Trends in Antibiotic Resistance of Escherichia coli Strains Isolated from Clinical Samples (2019–2023): A Hospital-Based Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Data Anonymization
2.2. Sample Collection
2.3. Bacterial Isolation
2.4. Bacterial Identification
2.5. Antibiotic Susceptibility Testing
2.6. Statistical Analysis
2.7. Representativeness and Limitations
3. Results
3.1. Distribution of Analyzed Clinical Specimens
3.2. Distribution of Isolated Microorganisms and the Impact of the Pandemic on Laboratory Activity
3.3. Urine Cultures and Infection Rates
3.4. E. coli Prevalence
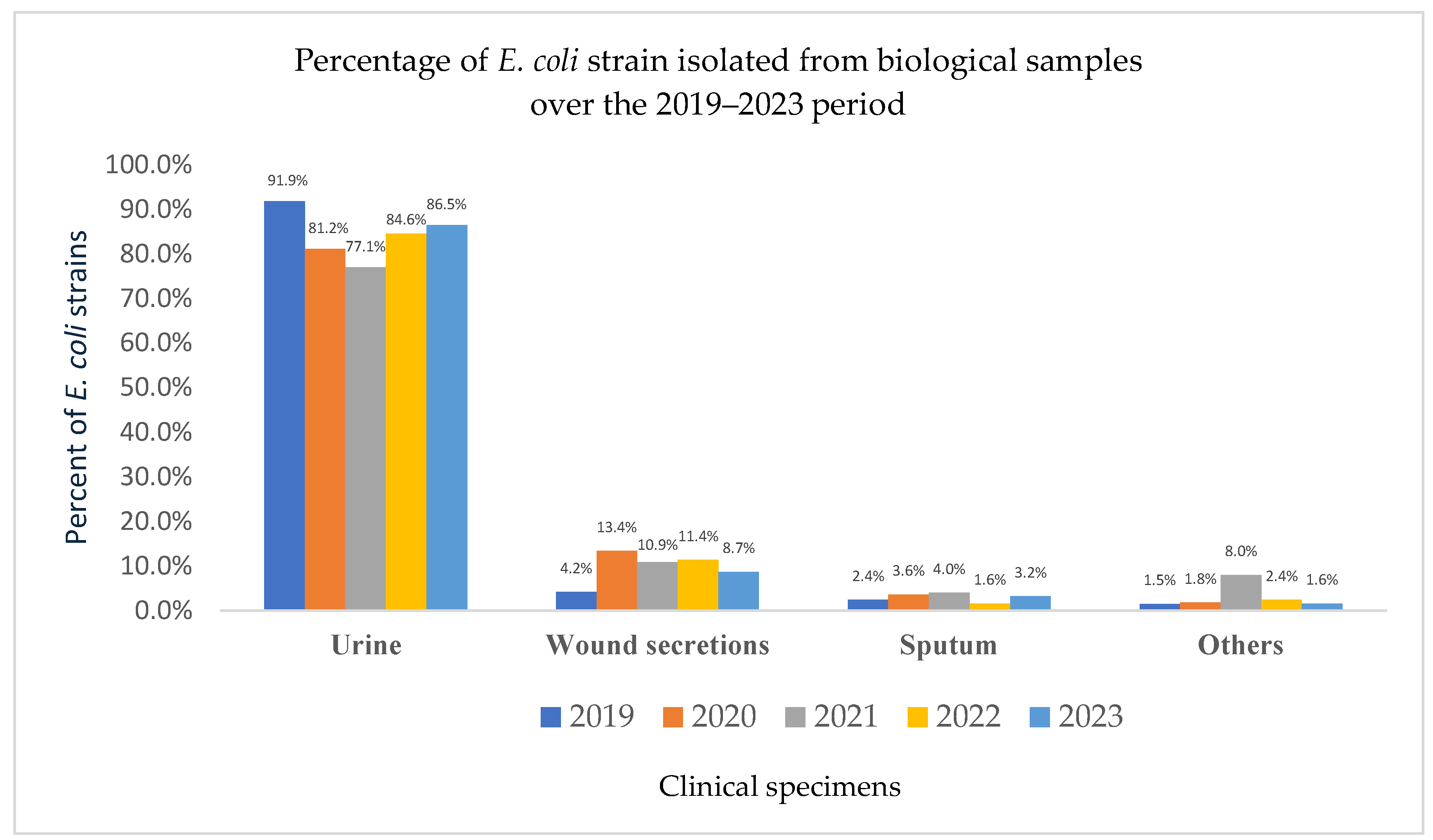
3.5. Source and Demographic Distribution of E. coli Isolates
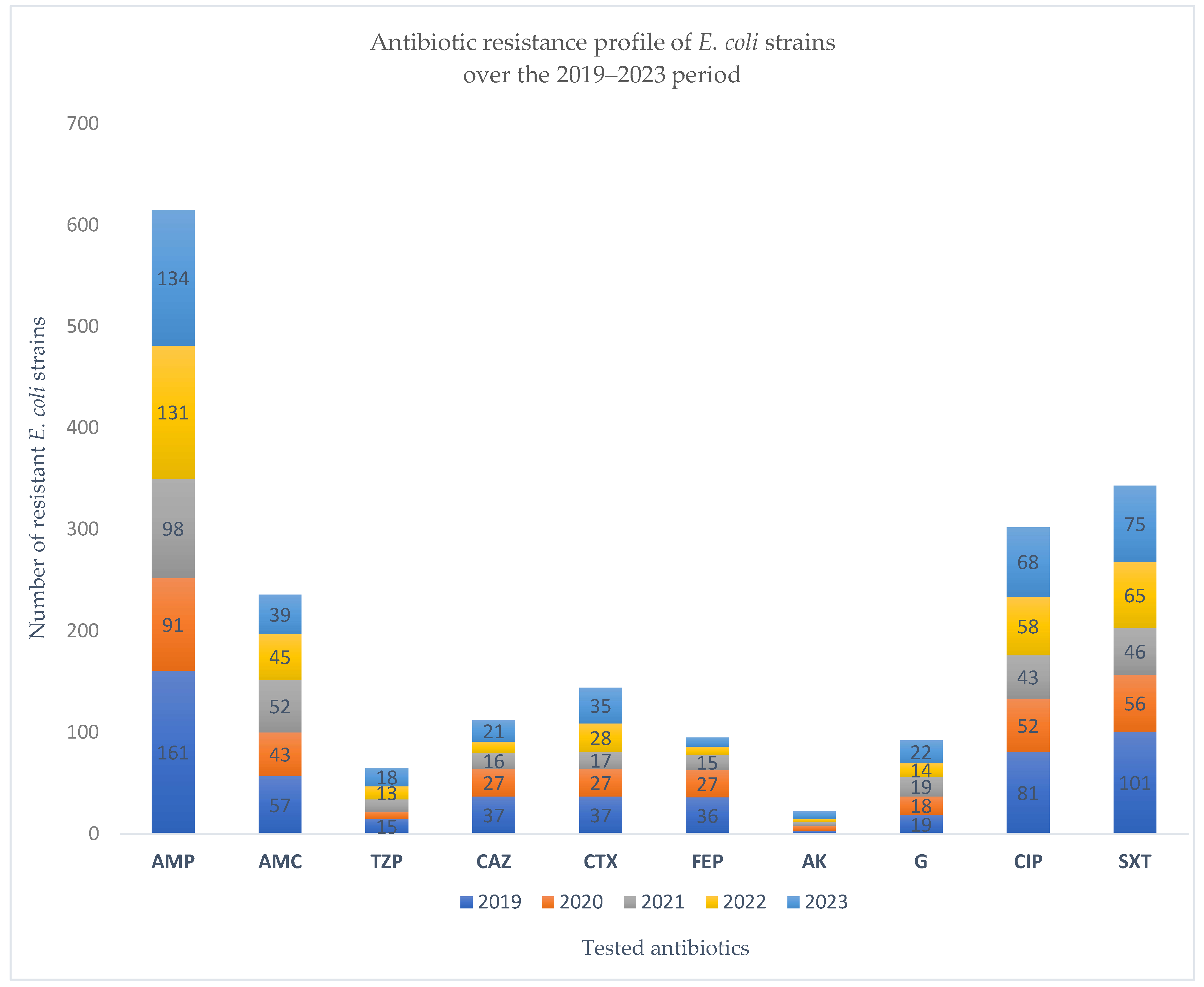
3.6. Five-Year Surveillance of Antibiotic Resistance Patterns in E. coli Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, S.; Chen, H.; Chen, Y.; Dong, S.; Mai, H.; Lopes, B.S.; Liu, S.; Wen, F. Trends in Antibiotic Resistance Patterns and Burden of Escherichia Coli Infections in Young Children: A Retrospective Cross-Sectional Study in Shenzhen, China from 2014–2018. Infect. Drug Resist. 2023, 16, 5501–5510. [Google Scholar] [CrossRef]
- Zagaglia, C.; Ammendolia, M.G.; Maurizi, L.; Nicoletti, M.; Longhi, C. Urinary Tract Infections Caused by Uropathogenic Escherichia coli Strains-New Strategies for an Old Pathogen. Microorganisms 2022, 10, 1425. [Google Scholar] [CrossRef]
- Trześniewska-Ofiara, Z.; Mendrycka, M.; Cudo, A.; Szmulik, M.; Woźniak-Kosek, A. Hospital Urinary Tract Infections in Healthcare Units on the Example of Mazovian Specialist Hospital Ltd. Front. Cell. Infect. Microbiol. 2022, 12, 891796. [Google Scholar] [CrossRef]
