Swine Practitioner Practices on Oral Fluid Sampling in U.S. Swine Farms: A Nationwide Survey
Abstract
1. Introduction
2. Materials and Methods
2.1. Questionnaire Development
2.2. Questionnaire Distribution
2.3. Data Cleaning and Analysis
3. Results
3.1. Demographics
3.2. Oral Fluid Implementation and Primary Use
3.3. Oral Fluid Sampling Protocols
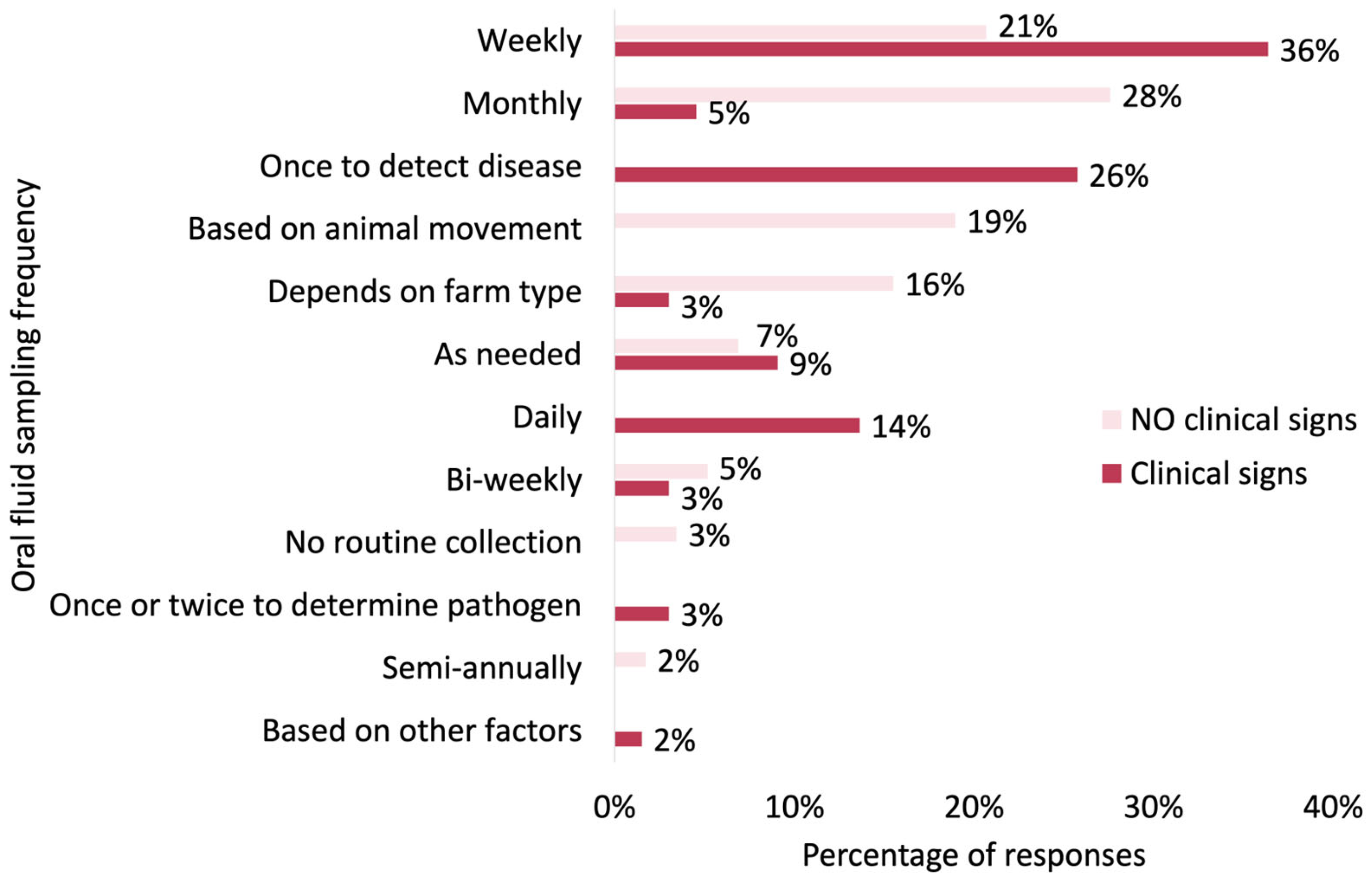
3.3.1. Sampling Frequency
3.3.2. Sample Size
3.3.3. Personnel Responsible for Oral Fluid Sample Collection
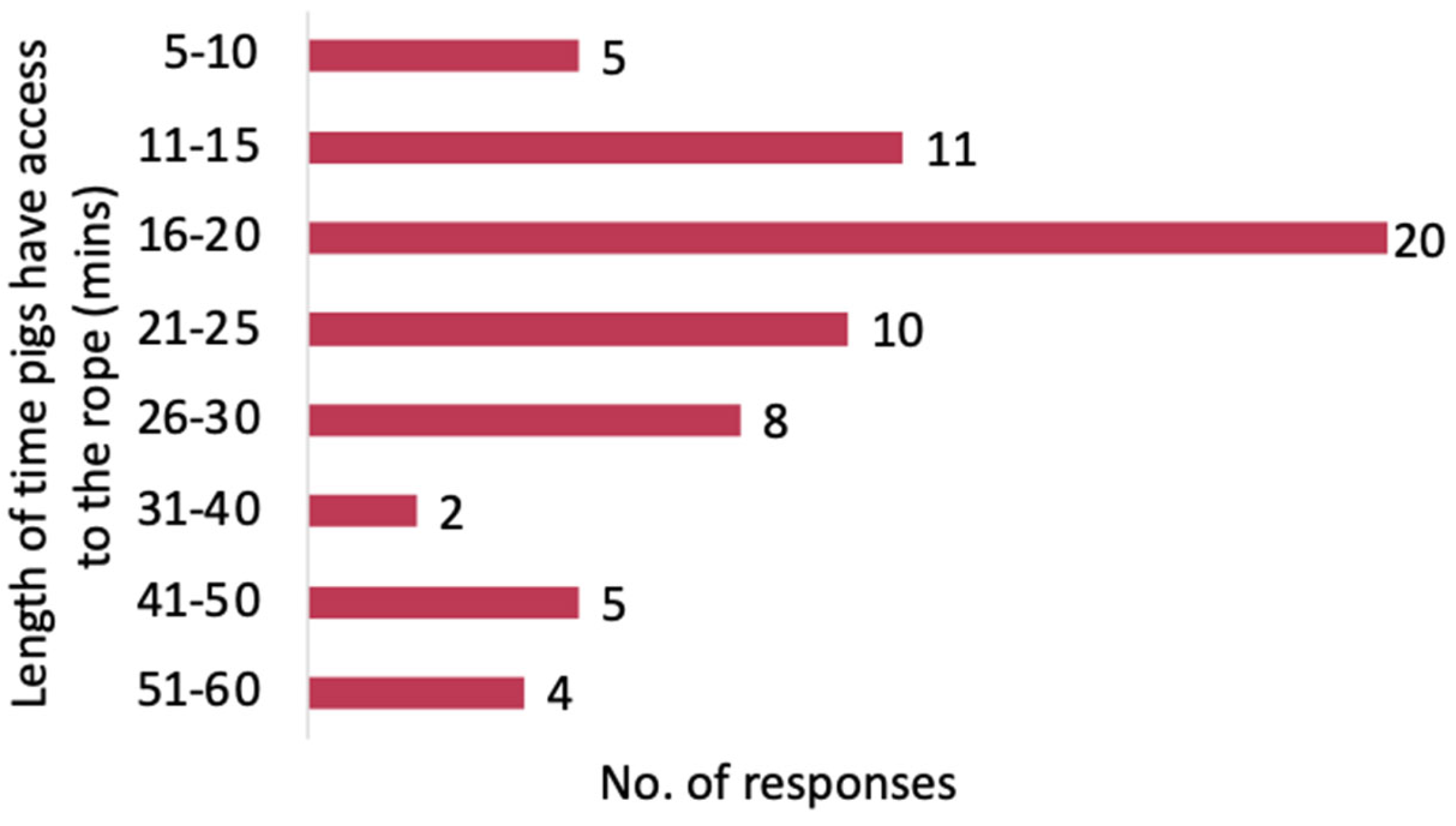
3.3.4. Length of Time Pigs Have Access to the Rope
3.3.5. Source and the Diameter of the Rope
3.4. Sample Handling, Processing and Submission
3.4.1. Availability of Written Protocol for Handling/Processing the Ropes and Oral Fluids
3.4.2. Storage of Collected Samples
3.4.3. The Time Range Between Collecting the Oral Fluid Sample and Submission to the Diagnostic Laboratory
3.4.4. Destination of Oral Fluid Samples upon Collection
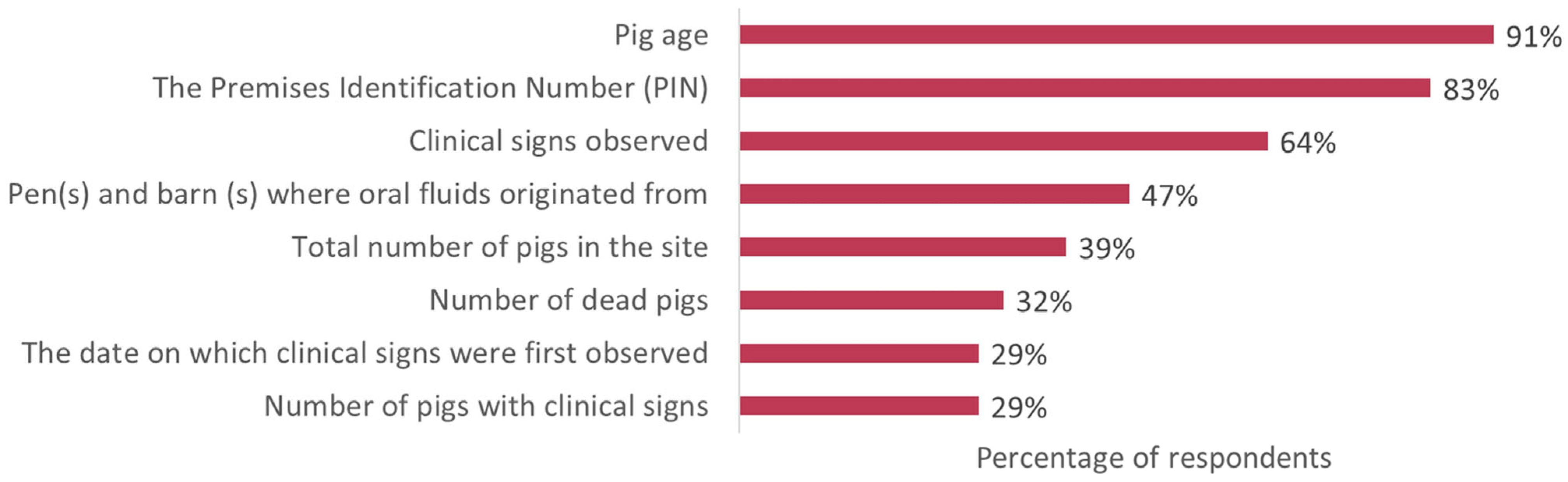
3.4.5. Information Included When Oral Fluid Samples Are Submitted
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AASV | American Association of Swine Veterinarians |
| ASF | African swine fever |
| FAD | Foreign animal disease |
| GDU | Gilt-development unit |
| IAV | Influenza A virus |
| IQR | Interquartile range |
| IRB | Institutional Review Board |
| PCV2 | Porcine circovirus type 2 |
| PDCoV | Porcine deltacoronavirus |
| PEDV | Porcine epidemic diarrhea virus |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| RT-PCR | Reverse transcription polymerase chain reaction |
| USDA | United States Department of Agriculture |
| USDA-APHIS-VS | United States Department of Agriculture-Animal and Plant Health Inspection Service-Veterinary Services |
