Hepatitis C (HCV) and Hepatitis Delta (HDV) Viruses in a Teaching Hospital in Southern Italy: What Is the Epidemiological Situation in the Era of New Drugs?
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Antibody (Ab) and Viral Load Detection
2.3. HCV and HDV Genotyping
2.4. Statistical Analysis
3. Results
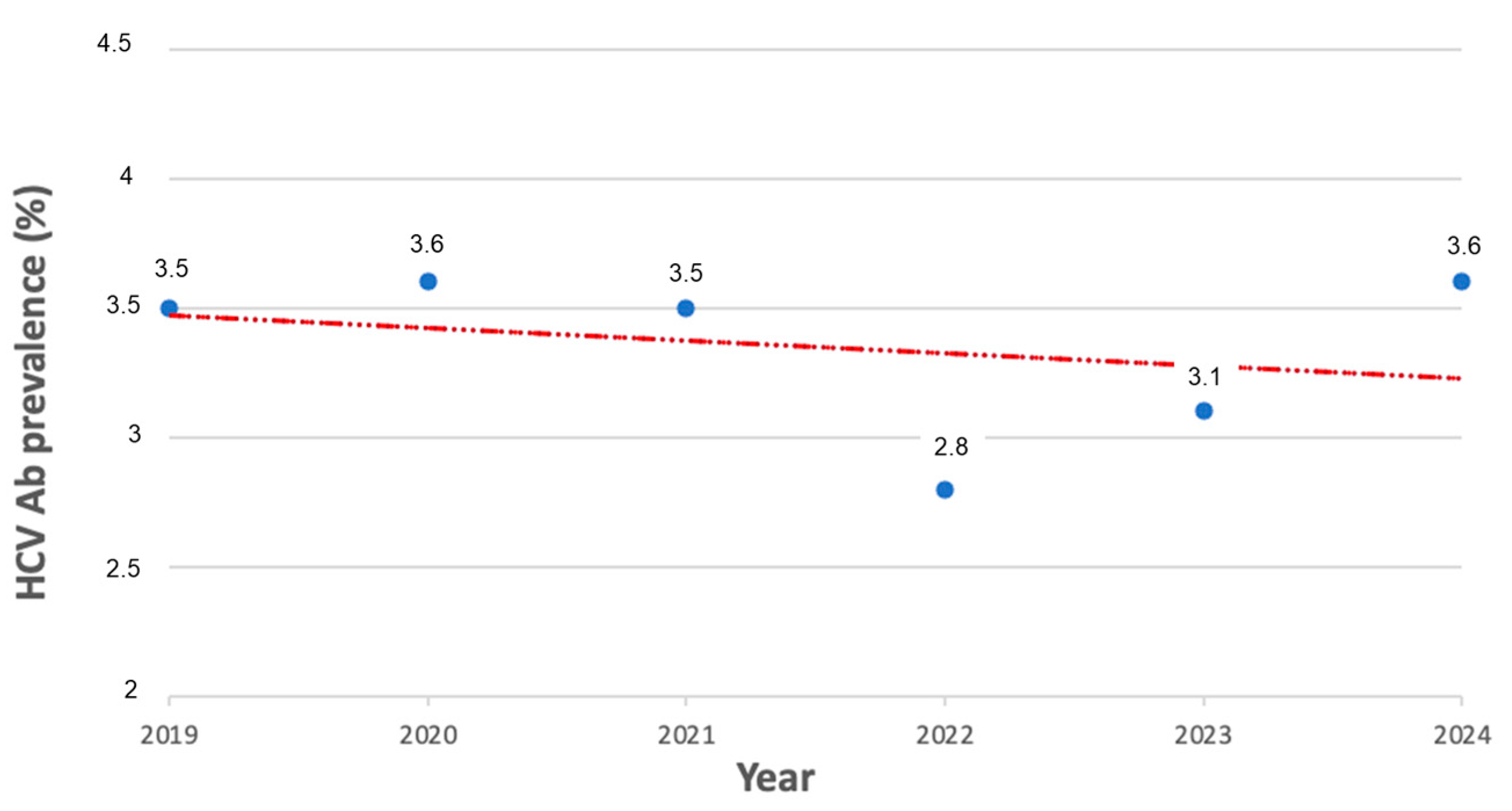