- Mahshouri, P.; Alikhani, M.Y.; Momtaz, H.E.; Doosti-Irani, A.; Shokoohizadeh, L. Analysis of phylogroups, biofilm formation, virulence factors, antibiotic resistance and molecular typing of uropathogenic Escherichia coli strains isolated from patients with recurrent and non-recurrent urinary tract infections. BMC Infect. Dis. 2025, 25, 267. [Google Scholar] [CrossRef] [PubMed]
- Sujith, S.; Solomon, A.P.; Rayappan, J.B.B. Comprehensive insights into UTIs: From pathophysiology to precision diagnosis and management. Front. Cell. Infect. Microbiol. 2024, 14, 1402941. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Zheng, L.; Gong, Z.; Li, Y.; Jin, Y.; Huang, Y.; Chi, M. Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options. Int. J. Mol. Sci. 2023, 24, 10537. [Google Scholar] [CrossRef]
- Palusiak, A. Proteus mirabilis and Klebsiella pneumoniae as pathogens capable of causing co-infections and exhibiting similarities in their virulence factors. Front. Cell. Infect. Microbiol. 2022, 12, 991657. [Google Scholar] [CrossRef]
- Lila, A.S.A.; Rajab, A.A.H.; Abdallah, M.H.; Rizvi, S.M.D.; Moin, A.; Khafagy, E.S.; Tabrez, S.; Hegazy, W.A.H. Biofilm Lifestyle in Recurrent Urinary Tract Infections. Life 2023, 13, 148. [Google Scholar] [CrossRef]
- Behzadi, P.; García-Perdomo, H.A.; Autrán Gómez, A.M.; Pinheiro, M.; Sarshar, M. Editorial: Uropathogens, urinary tract infections, the host-pathogen interactions and treatment. Front. Microbiol. 2023, 14, 1183236. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Fong Coronado, P.A.; Gastélum Cano, J.M.; Sánchez Chimeu, V.H.; Acosta Sandria, L.; Fragoso Flores, J. Comparative antibiotic resistance in urine cultures before and after the SARS-CoV-2 Pandemic. Microbe 2024, 4, 100134. [Google Scholar] [CrossRef]