| VDL | Veterinary diagnostic laboratory |
| MSHMP | Morrison Swine Health Monitoring Project |
References
- Prickett, J.R.; Zimmerman, J.J. The Development of Oral Fluid-Based Diagnostics and Applications in Veterinary Medicine. Anim. Health Res. Rev. 2010, 11, 207–216. [Google Scholar] [CrossRef]
- Henao-Diaz, A.; Giménez-Lirola, L.; Baum, D.H.; Zimmerman, J. Guidelines for Oral Fluid-Based Surveillance of Viral Pathogens in Swine. Porc. Health Manag. 2020, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.C.; Dawes, C.; Ericson, T.; Fox, P.C.; Gandara, B.K.; Malamud, D.; Mandel, I.D.; Navazesh, M.; Tabak, L.A. Guidelines for Saliva Nomenclature and Collection. Ann. N. Y. Acad. Sci. 1993, 694, XI+XII+. [Google Scholar]
- Bjustrom-Kraft, J.; Christopher-Hennings, J.; Daly, R.; Main, R.; Torrison, J.; Thurn, M.; Dvm, J.Z. The Use of Oral Fluid Diagnostics in Swine Medicine. J. Swine Health Prod. 2018, 26, 262–269. [Google Scholar] [CrossRef]
- Prickett, J.; Kim, W.; Simer, R.; Yoon, K.-J.; Zimmerman, J. Oral-Fluid Samples for Surveillance of Commercial Growing Pigs for Porcine Reproductive and Respiratory Syndrome Virus and Porcine Circovirus Type 2 Infections. JSHAP 2008, 16, 86–91. [Google Scholar] [CrossRef]
- Bjustrom-Kraft, J.; Woodard, K.; Giménez-Lirola, L.; Rotolo, M.; Wang, C.; Sun, Y.; Lasley, P.; Zhang, J.; Baum, D.; Gauger, P.; et al. Porcine Epidemic Diarrhea Virus (PEDV) Detection and Antibody Response in Commercial Growing Pigs. BMC Vet. Res. 2016, 12, 99. [Google Scholar] [CrossRef]
