Global Research Trends on Major Pathogenic Enteric Viruses (1990–2024): A Bibliometric Analysis of Epidemiology, Transmission, and Public Health Impact
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design (PICOS Criteria)
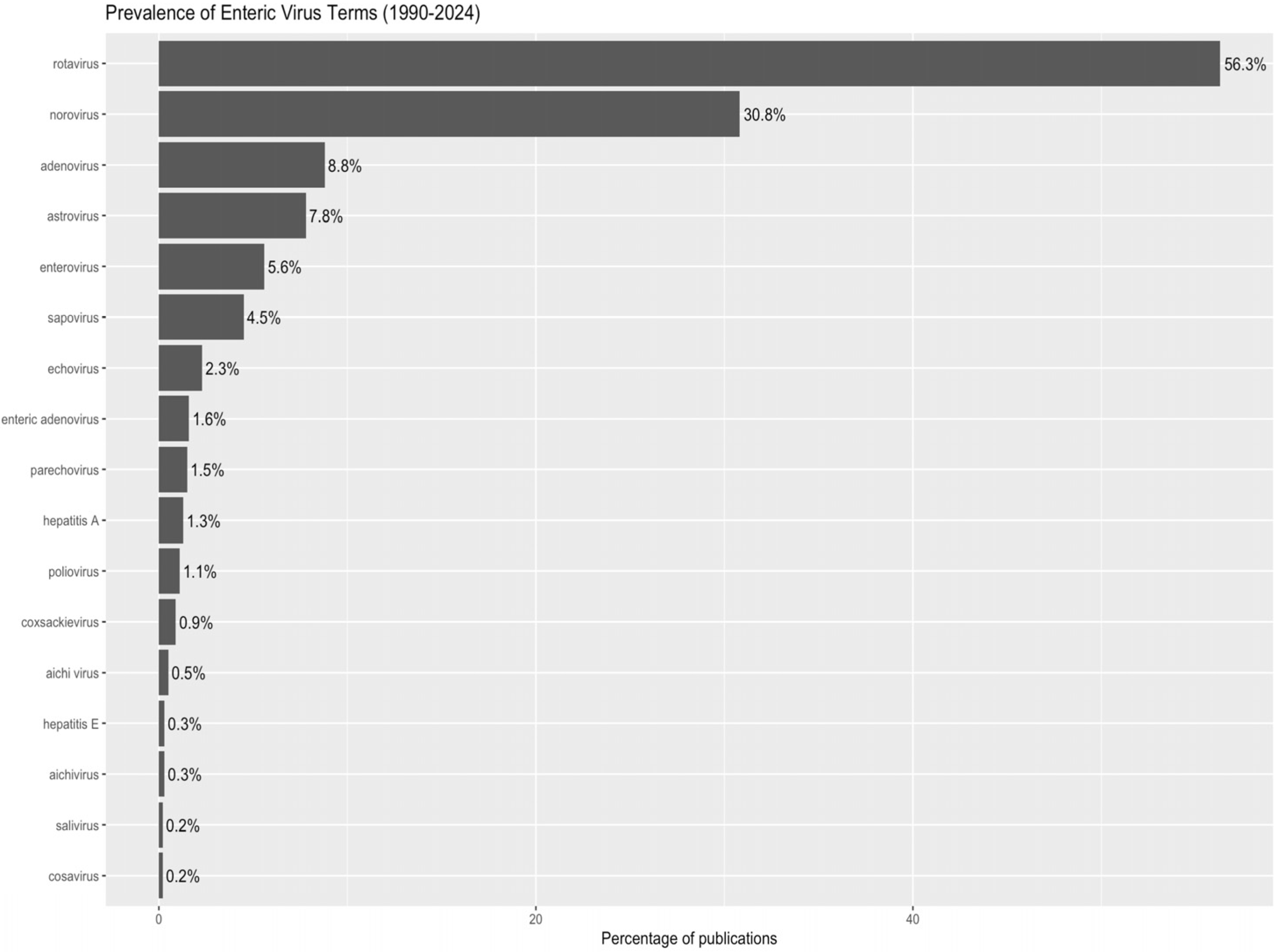
- Population (P): The scientific literature on the major pathogenic enteric viruses defined in this study was published between 1 January 1990, and 31 December 2024. We included publications addressing enteric viruses of significant public health importance, including rotavirus (family Sedoreoviridae), norovirus, sapovirus, and astrovirus (Caliciviridae, Astroviridae), as well as adenovirus (Adenoviridae). Within the Picornaviridae family, we considered enteroviruses (non-polio enteroviruses such as coxsackievirus and echovirus), as well as human parechoviruses (genus Parechovirus) and Aichi virus (genus Kobuvirus), both of which are increasingly recognized as enteric pathogens. For clarity, parechoviruses and Aichi virus were treated as distinct categories, not as subgroups of enteroviruses.
- Intervention (I): Bibliometric studies (i.e., analyses of publication trends, authorship, citations, keyword co-occurrence).
- Comparator (C): Not applicable.
- Outcomes (O): Research trends, including publication volume, geographic distribution, collaboration networks, thematic evolution, and methodological shifts in diagnostics and prevention.
- Study designs (S): Original research articles and review papers.
2.2. Information Sources and Search Strategy
Search Details
- Keywords and MeSH:
- o
- General: “enteric viruses”, “gastroenteritis”, “diarrhea”, “vomiting”, “dehydration”, “fecal-oral transmission”, “public health”, “epidemiology”, “transmission”, “diagnosis”, “prevention”, “vaccination”, “sanitation”, “hygiene”.
- o
- Specific: “rotavirus”, “norovirus”, “enteric adenovirus”, “astrovirus”, “sapovirus”, “viral gastroenteritis”, “waterborne diseases”, “foodborne diseases”, “outbreaks”, “clinical manifestations”, “molecular diagnostics”, “antigen-based tests”, “electron microscopy”, “oral rehydration therapy”, “vaccine efficacy”, “global burden”, “low-income countries”, “high-income countries”.
- o
- MeSH Terms: “Enterovirus Infections”[Mesh], “Gastroenteritis”[Mesh], “Diarrhea”[Mesh], “Vomiting”[Mesh], “Dehydration”[Mesh], “Fecal-Oral Route”[Mesh], “Public Health”[Mesh], “Epidemiology”[Mesh], “Disease Transmission, Infectious”[Mesh], “Diagnosis”[Mesh], “Prevention & Control”[Mesh], “Vaccination”[Mesh], “Sanitation”[Mesh], “Hygiene”[Mesh], “Rotavirus”[Mesh], “Norovirus”[Mesh], “Adenoviruses, Human”[Mesh], “Astroviridae”[Mesh], “Sapovirus”[Mesh].
- Boolean Example: (“enteric virus*” OR “gastroenter*” OR diarrh*) AND (“fecal-oral transmiss*” OR “public health” OR epidemiolog*).
- Wildcards Examples: “enteric virus*”, “enterovir*”, “gastroenter*”, “diarrh*”, “vomit*”, “dehydrat*”, “fecal-oral transmiss*”, “public health*”, “epidemiol*”, “transmiss*”, “diagnos*”, “prevent*”, “vaccinat*”, “sanitat*”, “hygien*”.
- Timeframe: 1990–2024 (search performed 1 February 2025).