- Stanley, J.; Sullivan, B.; Dowsey, A.W.; Jones, K.; Beck, C.R. Epidemiology of Escherichia coli bloodstream infection antimicrobial resistance trends across South West England during the first 2 years of the coronavirus disease 2019 pandemic response. Clin. Microbiol. Infect. 2024, 30, 1291–1297. [Google Scholar] [CrossRef]
- Ilmavirta, H.; Ollgren, J.; Räisänen, K.; Kinnunen, T.; Hakanen, A.J.; Rantakokko-Jalava, K.; Jalava, J.; Lyytikäinen, O. Impact of the COVID-19 pandemic on extended-spectrum β-lactamase producing Escherichia coli in urinary tract and blood stream infections: Results from a nationwide surveillance network, Finland, 2018 to 2022. Antimicrob. Resist. Infect. Control 2024, 13, 72. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.; Robson, J. COVID-19 restrictions limited interactions of people and resulted in lowered E. coli antimicrobial resistance rates. JAC Antimicrob. Resist. 2024, 6, dlae125. [Google Scholar] [CrossRef]
- Golli, A.L.; Popa, S.G.; Ghenea, A.E.; Turcu, F.L. The Impact of the COVID-19 Pandemic on the Antibiotic Resistance of Gram-Negative Pathogens Causing Bloodstream Infections in an Intensive Care Unit. Biomedicines 2025, 13, 379, Erratum in: Biomedicines 2025, 13, 848. https://doi.org/10.3390/biomedicines13040848. [Google Scholar] [CrossRef]
- INSP-CNSCBT. Analysis of the Evolution of Communicable Diseases Under Surveillance—Report for 2020 and 2021; National Institute of Public Health—National Center for Communicable Disease Surveillance and Control: Bucharest, Romania, 2022; pp. 2394–2537. [Google Scholar]