- Goodell, C.K.; Prickett, J.; Kittawornrat, A.; Zhou, F.; Rauh, R.; Nelson, W.; O’Connell, C.; Burrell, A.; Wang, C.; Yoon, K.-J.; et al. Probability of Detecting Influenza A Virus Subtypes H1N1 and H3N2 in Individual Pig Nasal Swabs and Pen-Based Oral Fluid Specimens over Time. Vet. Microbiol. 2013, 166, 450–460. [Google Scholar] [CrossRef]
- Homwong, N.; Jarvis, M.C.; Lam, H.C.; Diaz, A.; Rovira, A.; Nelson, M.; Marthaler, D. Characterization and Evolution of Porcine Deltacoronavirus in the United States. Prev. Vet. Med. 2016, 123, 168–174. [Google Scholar] [CrossRef]
- Kikuti, M.; Vilalta, C.; Sanhueza, J.; Melini, C.M.; Corzo, C.A. Porcine Reproductive and Respiratory Syndrome Prevalence and Processing Fluids Use for Diagnosis in United States Breeding Herds. Front. Vet. Sci. 2022, 9, 953918. [Google Scholar] [CrossRef]
- Prickett, J.; Simer, R.; Christopher-Hennings, J.; Yoon, K.-J.; Evans, R.B.; Zimmerman, J.J. Detection of Porcine Reproductive and Respiratory Syndrome Virus Infection in Porcine Oral Fluid Samples: A Longitudinal Study under Experimental Conditions. J. Vet. Diagn. Investig. 2008, 20, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Kittawornrat, A.; Prickett, J.; Chittick, W.; Wang, C.; Engle, M.; Johnson, J.; Patnayak, D.; Schwartz, T.; Whitney, D.; Olsen, C.; et al. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) in Serum and Oral Fluid Samples from Individual Boars: Will Oral Fluid Replace Serum for PRRSV Surveillance? Virus Res. 2010, 154, 170–176. [Google Scholar] [CrossRef]
- Rotolo, M.L.; Sun, Y.; Wang, C.; Giménez-Lirola, L.; Baum, D.H.; Gauger, P.C.; Harmon, K.M.; Hoogland, M.; Main, R.; Zimmerman, J.J. Sampling Guidelines for Oral Fluid-Based Surveys of Group-Housed Animals. Vet. Microbiol. 2017, 209, 20–29. [Google Scholar] [CrossRef]