- Search Queries:
- o
- Scopus Query:
TITLE-ABS-KEY( ( rotavirus OR norovirus OR “enteric adenovirus” OR “adenovirus F40” OR “adenovirus F41” OR astrovirus OR sapovirus OR enterovir* OR coxsackievirus* OR echovirus* OR “Aichi virus” OR kobuvirus OR parechovirus* OR picornaviridae ) AND ( “viral gastroenter*” OR gastroenteritis OR diarrh* OR vomit* OR dehydrat* OR “fecal-oral” OR waterborne OR foodborne ) AND ( epidemiol* OR transmiss* OR diagnos* OR “molecular surveillance” OR “genomic sequenc*” OR prevent* OR vaccinat* OR “antiviral therap*” OR WASH OR sanitati* OR “climate change” ) ) AND ( LIMIT-TO(DOCTYPE, “ar”) OR LIMIT-TO(DOCTYPE, “re”) ) AND ( EXCLUDE(SUBJAREA, “VETE”) OR EXCLUDE(SUBJAREA, “AGRI”) ) AND (PUBYEAR > 1989 AND PUBYEAR < 2025) AND ( LIMIT-TO(LANGUAGE, “English”) OR LIMIT-TO(LANGUAGE, “French”) ) - o
- PubMed Query:
( ( rotavirus[Title/Abstract] OR rotavirus[MeSH Terms] OR norovirus[Title/Abstract] OR norovirus[MeSH Terms] OR “enteric adenovirus”[Title/Abstract] OR “adenovirus F40”[Title/Abstract] OR “adenovirus F41”[Title/Abstract] OR “Adenoviridae”[MeSH Terms] OR astrovirus[Title/Abstract] OR “Astroviridae”[MeSH Terms] OR sapovirus[Title/Abstract] OR enterovirus[Title/Abstract] OR “Enterovirus Infections”[MeSH Terms] OR coxsackievirus[Title/Abstract] OR echovirus[Title/Abstract] OR “Aichi virus”[Title/Abstract] OR kobuvirus[Title/Abstract] OR parechovirus[Title/Abstract] OR “Parechovirus”[MeSH Terms] OR “human parechovirus”[Title/Abstract] OR picornaviridae[Title/Abstract] ) AND ( “gastroenteritis”[Title/Abstract] OR “Gastroenteritis”[MeSH Terms] OR diarrh*[Title/Abstract] OR “Diarrhea”[MeSH Terms] OR vomit*[Title/Abstract] OR “Dehydration”[MeSH Terms] OR “fecal-oral route”[MeSH Terms] OR “waterborne diseases”[MeSH Terms] OR “foodborne diseases”[MeSH Terms] ) AND ( epidemiology[MeSH Terms] OR “disease transmission”[MeSH Terms] OR diagnosis[MeSH Terms] OR “molecular surveillance”[Title/Abstract] OR “genomic sequencing”[Title/Abstract] OR prevention[MeSH Terms] OR vaccination[MeSH Terms] OR “antiviral agents”[MeSH Terms] OR sanitation[MeSH Terms] OR “climate change”[MeSH Terms] ) ) AND (english[Language] OR french[Language]) AND (“1990/01/01”[Date - Publication]: “2024/12/31”[Date - Publication]) AND (Humans[Mesh]).
2.3. Eligibility Criteria
- Inclusion Criteria: Research articles and reviews in English and French on the epidemiology, transmission, diagnosis, prevention, and public health of enteric viruses. We acknowledge that this language restriction may underrepresent research published in languages other than English, such as Spanish, Portuguese, Chinese, or in non-indexed regional journals, which is a potential source of selection bias.
- Exclusion Criteria: Studies should directly relate to epidemiology or public health and focus on human enteric viruses. Exclude animal-only research, non-English/French papers, and non-peer-reviewed sources such as conference proceedings, non-peer-reviewed book chapters, and editorials.
- Focus Specification: To maintain a focus on gastroenteritis, the analysis of enterovirus-related publications was restricted to those reporting on gastrointestinal manifestations or fecal viral excretion.
- Age: No age restrictions were applied; pediatric and adult studies were included.
- Co-infections: Studies reporting coinfections (viral, bacterial, and/or parasitic agents) were included only if virus-specific data could be unequivocally extracted (e.g., if results were stratified by pathogen or if viral outcomes were reported separately). If viral data could not be separated from non-viral outcomes, the article was excluded from virus-specific thematic analyses but documented in the selection journal.
2.4. Study Selection Process
2.5. Data Extraction and Management
2.5.1. Export and Import
- PubMed: Retrieved PMID, title, abstract, author names, affiliations, publication date, keywords, MeSH terms, and citation metadata (where available).
- Scopus: Retrieved EID/DOI, title, abstract, author names, affiliations, publication date, keywords, citation counts, journal name, and other bibliometric fields.
- All exports were saved as CSV and imported into RStudio (v4.3.3) using bibliometrix, readr, or data.table.
2.5.2. Deduplication and Cleaning
- Priority matching by DOI, when available; otherwise, exact title, first author, and year. Via distinct() and dplyr (v1.1.0) to remove precise duplicates.
- Standardized author names (“LastName Initials”), harmonized institution and country names (ISO standards).
- Institutions: Cleaned common variants/spelling differences (e.g., “Univ. of X” vs. “University of X”).
- Flagged records missing key metadata fields (e.g., no publication year, no affiliation data). A total of 108 records were excluded at this stage due to missing affiliation data, as noted in Section 2.4.
- All preprocessing steps, counts at each stage, and any manual corrections were logged in R scripts for reproducibility.
- During the pre-processing steps, 709 records that could not be retrieved during Biblioshiny export (as noted in Section 2.5) were logged and excluded. Then, 108 records (1.2% of the evaluated records) were excluded due to missing key metadata (specifically, affiliation data), and zero records were flagged for manual correction of import errors.
2.6. Subset and Thematic Filtering
2.6.1. Regional Subsets
- West Africa (Ghana, Nigeria, Senegal, Côte d’Ivoire, Mali, Burkina Faso, Benin, and Togo). Any record with at least one author affiliation in these countries was included; multi-affiliation papers were counted in each relevant subset but tracked to avoid double-counting when aggregating global metrics (204 records).
- Southern Africa (Botswana, Namibia, South Africa, Zimbabwe, Zambia, Malawi) with the same inclusion logic as above (309 records).
2.6.2. Risk-Factor Keywords
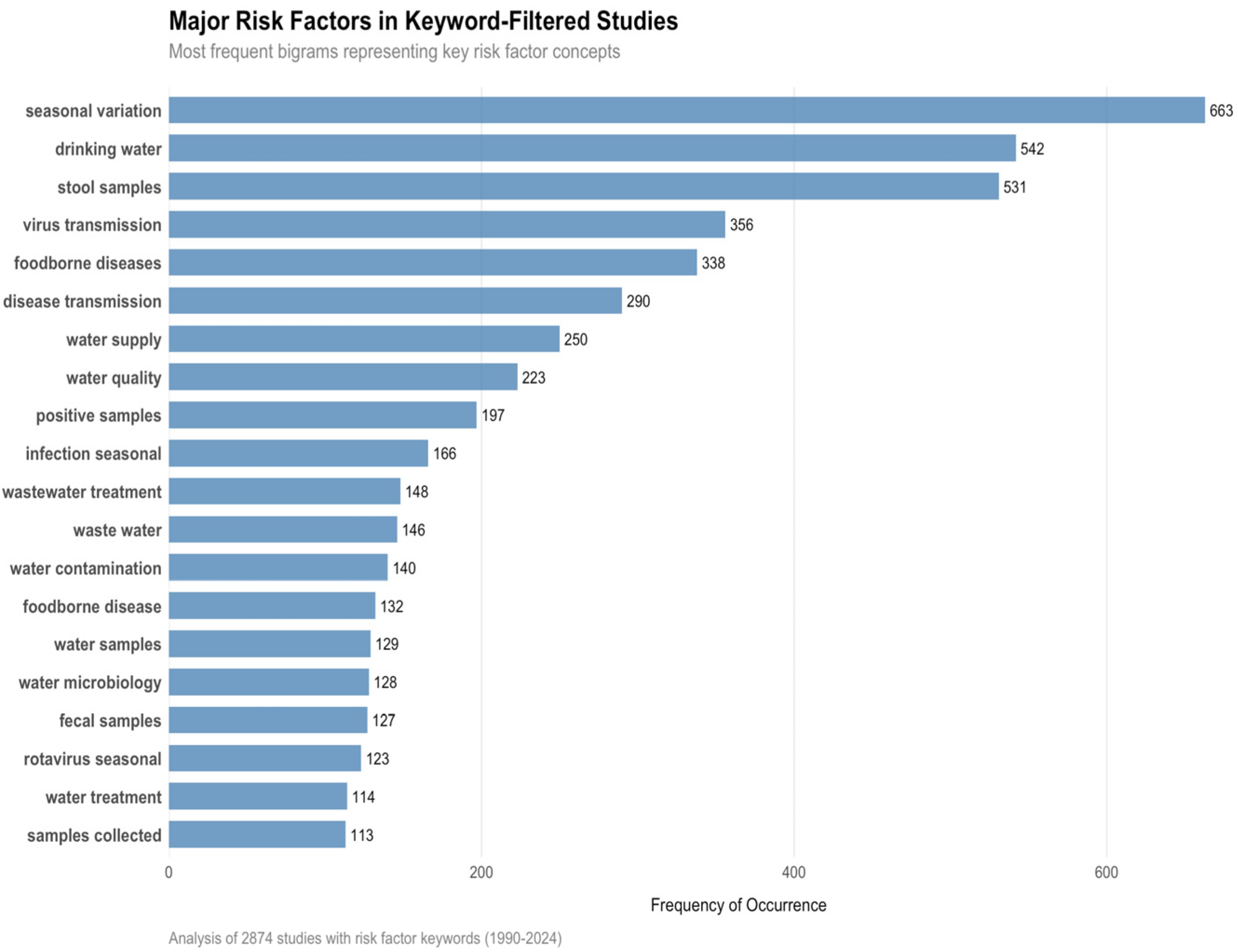
- Terms: “sanitation,” “water,” “population density,” “zoonotic” (and variants via wildcard truncation if relevant, e.g., ‘sanitat*’, ‘zoonot*’). Automated search in title/abstract/keywords flagged 7422 articles; each was manually validated.