- Rozwadowski, M.; Gawel, D. Molecular Factors and Mechanisms Driving Multidrug Resistance in Uropathogenic Escherichia coli—An Update. Genes 2022, 13, 1397. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A review of the mechanisms that confer antibiotic resistance in pathotypes of E. coli. Front. Cell. Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasani, H.M.; Al-Rubaye, D.S.; Abdelhameed, A. The emergence of multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR) in Iraqi clinical isolates of Escherichia coli. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 469–482. [Google Scholar] [CrossRef]
- Kasanga, M.; Kwenda, G.; Wu, J.; Kasanga, M.; Mwikisa, M.J.; Chanda, R.; Mupila, Z.; Yankonde, B.; Sikazwe, M.; Mwila, E.; et al. Antimicrobial Resistance Patterns and Risk Factors Associated with ESBL-Producing and MDR Escherichia coli in Hospital and Environmental Settings in Lusaka, Zambia: Implications for One Health, Antimicrobial Stewardship and Surveillance Systems. Microorganisms 2023, 11, 1951. [Google Scholar] [CrossRef]
- Mshana, H.J.; Kovacs, D.; Muro, F.; Zadoks, R.; Oravcova, K.; Matthews, L.; Mmbaga, B.T. The burden, risk factors, and antimicrobial susceptibility pattern associated with extended-spectrum beta-lactamase-producing E. coli and K. pneumoniae carriage among neonates and their surroundings at a referral hospital in the Moshi municipality. Front. Antibiot. 2025, 4, 1556842. [Google Scholar] [CrossRef]
- Borcan, A.M.; Radu, G.; Simoiu, M.; Costea, E.L.; Rafila, A. A Five-Year Analysis of Antibiotic Resistance Trends among Bacteria Identified in Positive Urine Samples in a Tertiary Care Hospital from Bucharest, Romania. Antibiotics 2024, 13, 160. [Google Scholar] [CrossRef]