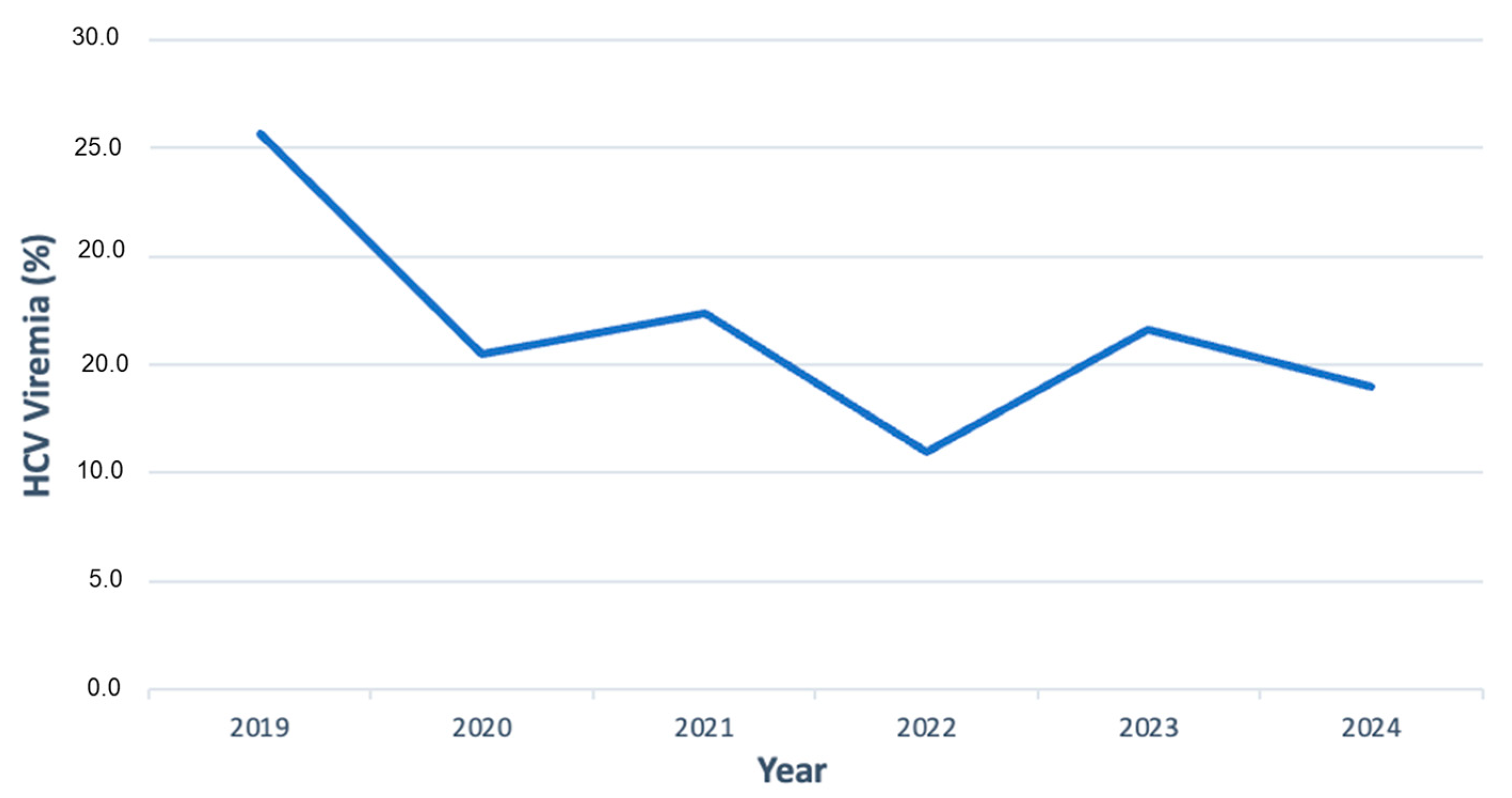
3.1. HCV Antibody Prevalence over Time
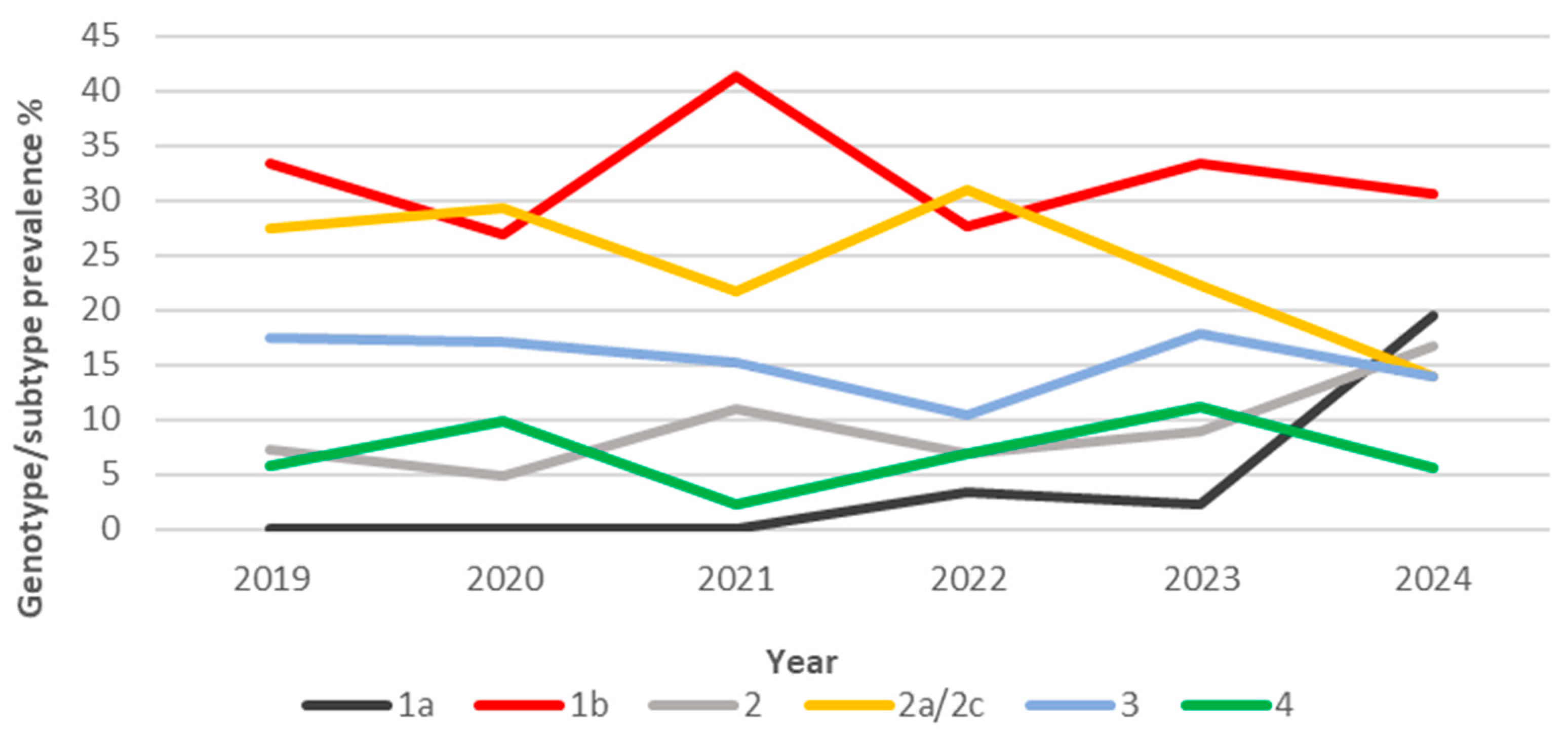
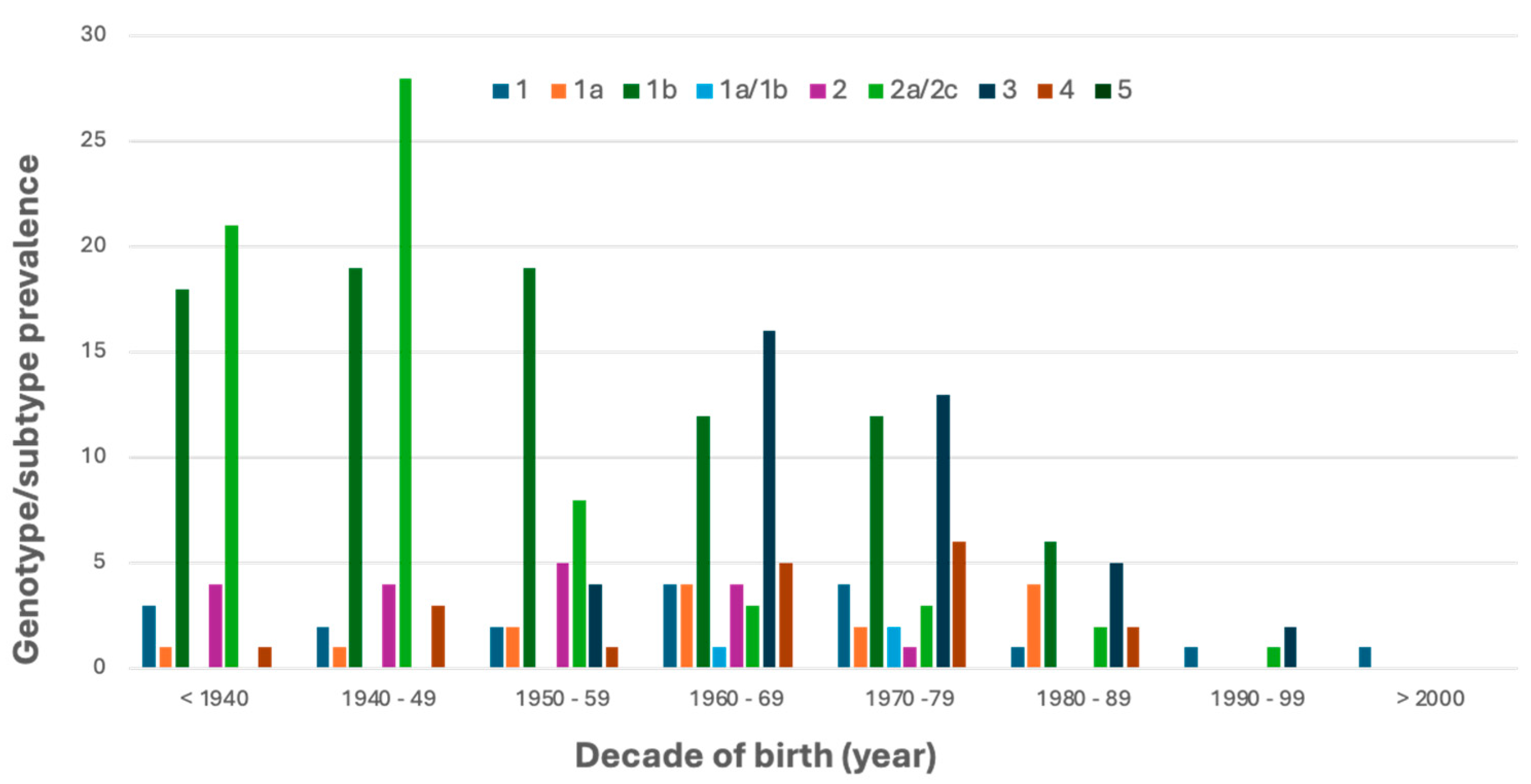
3.2. HCV Genotype/Subtype Distribution
3.3. HDV Antibody and Genotype/Subtype Prevalence
4. Discussion
4.1. Study Overview
4.2. Update on HCV Prevalence and Genotype Distribution
4.3. First Survey on HDV Prevalence and Genotype Distribution
4.4. Public Health and Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Hepatitis Report 2024: Action for Access in Low- and Middle-Income Countries. Available online: https://www.who.int/publications/i/item/9789240091672 (accessed on 13 July 2024).