2.7. Automated Epidemiological Extraction
- Targets: Incidence, mortality, and morbidity estimates mentioned in titles/abstracts.
- Method: Regex patterns capturing numeric-per-population expressions; ~10% randomly checked and refined iteratively. Results are reported descriptively.
2.8. Bibliometric Analyses
- Productivity trends: Yearly counts; CAGR (compound annual growth rate) with 95% CIs calculated per standard formulas.
- Key contributors:
- o
- Author-, institution-, and country-level metrics: total publications and average citations per document.
- Impact Metrics: H-index, total/average citations by author/institution/country based on the collected dataset snapshot (full counting).
- Journal ranking: By publications count and impact factor (sourced from Journal Citation Reports for the relevant year; details in script).
- Software:
2.9. Contextualization and Sensitivity Analyses
- Sensitivity analysis for rotavirus dominance: To assess whether the observed trends were strongly influenced by rotavirus research, we (i) excluded all publications explicitly mentioning rotavirus and (ii) applied a fractional counting method (where each article mentioning “k” viruses contributes “1/k” to each virus’s count).
- Contextualization of publication growth: to determine if the observed increase in publications in 2021 was specific to our field or part of a broader trend in biomedical publishing, we compared the annual growth of our corpus to the total number of publications indexed in PubMed each year. PubMed was chosen as the global reference because it offers free and exhaustive access to biomedical publications. The data on global PubMed production was obtained via the Entrez API.
3. Results
3.1. Study Selection
3.2. Descriptive Analysis
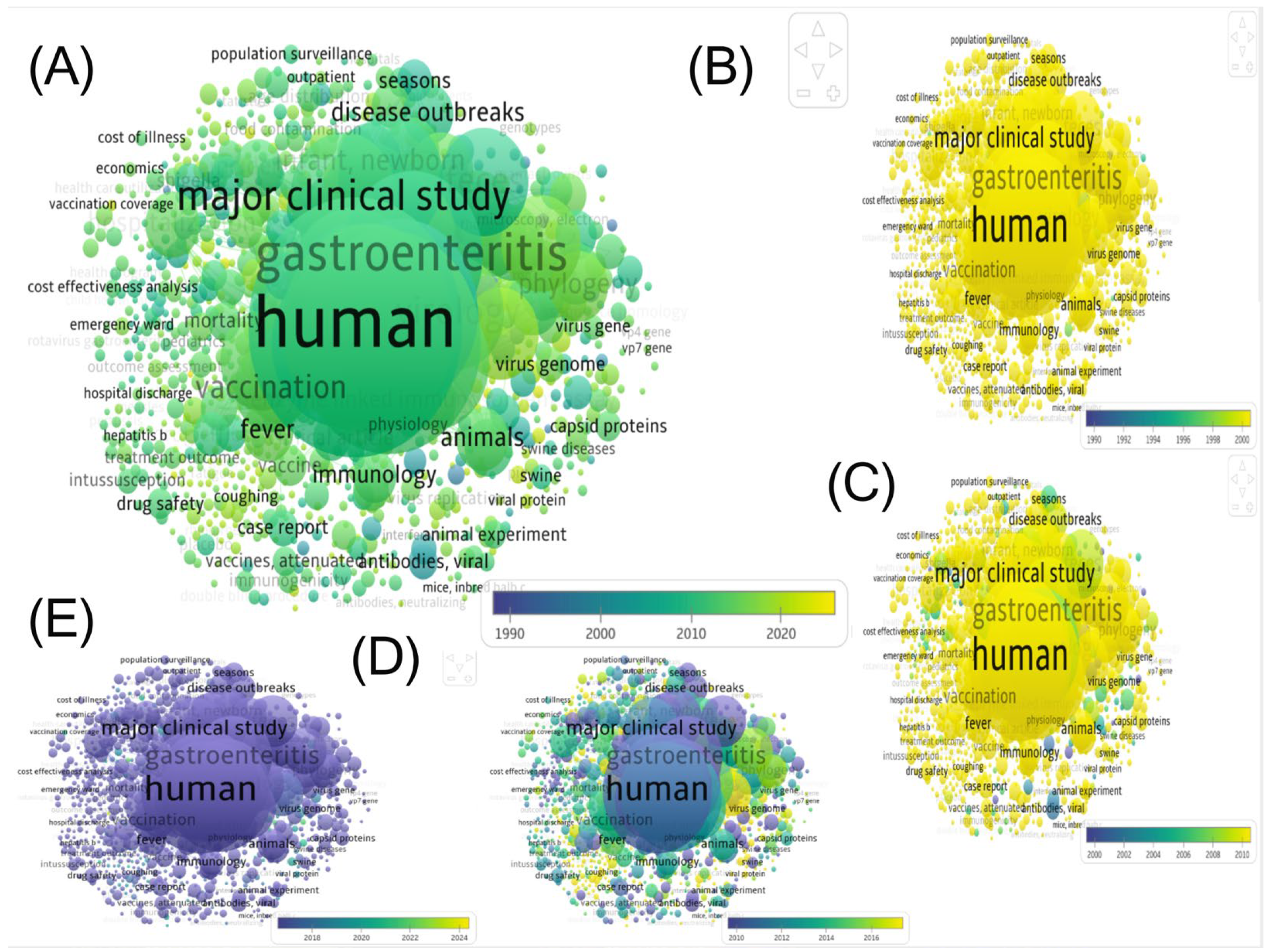
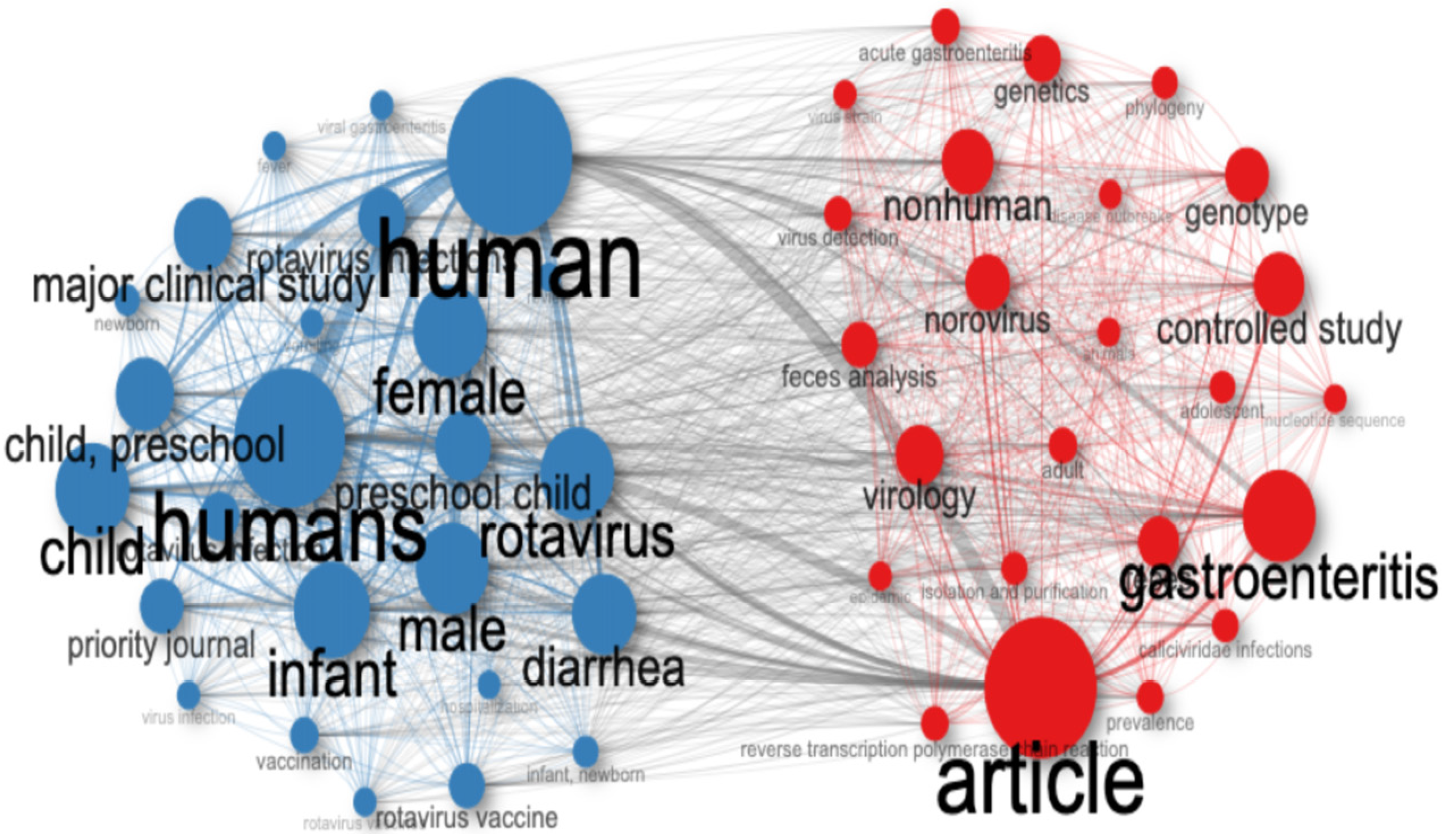
3.2.1. General Overview