- Topa, A.E.; Ionescu, C.; Pinzaru, A.; Mocanu, E.; Iancu, A.M.; Dumea, E.; Nitu, B.F.; Panculescu, F.G.; Cambrea, S.C. Challenges in the Treatment of Urinary Tract Infections: Antibiotic Resistance Profiles of Escherichia coli Strains Isolated from Young and Elderly Patients in a Southeastern Romanian Hospital. Biomedicines 2025, 13, 1066. [Google Scholar] [CrossRef]
- Duicu, C.; Cozea, I.; Delean, D.; Aldea, A.A.; Aldea, C. Antibiotic resistance patterns of urinary tract pathogens in children from Central Romania. Exp. Ther. Med. 2021, 22, 748. [Google Scholar] [CrossRef]
- Prastiyanto, M.E.; Iswara, A.; Khairunnisa, A.; Sofyantoro, F.; Siregar, A.R.; Mafiroh, W.U.; Setiawan, J.; Nadifah, F.; Wibowo, A.T.; Putri, W.A. Prevalence and antimicrobial resistance profiles of multidrug-resistant bacterial isolates from urinary tract infections in Indonesian patients: A cross-sectional study. Clin. Infect. Pract. 2024, 22, 100359. [Google Scholar] [CrossRef]
- Abdalla, B.A.; Anwar, K.A.; Fakhraldden, S.S.; Saida, B.S.; Bapir, R.; Ahmed, S.M.; Mustafa, A.M.; Abdullah, H.O.; Salih, R.Q.; Abdullah, H.S.; et al. Decoding urinary tract infections: Pathogen profiles and antimicrobial resistance in a cross-sectional study. Afr. J. Urol. 2025, 31, 37. [Google Scholar] [CrossRef]
- Vazouras, K.; Velali, K.; Tassiou, I.; Anastasiou-Katsiardani, A.; Athanasopoulou, K.; Barbouni, A.; Jackson, C.; Folgori, L.; Zaoutis, T.; Basmaci, R.; et al. Antibiotic treatment and antimicrobial resistance in children with urinary tract infections. J. Glob. Antimicrob. Resist. 