- Quirino, A.; Marascio, N.; Branda, F.; Ciccozzi, A.; Romano, C.; Locci, C.; Azzena, I.; Pascale, N.; Pavia, G.; Matera, G.; et al. Viral Hepatitis: Host Immune Interaction, Pathogenesis and New Therapeutic Strategies. Pathogens 2024, 13, 766. [Google Scholar] [CrossRef]
- CDC Global Viral Hepatitis. Available online: https://www.cdc.gov/hepatitis/global/index.html (accessed on 5 June 2025).
- Innes, H.; McDonald, S.A.; Hamill, V.; Yeung, A.; Dillon, J.F.; Hayes, P.C.; Went, A.; Fraser, A.; Bathgate, A.; Barclay, S.T.; et al. Declining Incidence of Hepatitis C Related Hepatocellular Carcinoma in the Era of Interferon-Free Therapies: A Population-Based Cohort Study. Liver Int. Off. J. Int. Assoc. Study Liver 2022, 42, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.D.; Willing, A.R.; Kairouz, A.; Cunningham, E.B.; Wheeler, A.; O’Brien, N.; Perera, V.; Ward, J.W.; Hiebert, L.; Degenhardt, L.; et al. Direct-Acting Antiviral Therapies for Hepatitis C Infection: Global Registration, Reimbursement, and Restrictions. Lancet Gastroenterol. Hepatol. 2024, 9, 366–382. [Google Scholar] [CrossRef]
- Artenie, A.; Trickey, A.; Looker, K.J.; Stone, J.; Lim, A.G.; Fraser, H.; Degenhardt, L.; Dore, G.J.; Grebely, J.; Cunningham, E.B.; et al. Global, Regional, and National Estimates of Hepatitis C Virus (HCV) Infection Incidence among People Who Inject Drugs and Number of New Annual HCV Infections Attributable to Injecting Drug Use: A Multi-Stage Analysis. Lancet Gastroenterol. Hepatol. 2025, 10, 315–331. [Google Scholar] [CrossRef]
- Marascio, N.; Mazzitelli, M.; Pavia, G.; Giancotti, A.; Barreca, G.S.; Costa, C.; Pisani, V.; Greco, G.; Serapide, F.; Trecarichi, E.M.; et al. Clinical, Virological Characteristics, and Outcomes of Treatment with Sofosbuvir/Ledipasvir in Two Pediatric Patients Infected by HCV Genotype 4. Cells 2019, 8, 416. [Google Scholar] [CrossRef]
- Marascio, N.; Pavia, G.; Strazzulla, A.; Dierckx, T.; Cuypers, L.; Vrancken, B.; Barreca, G.S.; Mirante, T.; Malanga, D.; Oliveira, D.M.; et al. Detection of Natural Resistance-Associated Substitutions by Ion Semiconductor Technology in HCV1b Positive, Direct-Acting Antiviral Agents-Naïve Patients. Int. J. Mol. Sci. 2016, 17, 1416. [Google Scholar] [CrossRef] [PubMed]
- Marascio, N.; Costantino, A.; Taffon, S.; Lo Presti, A.; Equestre, M.; Bruni, R.; Pisani, G.; Barreca, G.S.; Quirino, A.; Trecarichi, E.M.; et al. Phylogenetic and Molecular Analyses of More Prevalent HCV1b Subtype in the Calabria Region, Southern Italy. J. Clin. Med. 2021, 10, 1655. [Google Scholar] [CrossRef] [PubMed]
- Romeo, I.; Marascio, N.; Pavia, G.; Talarico, C.; Costa, G.; Alcaro, S.; Artese, A.; Torti, C.; Liberto, M.C.; Focà, A. Structural Modeling of New Polymorphism Clusters of HCV Polymerase Isolated from Direct-Acting Antiviral Naïve Patients: Focus on Dasabuvir and Setrobuvir Binding Affinity. ChemistrySelect 2018, 3, 6009–6017. [Google Scholar] [CrossRef]
- Blach, S.; Terrault, N.A.; Tacke, F.; Gamkrelidze, I.; Craxi, A.; Tanaka, J.; Waked, I.; Dore, G.J.; Abbas, Z.; Abdallah, A.R.; et al. Global Change in Hepatitis C Virus Prevalence and Cascade of Care between 2015 and 2020: A Modelling Study. Lancet Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef]
- Guadagnino, V.; Stroffolini, T.; Rapicetta, M.; Costantino, A.; Kondili, L.A.; Menniti-Ippolito, F.; Caroleo, B.; Costa, C.; Griffo, G.; Loiacono, L.; et al. Prevalence, Risk Factors, and Genotype Distribution of Hepatitis C Virus Infection in the General Population: A Community-Based Survey in Southern Italy. Hepatology 1997, 26, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S.; Pozzato, G.; Saccoccio, G.; Crovatto, M.; Crocè, L.S.; Mazzoran, L.; Masutti, F.; Cristianini, G.; Tiribelli, C. Clinical Course and Risk Factors of Hepatitis C Virus Related Liver Disease in the General Population: Report from the Dionysos Study. Gut 1999, 44, 874–880. [Google Scholar] [CrossRef]
- Maio, G.; D’Argenio, P.; Stroffolini, T.; Bozza, A.; Sacco, L.; Tosti, M.E.; Intorcia, M.; Fossi, E.; D’Alessio, G.; Kondili, L.A.; et al. Hepatitis C Virus Infection and Alanine Transaminase Levels in the General Population: A Survey in a Southern Italian Town. J. Hepatol. 2000, 33, 116–120. [Google Scholar] [CrossRef]