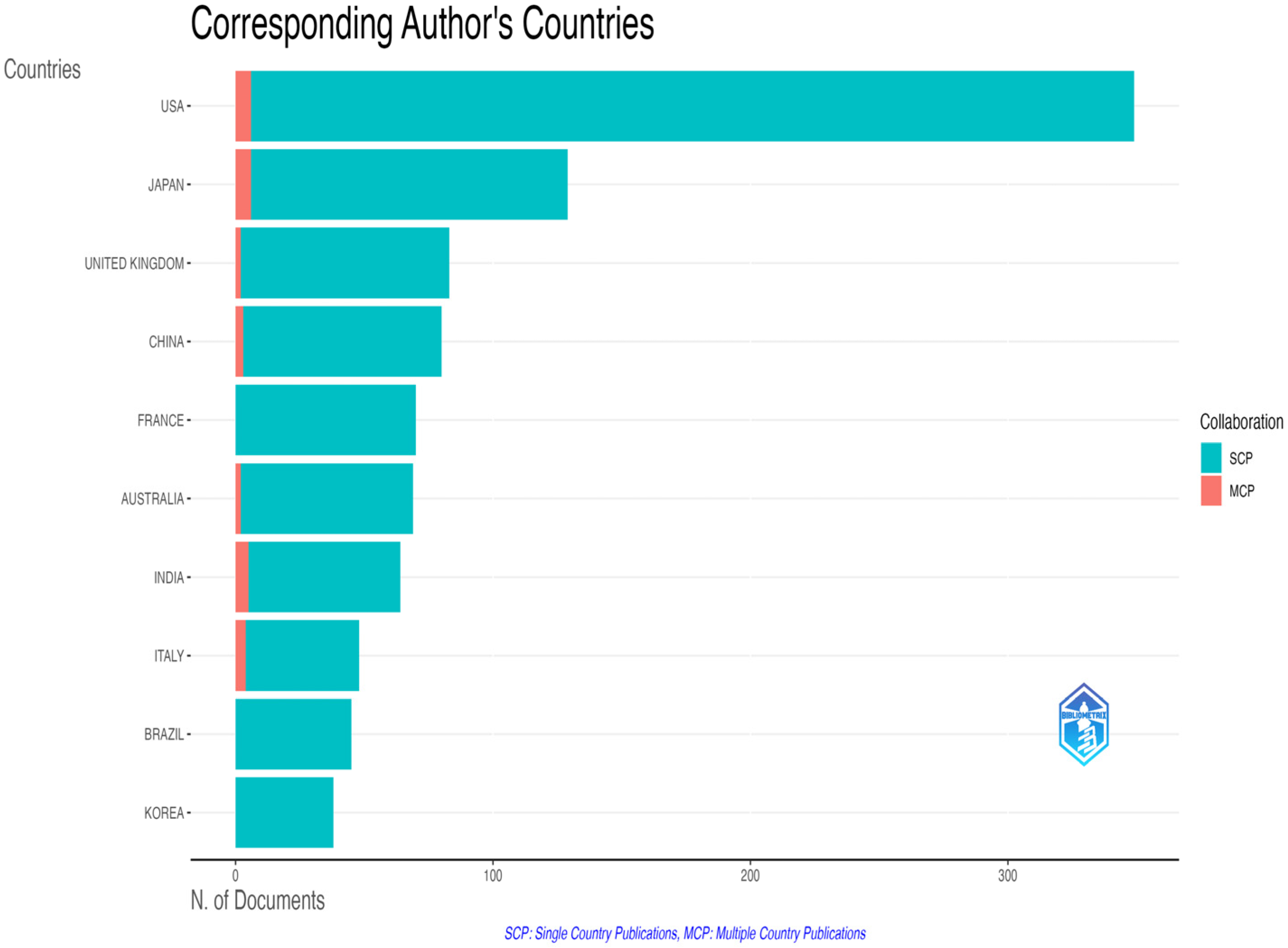
3.2.2. Geographic and Institutional Distribution
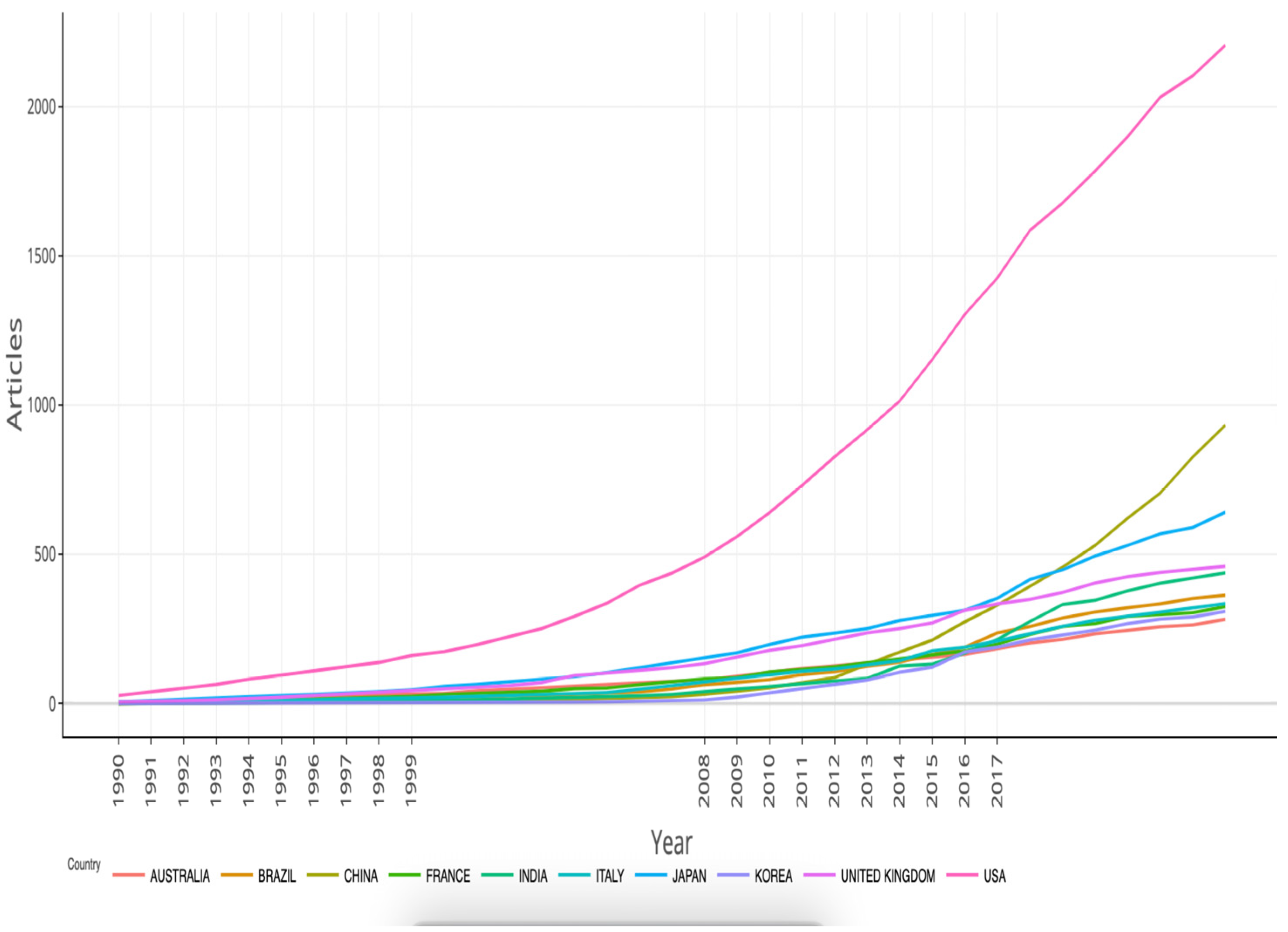
3.3. A Research Landscape Dominated by Rotavirus
Comparison with General Biomedical Publication Trends
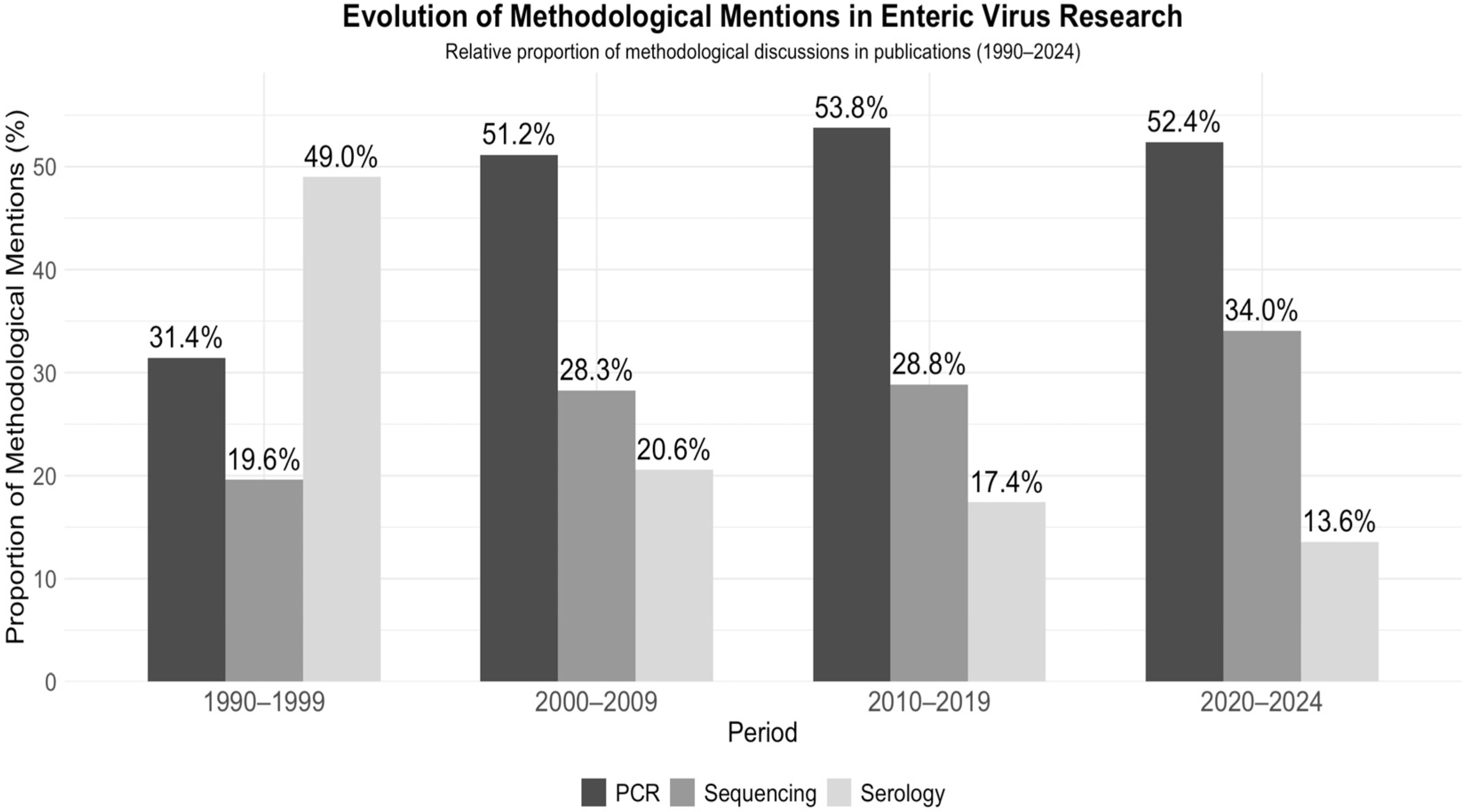
3.4. Trends in Epidemiology and Methods
3.5. Transmission Dynamics and Risk Factors
3.6. Public Health Impact
4. Discussion
4.1. Rotavirus Predominance: Causes and Implications
4.2. COVID-19 and the 2021 Publication Surge
4.3. Geographic Disparities
4.4. Methodological Trends
4.5. Transmission Dynamics and Risk Factors
4.6. Thematic Focus at the Expense of Antivirals
4.7. Policy Implications
- Expand equitable rotavirus immunization coverage, particularly in low- and middle-income countries (LMICs), through Gavi-supported platforms and reinforcement of cold-chain logistics;
- Scale genomic surveillance infrastructures in underserved regions by funding local laboratories, training scientists, and ensuring access to sequencing tools and data analysis platforms;
- Adopt integrated One Health surveillance in zoonotic hotspots by combining human, animal, and environmental monitoring to improve early detection and outbreak response;
- Invest in sustainable WASH (Water, Sanitation, and Hygiene) systems, recognizing that diagnostics and vaccines are insufficient without foundational public health infrastructure;
- Ensure inclusive knowledge sharing and data equity by strengthening open-access repositories, FAIR data standards, and inclusive platforms for LMIC researchers (e.g., preprints, community peer review);
- Incorporate climate and socio-demographic data into future surveillance models to anticipate outbreak risks in vulnerable populations and improve public health preparedness.