- Kittawornrat, A.; Prickett, J.; Wang, C.; Olsen, C.; Irwin, C.; Panyasing, Y.; Ballagi, A.; Rice, A.; Main, R.; Johnson, J.; et al. Detection of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Antibodies in Oral Fluid Specimens Using a Commercial PRRSV Serum Antibody Enzyme-Linked Immunosorbent Assay. J. Vet. Diagn. Investig. 2012, 24, 262–269. [Google Scholar] [CrossRef]
- Prost, K.; Kloeze, H.; Mukhi, S.; Bozek, K.; Poljak, Z.; Mubareka, S. Bioaerosol and Surface Sampling for the Surveillance of Influenza A Virus in Swine. Transbound. Emerg. Dis. 2019, 66, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Crum, W.; Bartels, M.; Drum, G.; Kayser, R.; Skoglund, L.; Munger, L.; Honkola, K.; Rotolo, M.; Pepin, B.; Havas, K. An Assessment of Rope Sampling Methodologies on Pen-Level Oral Fluid Samples for Detection of PRRSV Infection. JSHAP 2023, 31, 128–132. [Google Scholar] [CrossRef]
- White, D.; Rotolo, M.; Olsen, C.; Wang, C.; Prickett, J.; Kittawornrat, A.; Panyasing, Y.; Main, R.; Rademacher, C.; Hoogland, M.; et al. Recommendations for Pen-Based Oral-Fluid Collection in Growing Pigs. J. Swine Health Prod. 2014, 22, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Vosloo, W.; Morris, J.; Davis, A.; Giles, M.; Wang, J.; Nguyen, H.T.T.; Kim, P.V.; Quach, N.V.; Le, P.T.T.; Nguyen, P.H.N.; et al. Collection of Oral Fluids Using Cotton Ropes as a Sampling Method to Detect Foot-and-Mouth Disease Virus Infection in Pigs. Transbound. Emerg. Dis. 2015, 62, e71–e75. [Google Scholar] [CrossRef]
- Seddon, Y.M.; Guy, J.H.; Edwards, S.A. Optimising Oral Fluid Collection from Groups of Pigs: Effect of Housing System and Provision of Ropes. Vet. J. 2012, 193, 180–184. [Google Scholar] [CrossRef]