- Croce, D.; Bonfanti, M.; Restelli, U. Financial and Feasibility Implications of the Treatment of Hepatitis C Virus in Italy: Scenarios and Perspectives. Clin. Outcomes Res. CEOR 2016, 8, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Gardini, I.; Bartoli, M.; Conforti, M.; Mennini, F.S.; Marcellusi, A.; Lanati, E. HCV—Estimation of the Number of Diagnosed Patients Eligible to the New Anti-HCV Therapies in Italy. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Farci, P.; Niro, G.A. Clinical Features of Hepatitis D. Semin. Liver Dis. 2012, 32, 228–236. [Google Scholar] [CrossRef]
- Stockdale, A.J.; Kreuels, B.; Henrion, M.Y.R.; Giorgi, E.; Kyomuhangi, I.; de Martel, C.; Hutin, Y.; Geretti, A.M. The Global Prevalence of Hepatitis D Virus Infection: Systematic Review and Meta-Analysis. J. Hepatol. 2020, 73, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Alfaiate, D.; Clément, S.; Gomes, D.; Goossens, N.; Negro, F. Chronic Hepatitis D and Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis of Observational Studies. J. Hepatol. 2020, 73, 533–539. [Google Scholar] [CrossRef]
- Razavi-Shearer, D.; Child, H.; Razavi-Shearer, K.; Voeller, A.; Razavi, H.; Buti, M.; Tacke, F.; Terrault, N.; Zeuzem, S.; Abbas, Z.; et al. Adjusted Estimate of the Prevalence of Hepatitis Delta Virus in 25 Countries and Territories. J. Hepatol. 2024, 80, 232–242. [Google Scholar] [CrossRef]
- Salpini, R.; Piermatteo, L.; Torre, G.; D’Anna, S.; Khan, S.; Duca, L.; Bertoli, A.; La Frazia, S.; Malagnino, V.; Teti, E.; et al. Prevalence of Hepatitis D Virus Infection in Central Italy Has Remained Stable across the Last 2 Decades with Dominance of Subgenotypes 1 and Characterized by Elevated Viral Replication. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2024, 138, 1–9. [Google Scholar] [CrossRef]
- Stroffolini, T.; Ciancio, A.; Furlan, C.; Vinci, M.; Fontana, R.; Russello, M.; Colloredo, G.; Morisco, F.; Coppola, N.; Babudieri, S.; et al. Migratory Flow and Hepatitis Delta Infection in Italy: A New Challenge at the Beginning of the Third Millennium. J. Viral Hepat. 2020, 27, 941–947. [Google Scholar] [CrossRef]
- Brancaccio, G.; Nardi, A.; Madonia, S.; Fasano, M.; Verucchi, G.; Massari, M.; Maimone, S.; Contini, C.; Levantesi, F.; Alfieri, A.; et al. The Present Profile of Chronic Hepatitis B Virus Infection Highlights Future Challenges: An Analysis of the Multicenter Italian MASTER-B Cohort. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2019, 51, 438–442. [Google Scholar] [CrossRef]
- Wranke, A.; Pinheiro Borzacov, L.M.; Parana, R.; Lobato, C.; Hamid, S.; Ceausu, E.; Dalekos, G.N.; Rizzetto, M.; Turcanu, A.; Niro, G.A.; et al. Clinical and Virological Heterogeneity of Hepatitis Delta in Different Regions World-Wide: The Hepatitis Delta International Network (HDIN). Liver Int. Off. J. Int. Assoc. Study Liver 2018, 38, 842–850. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Palom, A.; Rando-Segura, A.; Vico, J.; Pacín, B.; Vargas, E.; Barreira-Díaz, A.; Rodríguez-Frías, F.; Riveiro-Barciela, M.; Esteban, R.; Buti, M. Implementation of Anti-HDV Reflex Testing among HBsAg-Positive Individuals Increases Testing for Hepatitis D. JHEP Rep. 2022, 4, 100547. [Google Scholar] [CrossRef] [PubMed]
- Cossiga, V.; Brusa, S.; Montalti, R.; De Conte, A.; Jannuzzi, G.; Ranieri, L.; Sorrentino, R.; Vallefuoco, L.; Pignata, L.; Guarino, M.; et al. Anti-HDV Reflex Testing in HBsAg-Positive Subjects: An Efficacious Strategy to Identify HDV Infection. Liver Int. Off. J. Int. Assoc. Study Liver 2024, 44, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, H.; Aleman, S.; Brunetto, M.; Blank, A.; Andreone, P.; Bogomolov, P.; Chulanov, V.; Mamonova, N.; Geyvandova, N.; Morozov, V.; et al. Bulevirtide Monotherapy in Patients with Chronic HDV: Efficacy and Safety Results through Week 96 from a Phase III Randomized Trial. J. Hepatol. 2024, 81, 621–629. [Google Scholar] [CrossRef]
- Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis. Available online: https://www.who.int/publications/i/item/WHO-HIV-2016.06 (accessed on 5 June 2025).