2020, 20, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Falup-Pecurariu, O.; Leibovitz, E.; Bucur, M.; Lixandru, R.; Bleotu, L.; Falup-Pecurariu, C. High resistance rates to 2nd and 3rd generation cephalosporins, ciprofloxacin and gentamicin of the uropathogens isolated in young infants hospitalized with first urinary tract infection. Biomed. Res. 2017, 28, 8774–8779. [Google Scholar]
- Yamba, K.; Chizimu, J.Y.; Chanda, R.; Mpundu, M.; Samutela, M.T.; Chanda, D.; Mudenda, S.; Finjika, M.; Chansa, B.N.; Siame, A.; et al. Antibiotic resistance profiles in Gram-negative bacteria causing bloodstream and urinary tract infections in paediatric and adult patients in Ndola District, Zambia, 2020–2021. Infect. Prev. Pract. 2025, 7, 100462. [Google Scholar] [CrossRef]
- Negri, M.; Lima, B.M.; Woloszynek, R.D.S.B.R.; Molina, R.A.S.; Germano, C.M.R.; Melo, D.G.; Souza, L.C.; Avó, L.R.D.S. Prevalence and antimicrobial resistance profile of pathogens isolated from patients with urine tract infections admitted to a university hospital in a medium-sized Brazilian city. Rev. Inst. Med. Trop. Sao Paulo 2024, 66, e3. [Google Scholar] [CrossRef] [PubMed]
- Raya, G.B.; Dhoubhadel, B.G.; Shrestha, D.; Raya, S.; Laghu, U.; Shah, A.; Raya, B.B.; Kafle, R.; Parry, C.M.; Ariyoshi, K. Multidrug-resistant and extended-spectrum beta-lactamase-producing uropathogens in children in Bhaktapur, Nepal. Trop. Med. Health 2020, 48, 65. [Google Scholar] [CrossRef]
- Farag, P.F.; Albulushi, H.O.; Eskembaji, M.H.; Habash, M.F.; Malki, M.S.; Albadrani, M.S.; Hanafy, A.M. Prevalence and antibiotic resistance profile of UTI-causing uropathogenic bacteria in diabetics and non-diabetics at the Maternity and Children Hospital in Jeddah, Saudi Arabia. Front. Microbiol. 