- Address gender and equity barriers in access to prevention, diagnosis, and care, with a focus on context-specific health education and resource allocation;
- Together, these measures provide a roadmap for stakeholders to strengthen surveillance, prevention, and research equity, thereby reducing the global burden of enteric viruses.
5. Conclusions and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lekana-Douki, S.E.; Kombila-Koumavor, C.; Nkoghe, D.; Drosten, C.; Drexler, J.F.; Leroy, E.M. Molecular Epidemiology of Enteric Viruses and Genotyping of Rotavirus A, Adenovirus and Astrovirus among Children under 5 Years Old in Gabon. Int. J. Infect. Dis. 2015, 34, 90–95. [Google Scholar] [CrossRef]
- Janko, M.M.; Joffe, J.; Michael, D.; Earl, L.; Rosettie, K.L.; Sparks, G.W.; Albertson, S.B.; Compton, K.; Pedroza Velandia, P.; Stafford, L.; et al. Cost-Effectiveness of Rotavirus Vaccination in Children under Five Years of Age in 195 Countries: A Meta-Regression Analysis. Vaccine 2022, 40, 3903–3917. [Google Scholar] [CrossRef] [PubMed]
- Burnett, E.M.; Parashar, U.; Tate, J. Global Impact of Rotavirus Vaccination on Diarrhea Hospitalizations and Deaths among Children <5 Years Old: 2006–2019. J. Infect. Dis. 2020, 222, 1731–1739. [Google Scholar] [CrossRef]
- Armah, G.; Lopman, B.A.; Vinjé, J.; O’Ryan, M.; Lanata, C.F.; Groome, M.; Ovitt, J.; Marshall, C.; Sajewski, E.; Riddle, M.S. Vaccine Value Profile for Norovirus. Vaccine 2023, 41, S134–S152. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Ugra, D. A Study of Efficacy of ‘Rota Virus Vaccination’ on Morbidity Due to Rotavirus Diarrhoea in Children Aged 6 Months to 5 Years. Int. J. Contemp. Pediatr. 2021, 8, 1819–1823. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention About Norovirus; Department of Health & Human Services: Atlanta, GA, USA, 2024; Available online: https://www.cdc.gov/norovirus/about/index.html (accessed on 14 September 2025).