- Marcellusi, A.; Mennini, F.S.; Andreoni, M.; Kondili, L.A.; PITER Collaboration Study Group. Screening Strategy to Advance HCV Elimination in Italy: A Cost-Consequence Analysis. Eur. J. Health Econ. HEPAC Health Econ. Prev. Care 2024, 25, 1261–1273. [Google Scholar] [CrossRef]
- Razavi, H.A.; Waked, I.; Qureshi, H.; Kondili, L.A.; Duberg, A.S.; Aleman, S. Number of People Treated for Hepatitis C Virus Infection in 2014–2023 and Applicable Lessons for New HBV and HDV Therapies. J. Hepatol. 2025, 83, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Salpini, R.; Svicher, V.; Kennedy, P.T. Editorial: The Current Challenges Underlying Hepatitis D Virus Infection. Front. Med. 2023, 10, 1355027. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Karimzadeh, H.; Usman, Z.; Frishman, D.; Roggendorf, M. Genetic Diversity of Hepatitis D Virus Genotype-1 in Europe Allows Classification into Subtypes. J. Viral Hepat. 2019, 26, 900–910. [Google Scholar] [CrossRef]
- FigTree. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 30 June 2025).
- Marascio, N.; Mazzitelli, M.; Scarlata, G.G.; Giancotti, A.; Barreca, G.S.; Lamberti, A.G.; Divenuto, F.; Costa, C.; Trecarichi, E.M.; Giovanni Matera, G.; et al. HCV Antibody Prevalence and Genotype Evolution in a Teaching Hospital, Calabria Region, Southern Italy Over A Decade (2008–2018). Open Microbiol. J. 2020, 14, 84–90. [Google Scholar] [CrossRef]
- Polaris Observatory HCV Collaborators. Global Prevalence and Genotype Distribution of Hepatitis C Virus Infection in 2015: A Modelling Study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Veneziano, C.; Marascio, N.; De Marco, C.; Quaresima, B.; Biamonte, F.; Trecarichi, E.M.; Santamaria, G.; Quirino, A.; Torella, D.; Quattrone, A.; et al. The Spread of SARS-CoV-2 Omicron Variant in CALABRIA: A Spatio-Temporal Report of Viral Genome Evolution. Viruses 2023, 15, 408. [Google Scholar] [CrossRef] [PubMed]
- De Marco, C.; Veneziano, C.; Massacci, A.; Pallocca, M.; Marascio, N.; Quirino, A.; Barreca, G.S.; Giancotti, A.; Gallo, L.; Lamberti, A.G.; et al. Dynamics of Viral Infection and Evolution of SARS-CoV-2 Variants in the Calabria Area of Southern Italy. Front. Microbiol. 2022, 13, 934993. [Google Scholar] [CrossRef]
- Pavia, G.; Quirino, A.; Marascio, N.; Veneziano, C.; Longhini, F.; Bruni, A.; Garofalo, E.; Pantanella, M.; Manno, M.; Gigliotti, S.; et al. Persistence of SARS-CoV-2 Infection and Viral Intra- and Inter- host Evolution in COVID-19 Hospitalized Patients. J. Med. Virol. 2024, 96, e29708. [Google Scholar] [CrossRef]
- Tramonti Fantozzi, M.P.; Ceccarelli, L.; Petri, D.; De Vita, E.; Agostini, A.; Colombatto, P.; Stasi, C.; Rossetti, B.; Brunetto, M.; Surace, L.; et al. Hepatitis C Epidemiology and Treatment Outcomes in Italy: Impact of the DAA Era and the COVID-19 Pandemic. J. Viral Hepat. 2024, 31, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Maticic, M.; Cernosa, J.; Loboda, C.; Tamse, J.; Rigoni, R.; Duffell, E.; Indave, I.; Zimmermann, R.; Darragh, L.; Moura, J.; et al. How Far Are We? Assessing Progress in Hepatitis C Response towards the WHO 2030 Elimination Goals by the Civil Society Monitoring in 25 European Countries, Period 2020 to 2023. Harm. Reduct. J. 2024, 21, 203. [Google Scholar] [CrossRef]
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global Epidemiology of Hepatitis C Virus Infection: An up-Date of the Distribution and Circulation of Hepatitis C Virus Genotypes. World J. Gastroenterol. 2016, 22, 7824–7840. [Google Scholar] [CrossRef]
- Vo-Quang, E.; Pawlotsky, J.-M. ‘Unusual’ HCV Genotype Subtypes: Origin, Distribution, Sensitivity to Direct-Acting Antiviral Drugs and Behaviour on Antiviral Treatment and Retreatment. Gut 2024, 73, 1570–1582. [Google Scholar] [CrossRef]
- Pawlotsky, J.M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G. EASL Recommendations on Treatment of Hepatitis C: Final Update of the Series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- Malandris, K.; Kalopitas, G.; Theocharidou, E.; Germanidis, G. The Role of RASs/RVs in the Current Management of HCV. Viruses 2021, 13, 2096. [Google Scholar] [CrossRef]
- Cunha, C.; Tavanez, J.P.; Gudima, S. Hepatitis Delta Virus: A Fascinating and Neglected Pathogen. World J. Virol. 2015, 4, 313–322. [Google Scholar] [CrossRef]
