Target Organelle In Vitro and Anti-Inflammatory Properties In Vivo" Pathogens 15, no. 1: 17. https://doi.org/10.3390/pathogens15010017
APA StyleDuarte, R. B., Ramos, V. F. S., Barbosa, J. M. C., Oliveira, G. M., Diogo, E. B. T., Almeida, R. G., Lennox, A. J. J., da Silva Júnior, E. N., Pedra-Rezende, Y., & Menna-Barreto, R. F. S. (2026). Trypanocidal Activity of Dual Redox-Active Quinones: Trypanosoma cruzi Mitochondrion as a Target Organelle In Vitro and Anti-Inflammatory Properties In Vivo. Pathogens, 15(1), 17. https://doi.org/10.3390/pathogens15010017