- Kumthip, K.; Khamrin, P.; Yodmeeklin, A.; Maneekarn, N. Prevalence and Genetic Characterization of Aichivirus in Environmental Waters in Thailand. Food Environ. Virol. 2020, 12, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.F.; Maqsood, R.; Sullins, R.A.; Driver, E.; Halden, R.U.; Lim, E.S. Seasonality of Respiratory, Enteric, and Urinary Viruses Revealed by Wastewater Genomic Surveillance. mSphere 2024, 9, e00105-24. [Google Scholar] [CrossRef]
- Sánchez, S.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Ávalos, M.; Guzmán-Trampe, S.; et al. Carbon Source Regulation of Antibiotic Production. J. Antibiot. 2010, 63, 442–459. [Google Scholar] [CrossRef]
- Dawley, C.; Gibson, K. Virus-Bacteria Interactions: Implications for Prevention and Control of Human Enteric Viruses from Environment to Host. Foodborne Pathog. Dis. 2019, 16, 81–89. [Google Scholar] [CrossRef]
- Juliao, P.; Guzman-Holst, A.; Gupta, V.; Velez, C.; Petrozzi, V.; Ochoa, T.J. Acute Gastroenteritis Morbidity and Mortality Trends Following Universal Rotavirus Vaccination in Children in Peru: Ecological Database Study with Time-Trend Analysis. Infect. Dis. Ther. 2021, 10, 2563–2574. [Google Scholar] [CrossRef]
- Pereira, V.; Assis, R.; Costa, K.; Silva, A.; Tavares, R.; Pires, F.; Simon, G.; Paiva Filho, S.; Ferreira, C.; Lopes, I. Root Growth Characteristics of Millet Cultivars and Sorghum Hybrids under Increasing Levels of Soil Compaction. Aust. J. Crop Sci. 2022, 16, 286–292. [Google Scholar] [CrossRef]
- Bose, T.; Borrow, R.; Arkwright, P.D. Impact of Rotavirus Vaccination on Diarrheal Disease Burden of Children in South America. Expert Rev. Vaccines 2024, 23, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Bouseettine, R.; Hassou, N.; Bessi, H.; Ennaji, M. Waterborne Transmission of Enteric Viruses and Their Impact on Public Health. In Emerging and Reemerging Viral Pathogens; Academic Press: Cambridge, MA, USA, 2019; pp. 907–932. [Google Scholar] [CrossRef]
- Isanaka, S.; Langendorf, C.; McNeal, M.; Meyer, N.; Plikaytis, B.; Garba, S.; Sayinzoga-Makombe, N.; Soumana, I.; Guindo, O.; Makarimi, R.; et al. Rotavirus Vaccine Efficacy up to 2 Years of Age and against Diverse Circulating Rotavirus Strains in Niger: Extended Follow-up of a Randomized Controlled Trial. PLoS Med. 2021, 18, e1003655. [Google Scholar] [CrossRef]
- Melhem, N.M.; Abou Hassan, F.F.; Ramadan, M. The Current Status of Norovirus Vaccine Development. In Norovirus; Melhem, N.M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 189–242. ISBN 978-3-030-27209-8. [Google Scholar]
- Sun, Y.; Yuan, Y.; Mao, H.; Su, L.; Ge, Q.; Gao, J.; Xu, C.; Gong, L. Molecular Epidemiology of Human Norovirus Variants from Outbreaks in Zhejiang Province, China, during 2021. Adv. Virol. 2024, 2024, 7972494. [Google Scholar] [CrossRef] [PubMed]
- Alidjinou, E.K.; Sane, F.; Firquet, S.; Lobert, P.; Hober, D. Resistance of Enteric Viruses on Fomites. Intervirology 2017, 61, 205–213. [Google Scholar] [CrossRef]
- Farkas, K.; Cooper, D.; McDonald, J.; Malham, S.; de Rougemont, A.; Jones, D.L. Seasonal and Spatial Dynamics of Enteric Viruses in Wastewater and in Riverine and Estuarine Receiving Waters. Sci. Total Environ. 2018, 634, 1174–1183. [Google Scholar] [CrossRef]
- Meister, S.; Klinger, M.; Kohn, T. Isolation and Characterization of Disinfection-Resistant Enteroviruses. In Proceedings of the 74th Annual Swiss Society for Microbiology Meeting, Bern, Switzerland, 13–16 June 2016. [Google Scholar]
- Al-Shammari, A.; Naji, N.; Tawfeeq, Y.; Al-Badri, S.; Al-Fatlawi, N.; Hasan, H.; Al-Gizey, N.; Tawfeeq, R.; Al-Jorani, M.S.; Al-Ibraheem, A. Unraveling Coxsackie and Echovirus, Epidemiology, Pathogenesis, and Global Challenges: From Molecular Mechanisms to Clinical Realities and Global Health Implications. In Distribution and Treatment of Infectious Diseases: Challenges for Developed and Developing Nations; IGI Global Scientific Publishing: Hershey, PA, USA, 2025. [Google Scholar]
- Harvala, H.; Robertson, I.; McWilliam Leitch, E.C.; Benschop, K.; Wolthers, K.C.; Templeton, K.; Simmonds, P. Epidemiology and Clinical Associations of Human Parechovirus Respiratory Infections. J. Clin. Microbiol. 2008, 46, 3446–3453. [Google Scholar] [CrossRef]
- Alam, F.; Li, Y.; Vogt, M.R. Parechovirus: Neglected for Too Long? J. Virol. 2025, 99, e01846-24. [Google Scholar] [CrossRef]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef] [PubMed]
- Elois, M.A.; Pavi, C.P.; Jempierre, Y.F.S.H.; Pilati, G.V.T.; Zanchetta, L.; Grisard, H.B.d.S.; García, N.; Rodríguez-Lázaro, D.; Fongaro, G. Trends and Challenges in the Detection and Environmental Surveillance of the Hepatitis E Virus. Microorganisms 2025, 13, 998. [Google Scholar] [CrossRef] [PubMed]
- Gloriani, N.G.; de Paz-Silava, S.L.M.; Allison, R.D.; Takashima, Y.; Avagyan, T. The Shifting Epidemiology of Hepatitis A in the World Health Organization Western Pacific Region. Vaccines 2024, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Ciarlet, M.; Estes, M.K. Rotavirus and Calicivirus Infections of the Gastrointestinal Tract. Curr. Opin. Gastroenterol. 2001, 17, 10. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to Conduct a Bibliometric Analysis: An Overview and Guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Chacón, L.; Morales, E.; Valiente, C.; Reyes, L.; Barrantes, K. Wastewater-Based Epidemiology of Enteric Viruses and Surveillance of Acute Gastrointestinal Illness Outbreaks in a Resource-Limited Region. Am. J. Trop. Med. Hyg. 2021, 105, 1004–1012. [Google Scholar] [CrossRef]
- Verani, M.; Pagani, A.; Federigi, I.; Lauretani, G.; Atomsa, N.T.; Rossi, V.; Viviani, L.; Carducci, A. Wastewater-Based Epidemiology for Viral Surveillance from an Endemic Perspective: Evidence and Challenges. Viruses 2024, 16, 482. [Google Scholar] [CrossRef]
- Clark, M.; Severn, M. Wastewater Surveillance for Communicable Diseases; Emerging Health Technologies; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2023. [Google Scholar]
- Huang, Y.; Zhou, N.; Zhang, S.; Yi, Y.; Han, Y.; Liu, M.; Han, Y.; Shi, N.; Yang, L.; Wang, Q.; et al. Norovirus Detection in Wastewater and Its Correlation with Human Gastroenteritis: A Systematic Review and Meta-Analysis. Environ. Sci. Pollut. Res. Int. 2022, 29, 22829–22842. [Google Scholar] [CrossRef]
- Kilmarx, P.H.; Glass, R.I. Building Global Health Research Capacity to Address Research Imperatives Following the COVID-19 Pandemic. PLoS Med. 2021, 18, e1003753. [Google Scholar] [CrossRef]