- Sagnelli, E.; Stroffolini, T.; Ascione, A.; Chiaramonte, M.; Craxì, A.; Giusti, G.; Piccinino, F. Decrease in HDV Endemicity in Italy. J. Hepatol. 1997, 26, 20–24. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Ciancio, A.; Rizzetto, M. A Review of HDV Infection. Viruses 2022, 14, 1749. [Google Scholar] [CrossRef]
- Sagnelli, E.; Stroffolini, T.; Mele, A.; Imparato, M.; Almasio, P.L.; Italian Hospitals’ Collaborating Group. Chronic Hepatitis B in Italy: New Features of an Old Disease—Approaching the Universal Prevalence of Hepatitis B e Antigen-Negative Cases and the Eradication of Hepatitis D Infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 46, 110–113. [Google Scholar] [CrossRef][Green Version]
- EpiCentro Archivio del Bollettino SEIEVA. Available online: https://www.epicentro.iss.it/epatite/dati-seieva-archivio (accessed on 30 June 2025).[Green Version]
- Rizzetto, M. Hepatitis D Virus: Introduction and Epidemiology. Cold Spring Harb. Perspect. Med. 2015, 5, a021576. [Google Scholar] [CrossRef] [PubMed]
- Coghill, S.; McNamara, J.; Woods, M.; Hajkowicz, K. Epidemiology and Clinical Outcomes of Hepatitis Delta (D) Virus Infection in Queensland, Australia. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2018, 74, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Sausen, D.G.; Shechter, O.; Bietsch, W.; Shi, Z.; Miller, S.M.; Gallo, E.S.; Dahari, H.; Borenstein, R. Hepatitis B and Hepatitis D Viruses: A Comprehensive Update with an Immunological Focus. Int. J. Mol. Sci. 2022, 23, 15973. [Google Scholar] [CrossRef] [PubMed]
- Zachou, K.; Yurdaydin, C.; Drebber, U.; Dalekos, G.N.; Erhardt, A.; Cakaloglu, Y.; Degertekin, H.; Gurel, S.; Zeuzem, S.; Bozkaya, H.; et al. Quantitative HBsAg and HDV-RNA Levels in Chronic Delta Hepatitis. Liver Int. Off. J. Int. Assoc. Study Liver 2010, 30, 430–437. [Google Scholar] [CrossRef]
- Degasperi, E.; Anolli, M.P.; Lampertico, P. Bulevirtide-Based Treatment Strategies for Chronic Hepatitis Delta: A Review. J. Viral Hepat. 2023, 30 (Suppl. 1), 26–32. [Google Scholar] [CrossRef]
- Liaw, Y.F.; Tsai, S.L.; Sheen, I.S.; Chao, M.; Yeh, C.T.; Hsieh, S.Y.; Chu, C.M. Clinical and Virological Course of Chronic Hepatitis B Virus Infection with Hepatitis C and D Virus Markers. Am. J. Gastroenterol. 1998, 93, 354–359. [Google Scholar] [CrossRef]
- Jardi, R.; Rodriguez, F.; Buti, M.; Costa, X.; Cotrina, M.; Galimany, R.; Esteban, R.; Guardia, J. Role of Hepatitis B, C, and D Viruses in Dual and Triple Infection: Influence of Viral Genotypes and Hepatitis B Precore and Basal Core Promoter Mutations on Viral Replicative Interference. Hepatology 2001, 34, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Dwyre, D.M.; Fernando, L.P.; Holland, P.V. Hepatitis B, Hepatitis C and HIV Transfusion-Transmitted Infections in the 21st Century. Vox Sang. 2011, 100, 92–98. [Google Scholar] [CrossRef]
- Riaz, M.; Idrees, M.; Kanwal, H.; Kabir, F. An Overview of Triple Infection with Hepatitis B, C and D Viruses. Virol. J. 2011, 8, 368. [Google Scholar] [CrossRef]
- Feld, J.J.; Forns, X.; Dylla, D.E.; Kumada, H.; de Ledinghen, V.; Wei, L.; Brown, R.S.; Flisiak, R.; Lampertico, P.; Thabut, D.; et al. Safety Analysis of Glecaprevir/Pibrentasvir in Patients with Markers of Advanced Liver Disease in Clinical and Real-World Cohorts. J. Viral Hepat. 2022, 29, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Serraino, R.; Mazzitelli, M.; Greco, G.; Serapide, F.; Scaglione, V.; Marascio, N.; Trecarichi, E.M.; Torti, C. Risk Factors for Hepatitis B and C among Healthy Population: A Community-Based Survey from Four Districts of Southern Italy. Infez. Med. 2020, 28, 223–226. [Google Scholar] [PubMed]
- Spada, E.; Marcantonio, C.; Vescio, M.F.; Marascio, N.; Villano, U.; Pisani, G.; Tritarelli, E.; Bruni, R.; Barreca, G.S.; Torti, C.; et al. Changing Epidemiology of Hepatitis C in Italy: A Population-Based Survey in a Historically High Endemic Area. Minerva Med. 2023, 114, 191–202. [Google Scholar] [CrossRef]
- Krassenburg, L.A.P.; Maan, R.; Ramji, A.; Manns, M.P.; Cornberg, M.; Wedemeyer, H.; de Knegt, R.J.; Hansen, B.E.; Janssen, H.L.A.; de Man, R.A.; et al. Clinical Outcomes Following DAA Therapy in Patients with HCV-Related Cirrhosis Depend on Disease Severity. J. Hepatol. 2021, 74, 1053–1063. [Google Scholar] [CrossRef]
- CDC Viral Hepatitis. Available online: https://www.cdc.gov/immigrant-refugee-health/hcp/domestic-guidance/viral-hepatitis.html (accessed on 29 July 2025).