- Pol, F.; Dorenlor, V.; Eono, F.; Eudier, S.; Eveno, E.; Liégard-Vanhecke, D.; Rose, N.; Fablet, C. Individual and Pen-Based Oral Fluid Sampling: A Welfare-Friendly Sampling Method for Group-Housed Gestating Sows. Prev. Vet. Med. 2017, 147, 58–65. [Google Scholar] [CrossRef]
- Tarasiuk, G.; Remmenga, M.D.; O’Hara, K.C.; Talbert, M.K.; Mielke, S.; Rotolo, M.L.; Zaabel, P.; Zhang, D.; Zimmerman, J.J. Optimizing Swine Oral Fluid Sampling Procedures for Growing Pigs in Commercial Settings. Pathogens 2024, 13, 1097. [Google Scholar] [CrossRef] [PubMed]
- USDA-APHIS-VS. African Swine Fever Response Plan: The Red Book; USDA: Washington, DC, USA, 2023. [Google Scholar]
- Spiegel, K.; O’Hara, K.; Vanicek, C.; Beemer, O. Swine Hemorrhagic Fevers: African and Classical Swine Fevers Integrated Surveillance Plan; USDA-APHIS-VS-Center for Epidemiology and Animal Health-Surveillance Design and Analysis Unit: Washington, DC, USA, 2022. [Google Scholar]
- Krug, P.W.; Davis, T.; O’Brien, C.; LaRocco, M.; Rodriguez, L.L. Disinfection of Transboundary Animal Disease Viruses on Surfaces Used in Pork Packing Plants. Vet. Microbiol. 2018, 219, 219–225. [Google Scholar] [CrossRef] [PubMed]
- McVicar, J.W. Quantitative Aspects of the Transmission of African Swine Fever. Am. J. Vet. Res. 1984, 45, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.; Gallardo, C.; Soler, A.; Zimmermman, J.; Pelayo, V.; Nieto, R.; Sánchez-Vizcaíno, J.M.; Arias, M. Potential Use of Oral Fluid Samples for Serological Diagnosis of African Swine Fever. Vet. Microbiol. 2013, 165, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.H. Design a Questionnaire. Br. Med. J. 1993, 307, 1264–1266. [Google Scholar] [CrossRef]
- MSHMP Morrison Swine Health Monitoring Program. Available online: https://mshmp.umn.edu/ (accessed on 29 March 2024).