- Wao, H.; Wang, Y.; Wao, M.A.; Were, J.A. Factors Associated with North–South Research Collaboration Focusing on HIV/AIDS: Lessons from ClinicalTrials.Gov. AIDS Res. Ther. 2021, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Charani, E.; Abimbola, S.; Pai, M.; Adeyi, O.; Mendelson, M.; Laxminarayan, R.; Rasheed, M.A. Funders: The Missing Link in Equitable Global Health Research? PLoS Glob. Public Health 2022, 2, e0000583. [Google Scholar] [CrossRef]
- Global Burden of Disease 2021 Health Financing Collaborator Network Global Investments in Pandemic Preparedness and COVID-19: Development Assistance and Domestic Spending on Health between 1990 and 2026. Lancet Glob. Health 2023, 11, e385–e413. [CrossRef]
- Li, T.; Qiang, N.; Bao, Y.; Li, Y.; Zhao, S.; Chong, K.C.; Deng, X.; Zhang, X.; Ran, J.; Han, L. Global Burden of Enteric Infections Related Foodborne Diseases, 1990–2021: Findings from the Global Burden of Disease Study 2021. Sci. One Health 2024, 3, 100075. [Google Scholar] [CrossRef]
- World Health Organization Global Observatory on Health R&D. Bridging the Gap in Global Health Research and Development; World Health Organization Global Observatory on Health R&D: Geneva, Switzerland, 2023. [Google Scholar]
- Adam, T.; Ralaidovy, A.H.; Ross, A.L.; Reeder, J.C.; Swaminathan, S. Tracking Global Resources and Capacity for Health Research: Time to Reassess Strategies and Investment Decisions. Health Res. Policy Syst. 2023, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Odeny, B.; Bosurgi, R. Time to End Parachute Science. PLoS Med. 2022, 19, e1004099. [Google Scholar] [CrossRef]
- Hedt-Gauthier, B.L.; Jeufack, H.M.; Neufeld, N.H.; Alem, A.; Sauer, S.; Odhiambo, J.; Boum, Y.; Shuchman, M.; Volmink, J. Stuck in the Middle: A Systematic Review of Authorship in Collaborative Health Research in Africa, 2014–2016. BMJ Glob. Health 2019, 4, e001853. [Google Scholar] [CrossRef]
- Mbaye, R.; Gebeyehu, R.; Hossmann, S.; Mbarga, N.; Bih-Neh, E.; Eteki, L.; Thelma, O.-A.; Oyerinde, A.; Kiti, G.; Mburu, Y.; et al. Who Is Telling the Story? A Systematic Review of Authorship for Infectious Disease Research Conducted in Africa, 1980–2016. BMJ Glob. Health 2019, 4, e001855. [Google Scholar] [CrossRef] [PubMed]
- Tohma, K.; Lepore, C.J.; Martinez, M.; Degiuseppe, J.I.; Khamrin, P.; Saito, M.; Mayta, H.; Nwaba, A.U.A.; Ford-Siltz, L.A.; Green, K.Y.; et al. Genome-Wide Analyses of Human Noroviruses Provide Insights on Evolutionary Dynamics and Evidence of Coexisting Viral Populations Evolving under Recombination Constraints. PLOS Pathog. 2021, 17, e1009744. [Google Scholar] [CrossRef]
- Bellandi, D. Rotavirus Leads Global Diarrhea Hospitalizations Among Young Children. JAMA 2022, 328, 1490. [Google Scholar] [CrossRef]
- Njifon, H.; Kenmoe, S.; Ahmed, S.M.; Takuissu, G.R.; Ebogo-Belobo, J.; Njile, D.K.; Bowo-Ngandji, A.; Mbaga, D.S.; Kengne-Ndé, C.; Mouiche, M.M.; et al. Epidemiology of Rotavirus in Humans, Animals, and the Environment in Africa: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2023, 229, 1470–1480. [Google Scholar] [CrossRef]
- Ghonaim, A.H.; Rouby, S.R.; Nageeb, W.M.; Elgendy, A.A.; Xu, R.; Jiang, C.; Ghonaim, N.H.; He, Q.; Li, W. Insights into Recent Advancements in Human and Animal Rotavirus Vaccines: Exploring New Frontiers. Virol. Sin. 2025, 40, 1–14. [Google Scholar] [CrossRef]
- Ghonaim, A.H.; Hopo, M.G.; Ghonaim, N.H.; Jiang, Y.; He, Q.; Li, W. The Epidemiology of Circulating Rotavirus Associated with Diarrhea in Egyptian Kids and Calves: A Review. Zoonoses 2023, 3, 985. [Google Scholar] [CrossRef]
- França, Y.; Medeiros, R.S.; Viana, E.; de Azevedo, L.S.; Guiducci, R.; da Costa, A.C.; Luchs, A. Genetic Diversity and Evolution of G12P[6] DS-1-like and G12P[9] AU-1-like Rotavirus Strains in Brazil. Funct. Integr. Genomics 2024, 24, 92. [Google Scholar] [CrossRef]
- Grassi, T.; Donno, A.D.; Guido, M.; Gabutti, G.; Familyyok, G. The Epidemiology and Disease Burden of Rotavirus Infection in the Salento Peninsula, Italy. Turk. J. Pediatr. 2008, 50, 132–136. [Google Scholar] [PubMed]
- Angel, J.; Franco, M.A.; Greenberg, H.B. Rotavirus Vaccines: Recent Developments and Future Considerations. Nat. Rev. Microbiol. 2007, 5, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; McKenzie, T.; Gaw, I.M.; Dean, J.M.; von Hammerstein, H.; Knudson, T.A.; Setter, R.O.; Smith, C.Z.; Webster, K.M.; Patz, J.A.; et al. Over Half of Known Human Pathogenic Diseases Can Be Aggravated by Climate Change. Nat. Clim. Change 2022, 12, 869–875. [Google Scholar] [CrossRef]
- Carmo dos Santos, M.; Cerqueira Silva, A.C.; dos Reis Teixeira, C.; Pinheiro Macedo Prazeres, F.; Fernandes dos Santos, R.; de Araújo Rolo, C.; de Souza Santos, E.; Santos da Fonseca, M.; Oliveira Valente, C.; Saraiva Hodel, K.V.; et al. Wastewater Surveillance for Viral Pathogens: A Tool for Public Health. Heliyon 2024, 10, e33873. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, L.; Sha, Y.; Zhang, B.; Zhang, K. Impact of Dual Climatic and Socioeconomic Factors on Global Trends in Infectious Disease Outbreaks. Sci. Rep. 2025, 15, 16092. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, A.; Song, L.; Tong, Y.; Fan, H. Advances in the Development of Antivirals for Rotavirus Infection. Front. Immunol. 2023, 14, 1041149. [Google Scholar] [CrossRef]
- Pevear, D.C.; Tull, T.M.; Seipel, M.E.; Groarke, J.M. Activity of Pleconaril against Enteroviruses. Antimicrob. Agents Chemother. 1999, 43, 2109–2115. [Google Scholar] [CrossRef]
- Rossignol, J.-F.; El-Gohary, Y.M. Nitazoxanide in the Treatment of Viral Gastroenteritis: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Aliment. Pharmacol. Ther. 2006, 24, 1423–1430. [Google Scholar] [CrossRef]
- Rossignol, J.-F.; Abu-Zekry, M.; Hussein, A.; Santoro, M.G. Effect of Nitazoxanide for Treatment of Severe Rotavirus Diarrhoea: Randomised Double-Blind Placebo-Controlled Trial. Lancet 2006, 368, 124–129. [Google Scholar] [CrossRef]
- Ebenezer, O.; Jordaan, M.A.; Damoyi, N.; Shapi, M. Discovery of Potential Inhibitors for RNA-Dependent RNA Polymerase of Norovirus: Virtual Screening, and Molecular Dynamics. Int. J. Mol. Sci. 2020, 22, 171. [Google Scholar] [CrossRef]
- Gingerich, A.; Mahoney, L.; McCormick, A.L.; Miller, R.J.; Mousa, J. Human Monoclonal Antibodies Protect against Viral-Mediated Pneumococcal Superinfection. Front. Immunol. 2024, 15, 1364622. [Google Scholar] [CrossRef]
- Koszalka, P.; Tilmanis, D.; Hurt, A.C. Influenza Antivirals Currently in Late-Phase Clinical Trial. Influenza Other Respir. Viruses 2017, 11, 240–246. [Google Scholar] [CrossRef]