| Genotype/Subtype | Male | Female | p-Value | ||
|---|---|---|---|---|---|
| N. | (%) | N. | (%) | ||
| 1 | 11 | (61.2) | 7 | (38.8) | ns |
| 1a | 9 | (100) | 0 | (0) | p < 0.05 |
| 1b | 45 | (51.7) | 42 | (48.3) | ns |
| 1a/1b | 3 | (100) | 0 | (0) | ns |
| 2 | 14 | (58.3) | 10 | (41.7) | ns |
| 2a/2c | 22 | (33.8) | 43 | (66.2) | p < 0.05 |
| 3 | 30 | (73.2) | 11 | (26.8) | p < 0.05 |
| 4 | 13 | (72.2) | 5 | (27.8) | ns |
| Total | 147 | (55.5) | 118 | (44.5) | ns |
| Demographic characteristics | |||||||
| Patient ID | ID1 | ID2 | ID3 | ID4 | ID5 | ID6 | ID7 |
| Age | 61 | 58 | 49 | 61 | 54 | 39 | 59 |
| Gender | Female | Female | Male | Female | Male | Female | Female |
| Virological characteristics | |||||||
| HBcAb/HDAb | R | R | R | R | R | R | R |
| HBeAg | NR | NR | NR | NR | NR | NR | NR |
| qHBsAg (IU/mL) | 5.81 | 1.74 | 3.78 | 19.16 | 20 | 1461 | - |
| HBV DNA (IU/mL) | <10 | <10 | <10 | <10 | 450 | 40,500 | <10 |
| HDV RNA (IU/mL) | 162,470 | 60,159 | 752,739 | 8,203,800 | 20 | 5114 | 245,960 |
| HDV Genotype | 1c | 1e | 1e | 1c | NG | 1c | 1c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marascio, N.; Pavia, G.; Mazzei, C.; Manno, M.; Barreca, G.S.; Peronace, C.; Ciurleo, C.; Trimboli, F.; Pantanella, M.; Lamberti, A.G.; et al. Hepatitis C (HCV) and Hepatitis Delta (HDV) Viruses in a Teaching Hospital in Southern Italy: What Is the Epidemiological Situation in the Era of New Drugs? Pathogens 2025, 14, 941. https://doi.org/10.3390/pathogens14090941
Marascio N, Pavia G, Mazzei C, Manno M, Barreca GS, Peronace C, Ciurleo C, Trimboli F, Pantanella M, Lamberti AG, et al. Hepatitis C (HCV) and Hepatitis Delta (HDV) Viruses in a Teaching Hospital in Southern Italy: What Is the Epidemiological Situation in the Era of New Drugs? Pathogens. 2025; 14(9):941. https://doi.org/10.3390/pathogens14090941
Chicago/Turabian StyleMarascio, Nadia, Grazia Pavia, Chiara Mazzei, Michele Manno, Giorgio Settimo Barreca, Cinzia Peronace, Carmela Ciurleo, Francesca Trimboli, Marta Pantanella, Angelo Giuseppe Lamberti, and et al. 2025. "Hepatitis C (HCV) and Hepatitis Delta (HDV) Viruses in a Teaching Hospital in Southern Italy: What Is the Epidemiological Situation in the Era of New Drugs?" Pathogens 14, no. 9: 941. https://doi.org/10.3390/pathogens14090941
APA StyleMarascio, N., Pavia, G., Mazzei, C., Manno, M., Barreca, G. S., Peronace, C., Ciurleo, C., Trimboli, F., Pantanella, M., Lamberti, A. G., Matera, G., & Quirino, A. (2025). Hepatitis C (HCV) and Hepatitis Delta (HDV) Viruses in a Teaching Hospital in Southern Italy: What Is the Epidemiological Situation in the Era of New Drugs? Pathogens, 14(9), 941. https://doi.org/10.3390/pathogens14090941