- Vilalta, C.; Arruda, A.G.; Tousignant, S.J.P.; Valdes-Donoso, P.; Muellner, P.; Muellner, U.; Alkhamis, M.A.; Morrison, R.B.; Perez, A.M. A Review of Quantitative Tools Used to Assess the Epidemiology of Porcine Reproductive and Respiratory Syndrome in U.S. Swine Farms Using Dr. Morrison’s Swine Health Monitoring Program Data. Front. Vet. Sci. 2017, 4, 94. [Google Scholar] [CrossRef]
- USDA, National Agricultural Statistics Service. Quarterly Hogs and Pigs 12/22/2023; USDA: Washington, DC, USA, 2023. [Google Scholar]
- Ramirez, A.; Wang, C.; Prickett, J.R.; Pogranichniy, R.; Yoon, K.-J.; Main, R.; Johnson, J.K.; Rademacher, C.; Hoogland, M.; Hoffmann, P.; et al. Efficient Surveillance of Pig Populations Using Oral Fluids. Prev. Vet. Med. 2012, 104, 292–300. [Google Scholar] [CrossRef]
- Farmer, C. The Gestating and Lactating Sow; BRILL: Leiden, The Netherlands, 2023; ISBN 978-90-8686-803-2. [Google Scholar]
- Secure Pork Supply Plan. Oral Fluid Collection in Pigs; United States Department of Agriculture (USDA), American Association Swine Veterinarians (AASV), National Pork Board, The Center for Food Security & Public Health: Washington, DC, USA, 2016. [Google Scholar]
- University of Minnesota Veterinary Diagnostic Lab Oral Fluids Submission|University of Minnesota. Available online: https://vdl.umn.edu/submission-guidelines/oral-fluids/oral-fluids-submission (accessed on 26 October 2024).
- The Center for Food Security & Public Health. Oral Fluid Collection in Pigs; College of Veterinary Medicine, Iowa State University: Ames, IA, USA, 2016. [Google Scholar]
- Moraes, D.C.d.A.; Osemeke, O.H.; Gauger, P.C.; Moura, C.A.; Trevisan, G.; Silva, G.S.; Linhares, D.C. Probability of Influenza A virus RNA detection at different pooling levels for commonly used sample types in swine breeding herds. Prev. Vet. Med. 2025, 245, 106671. [Google Scholar] [CrossRef]
- Osemeke, O.H.; de Freitas Costa, E.; Almeida, M.N.; Trevisan, G.; Ghosh, A.P.; Silva, G.S.; Linhares, D.C.L. Effect of Pooling Family Oral Fluids on the Probability of PRRSV RNA Detection by RT-rtPCR. Prev. Vet. Med. 2022, 206, 105701. [Google Scholar] [CrossRef]
- Prickett, J.R.; Cutler, S.; Kinyon, J.M.; Naberhaus, N.; Stensland, W.R.; Yoon, K.-J.; Zimmerman, J.J. Stability of Porcine Reproductive and Respiratory Syndrome Virus and Antibody in Swine Oral Fluid. J. Swine Health Prod. 2010, 18, 187–195. [Google Scholar] [CrossRef]
- Faburay, B.; O’Hara, K.; Remmenga, M.; Odoom, T.; Johnson, S.; Tasiame, W.; Ayim-Akonor, M.; Anderson, B.; Kwabena Amoako, K.; Holder, D.; et al. Evaluation of Aggregate Oral Fluid Sampling for Early Detection of African Swine Fever Virus Infection. Viruses 2025, 17, 1089. [Google Scholar] [CrossRef]
- Mai, T.N.; Tran, T.H.G.; Dong, V.H.; Le, V.P.; Huynh, T.M.L.; Bui, T.A.D.; Uemura, R.; Yamazaki, Y.; Nguyen, T.L.; Yamazaki, W. A Highly Sensitive Method for Detecting African Swine Fever Virus in Oral Fluids from Naturally Infected Pigs in Northern Vietnam. Sci. Rep. 2025, 15, 27855. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.C.; Reoyo, A.d.l.T.; Fernández-Pinero, J.; Iglesias, I.; Muñoz, M.J.; Arias, M.L. African Swine Fever: A Global View of the Current Challenge. Porc. Health Manag. 2015, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Tarasiuk, G.; Zaabel, P.; Remmenga, M.; O’Hara, K.; Ye, F.; Rotolo, M.; Zimmerman, J. The Evolving US Swine Industry. J. Swine Health Prod. 2024, 32, 105–110. [Google Scholar] [CrossRef] [PubMed]


| Sections | Details (Corresponding Question Number in the Questionnaire *) | |
|---|---|---|