- Brendish, N.J.; Clark, T.W. Antiviral Treatment of Severe Non-Influenza Respiratory Virus Infection. Curr. Opin. Infect. Dis. 2017, 30, 573. [Google Scholar] [CrossRef]





| Family | Virus | Key Epidemiological Features | Transmission Routes | Public Health Impact | Prevention/Treatment | Research Challenges | References |
|---|---|---|---|---|---|---|---|
| Sedoreoviridae | Rotavirus | Leading cause of severe pediatric diarrhea globally; peak incidence in children <5 years | Fecal–oral, contaminated water/food | High morbidity and mortality in LMICs; seasonal epidemics | Vaccination, WASH interventions, supportive care | Strain diversity, vaccine escape variants, limited surveillance in LMICs | [3] |
| Caliciviridae | Norovirus, Sapovirus | Major cause of gastroenteritis across all ages; frequent outbreaks in closed settings | Fecal–oral, contaminated food/water, fomites | High burden in both HICs and LMICs; outbreaks in hospitals, cruise ships, schools | Hygiene, outbreak control measures, vaccine development ongoing | High genetic diversity, short-lived immunity, limited vaccines | [16,17] |
| Adenoviridae | Enteric adenovirus (F40/F41) | Causes diarrhea primarily in infants and young children | Fecal–oral | Moderate global burden; associated with sporadic outbreaks | Supportive care, hygiene measures | Limited epidemiological data, few diagnostics in LMICs | [18,19] |
| Astroviridae | Astrovirus | Often mild diarrhea in children; some severe cases in immunocompromised | Fecal–oral | Underrecognized; global incidence unclear | Hygiene, supportive care | Limited surveillance, low research attention | [14,20] |
| Picornaviridae 1 | Enteroviruses (genus Enterovirus; e.g., coxsackievirus, echovirus). Other enteric picornaviruses include human parechoviruses (HPeV; genus Parechovirus) and Aichi virus (AiV; genus Kobuvirus) | Clinical spectrum ranges from gastroenteritis to occasional systemic/CNS disease; some types associated with severe neonatal disease (e.g., certain HPeV types) | Fecal–oral, waterborne | Significant pediatric morbidity in outbreaks; variable detection across settings | Supportive care; some experimental antivirals | Diagnostic heterogeneity, limited surveillance, taxonomic and nomenclature confusion in older literature | [21,22,23,24] |
| Hepeviridae | Hepatitis E virus (HEV) | Enterically transmitted hepatitis; high risk in pregnant women | Fecal–oral, contaminated water | Severe outbreaks in LMICs; high mortality in pregnancy | Vaccination (restricted availability), WASH | Poor surveillance, limited vaccine coverage outside China | [25] |
| Hepatoviridae | Hepatitis A virus (HAV) | Self-limiting acute hepatitis; widespread global exposure | Fecal–oral | Moderate burden; epidemics in regions with poor sanitation | Vaccination, WASH | Limited genomic surveillance, outbreak prediction challenges | [26] |
| Country | Total Publications | Corresponding Authorships | Leadership Share (%) * |
|---|---|---|---|
| USA | 2205 | 349 | 15.8 |
| China | 932 | 80 | 8.6 |
| Japan | 641 | 129 | 20.1 |
| India | 438 | 64 | 14.6 |
| Brazil | 363 | 45 | 12.4 |
| Italy | 335 | 48 | 14.3 |
| France | 325 | 70 | 21.5 |
| South Korea | 309 | 38 | 12.3 |
| Australia | 282 | 69 | 24.5 |
| Germany | 223 | 24 | 10.8 |
| Country | Articles | Institution | Articles | Journal | Articles |
|---|---|---|---|---|---|
| USA | 3180 | Centers for Disease Control and Prevention | 902 | Journal of Medical Virology | 432 |
| China | 1912 | National Center for Immunization and Respiratory Diseases | 521 | Vaccine | 378 |
| Japan | 1034 | University of Liverpool | 362 | Journal of Clinical Microbiology | 246 |
| India | 874 | Christian Medical College | 341 | Pediatric Infectious Disease Journal | 214 |
| Brazil | 689 | Baylor College of Medicine | 318 | PLOS One | 195 |
| Italy | 602 | International Centre for Diarrhoeal Disease Research | 287 | Viruses | 168 |
| UK | 511 | Emory University | 263 | Epidemiology and Infection | 151 |
| Spain | 498 | University of Virginia | 244 | Archives of Virology | 147 |
| France | 472 | Chiang Mai University | 229 | Journal of Infectious Diseases | 139 |
| South Korea | 438 | Nihon University School of Medicine | 196 | BMC Infectious Diseases | 133 |
| Keyword | 1990–1999 % | 2000–2009 % | 2010–2019 % | 2020–2024 % |
|---|---|---|---|---|
| Children | 54.0 | 48.5 | 51.7 | 50.8 |
| Gastroenteritis | 42.3 | 52.5 | 55.1 | 46.0 |
| Diarrhea | 56.9 | 44.7 | 42.0 | 41.1 |
| Norovirus | 0.0 | 26.6 | 35.9 | 32.7 |
| Genome sequencing | 10.6 | 16.5 | 19.4 | 26.2 |
| Gastroenteritis | 42.3 | 52.5 | 55.1 | 46.0 |
| Rotavirus vaccines | 18.6 | 17.0 | 19.2 | 16.7 |
| Outbreak | 10.8 | 24.3 | 18.0 | 15.6 |
| RT-PCR | 4.7 | 15.0 | 13.8 | 10.4 |
| Adenovirus | 9.5 | 7.4 | 7.1 | 9.8 |
| Astrovirus | 11.1 | 9.5 | 6.7 | 7.8 |
| Sapovirus | 0.0 | 3.5 | 4.7 | 6.0 |
| Molecular epidemiology | 2.7 | 6.1 | 5.8 | 4.9 |
| Wastewater | 0.9 | 1.8 | 1.9 | 2.5 |
| Serotyping | 26.6 | 9.9 | 3.6 | 1.8 |
| RT-PCR | 4.7 | 15.0 | 13.8 | 10.4 |
| Study Type | Number of Studies | Percentage |
|---|---|---|
| Vaccine | 3311 | 39.8% |
| Morbidity | 2045 | 24.6% |
| Molecular/Genetic | 1890 | 22.7% |
| Incidence/Prevalence | 1042 | 12.5% |
| Mortality | 31 | 0.4% |
| Other | 1 | 0.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, M.; Al-Khalaifah, H.; Bouhoudan, A. Global Research Trends on Major Pathogenic Enteric Viruses (1990–2024): A Bibliometric Analysis of Epidemiology, Transmission, and Public Health Impact. Pathogens 2025, 14, 938. https://doi.org/10.3390/pathogens14090938
Alotaibi M, Al-Khalaifah H, Bouhoudan A. Global Research Trends on Major Pathogenic Enteric Viruses (1990–2024): A Bibliometric Analysis of Epidemiology, Transmission, and Public Health Impact. Pathogens. 2025; 14(9):938. https://doi.org/10.3390/pathogens14090938
Chicago/Turabian StyleAlotaibi, Mohammad, Hanan Al-Khalaifah, and Assia Bouhoudan. 2025. "Global Research Trends on Major Pathogenic Enteric Viruses (1990–2024): A Bibliometric Analysis of Epidemiology, Transmission, and Public Health Impact" Pathogens 14, no. 9: 938. https://doi.org/10.3390/pathogens14090938
APA StyleAlotaibi, M., Al-Khalaifah, H., & Bouhoudan, A. (2025). Global Research Trends on Major Pathogenic Enteric Viruses (1990–2024): A Bibliometric Analysis of Epidemiology, Transmission, and Public Health Impact. Pathogens, 14(9), 938. https://doi.org/10.3390/pathogens14090938