| Demographics | Name (Q1), contact (Q2), respondent role (Q3), affiliated companies/clinics (Q4), job title (Q5), number of sows and growing pigs they are responsible for (Q6) | |
| Oral fluid implementation and primary use | Use oral fluid for diagnostics (Yes/No) (Q7) | |
| Primary use of oral fluids as a diagnostic sample (testing for active surveillance/passive surveillance based on clinical signs/other factors) (Q12) | ||
| Oral fluid sampling protocols | Sampling frequency | Frequency of sampling per farm type—active surveillance (Q8) |
| Frequency of sampling—no clinical signs (Q9) | ||
| Frequency of sampling—with clinical signs (Q10) | ||
| Other situations impact the frequency (Q11) | ||
| Sample size | Number of ropes per barn and pigs when collecting oral fluids for active surveillance (Q13) | |
| Number of ropes per barn and pigs when collecting oral fluids based on clinical signs (Q14) | ||
| How to/who determine(s) the number of ropes to hang (Q15) | ||
| Average number of ropes used per sampled pen (Q16) | ||
| Average number of pens sampled per barn (Q17) | ||
| Average number of pigs represented by a single oral fluid–rope sample (Q18) | ||
| Sampling process | Personnel collecting oral fluid samples (Q19) | |
| Length of time pigs have access to the rope (Q20) | ||
| Source and the diameter of the rope (Q21) | ||
| Sample handling/processing and submission | Availability of written protocol for handling/processing the ropes (Q22) | |
| Storage of collected sample (Q23) | ||
| Time range between collecting the oral fluid and submission to the diagnostic laboratory (Q24) | ||
| Destination of oral fluid samples upon collection (Q25) | ||
| Information includes when oral fluid samples are submitted (Q26) | ||
| Median | Min; Max | IQR (Interquartile Range) | Number of NA Answer * | |
|---|---|---|---|---|
| Number of ropes hung per barn | 2 | 1; 15 | 1; 4 | 5 |
| Number of pens sampled per barn | 6 | 1; 30 | 4; 10 | 3 |
| Number of pigs per rope in the airspace **—active surveillance | 500 | 20; 1700 | 263; 650 | 24 |
| Number of pigs per rope in the airspace **—clinical sign | 500 | 38; 2800 | 288; 700 | 22 |
| Number of pigs represented by a single oral fluid—rope sample *** | 138 | 5; 1200 | 60; 294 | 0 |
| Time Between Sample Collection and Submission | Median | Minimum | Maximum | IQR |
|---|---|---|---|---|
| Average (h) | 24 | 2 | 48 | 12; 24 |
| Minimum (h) | 4.5 | 0 | 48 | 2; 16 |
| Maximum (h) | 36 | 4 | 96 | 24; 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, X.; Leonard, M.; Tavai-Tuisalo’o, M.; Malladi, S.; Kikuti, M.; Melini, C.M.; Zaabel, P.; Culhane, M.R.; Corzo, C.A. Swine Practitioner Practices on Oral Fluid Sampling in U.S. Swine Farms: A Nationwide Survey. Pathogens 2025, 14, 940. https://doi.org/10.3390/pathogens14090940
Yue X, Leonard M, Tavai-Tuisalo’o M, Malladi S, Kikuti M, Melini CM, Zaabel P, Culhane MR, Corzo CA. Swine Practitioner Practices on Oral Fluid Sampling in U.S. Swine Farms: A Nationwide Survey. Pathogens. 2025; 14(9):940. https://doi.org/10.3390/pathogens14090940
Chicago/Turabian StyleYue, Xiaomei, Mickey Leonard, Margret Tavai-Tuisalo’o, Sasidhar Malladi, Mariana Kikuti, Claudio Marcello Melini, Pam Zaabel, Marie R. Culhane, and Cesar A. Corzo. 2025. "Swine Practitioner Practices on Oral Fluid Sampling in U.S. Swine Farms: A Nationwide Survey" Pathogens 14, no. 9: 940. https://doi.org/10.3390/pathogens14090940
APA StyleYue, X., Leonard, M., Tavai-Tuisalo’o, M., Malladi, S., Kikuti, M., Melini, C. M., Zaabel, P., Culhane, M. R., & Corzo, C. A. (2025). Swine Practitioner Practices on Oral Fluid Sampling in U.S. Swine Farms: A Nationwide Survey. Pathogens, 14(9), 940. https://doi.org/10.3390/pathogens14090940