2024, 15, 1507505. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.M.; Flores, V.R.; do Rosario, G.G.; Succar, J.B.; Berbert, L.C.; Oliveira, M.C.d.F.; Canellas, A.L.B.; Laport, M.S.; Souza, C.R.V.M.; Chagas, T.P.G.; et al. Antimicrobial Susceptibility of Escherichia coli Isolates Causing Community-Acquired Urinary Tract Infections: Comparison of Methods. Microorganisms 2025, 13, 231. [Google Scholar] [CrossRef] [PubMed]
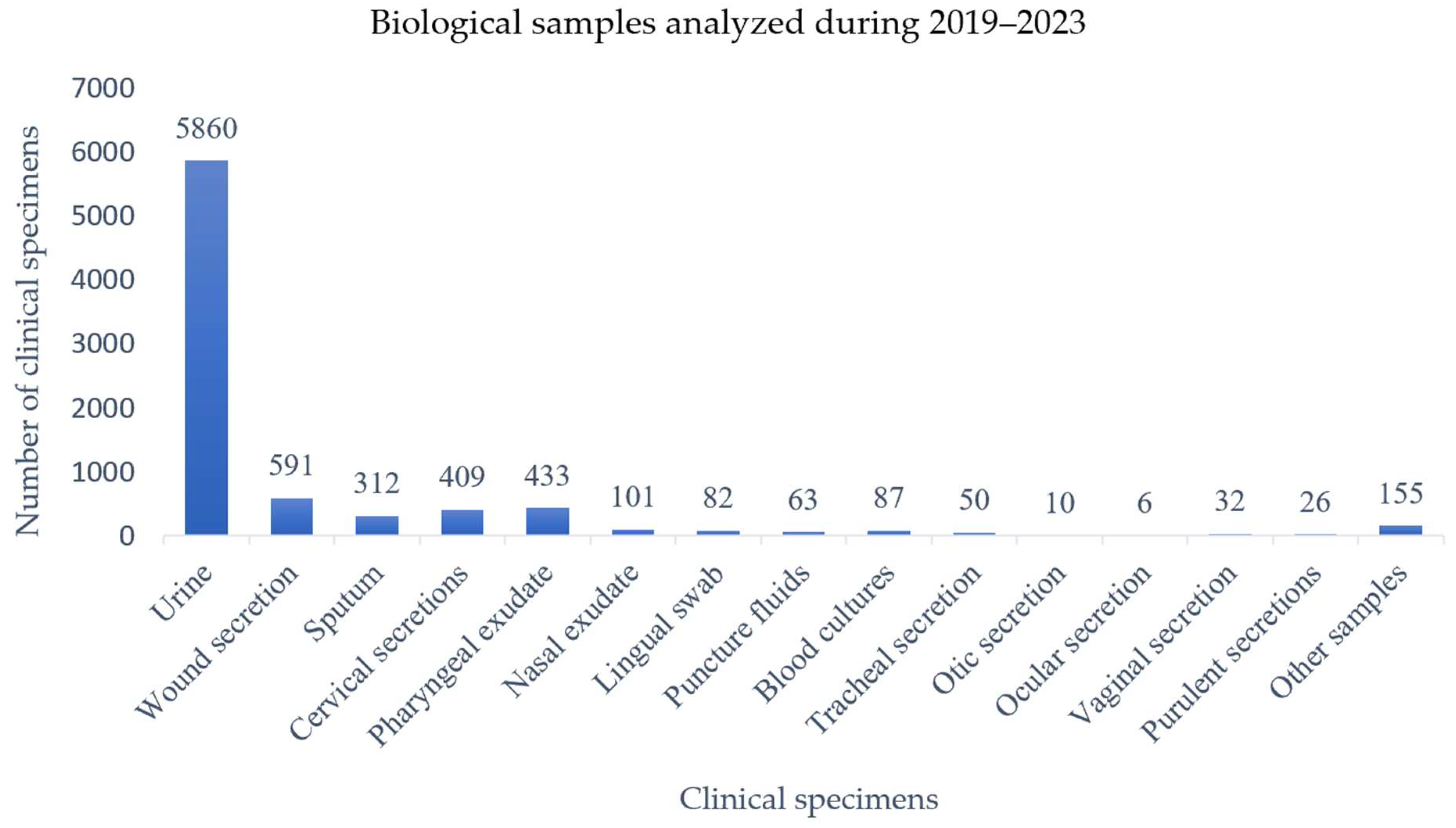
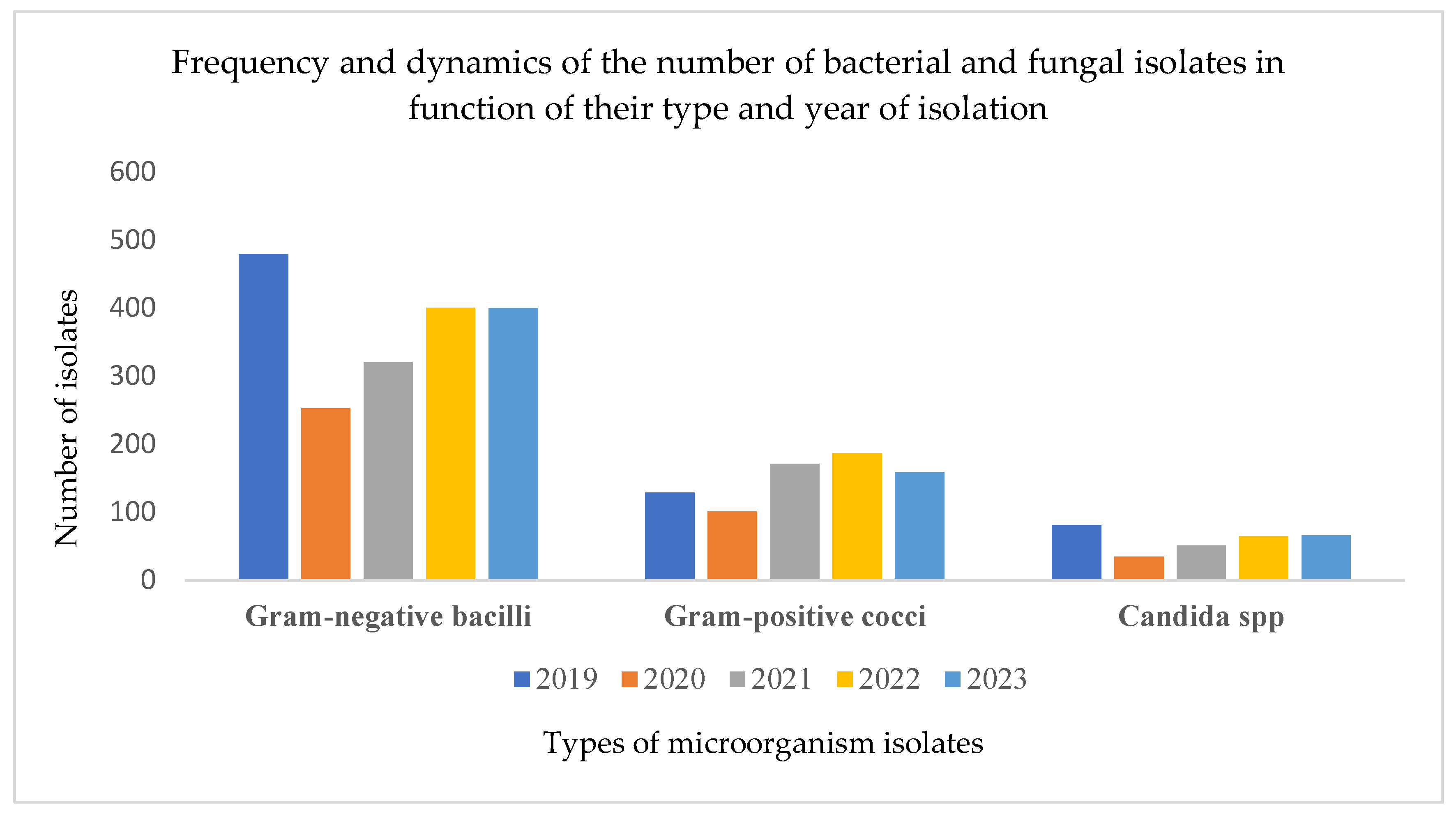
| Year | Urine | Wound Secretions | Sputum | Pharyngeal Exudate | Nasal Exudate | Tracheal Secretion | Otic Secretion | Vaginal Secretion | Cervical Secretion | Puncture Fluid | Blood Culture | Lingual Swab | Ocular Secretion | Purulent Secretion | Other Products * | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 1542 | 87 | 79 | 126 | 18 | 8 | 7 | 5 | 71 | 18 | 14 | 28 | - | 6 | 14 | 2023 (24.6%) |
| 2020 | 627 | 89 | 38 | 45 | 10 | 9 | - | 14 | 62 | 17 | 16 | 14 | - | 5 | 11 | 957 (11.6%) |
| 2021 | 988 | 131 | 62 | 61 | 22 | 3 | - | 1 | 124 | 8 | 26 | 14 | 1 | 3 | 42 | 1486 (18.1%) |
| 2022 | 1183 | 153 | 50 | 86 | 26 | 10 | 2 | 6 | 97 | 10 | 18 | 16 | 3 | 5 | 42 | 1707 (20.7%) |
| 2023 | 1520 | 131 | 83 | 115 | 25 | 20 | 1 | 6 | 55 | 10 | 13 | 10 | 2 | 7 | 46 | 2044 (24.8%) |
| Total | 5860 | 591 | 312 | 433 | 101 | 50 | 10 | 32 | 409 | 63 | 87 | 82 | 6 | 26 | 155 | 8217 |
| Year | Urine Samples | Positive Cultures | % Positive Cultures |
|---|---|---|---|
| 2019 | 1542 | 443 | 28.7% |
| 2020 | 627 | 188 | 30% |
| 2021 | 988 | 247 | 25% |
| 2022 | 1183 | 321 | 27% |
| 2023 | 1520 | 331 | 22% |
| Total 2019–2023 | 5860 | 1530 | 26% |
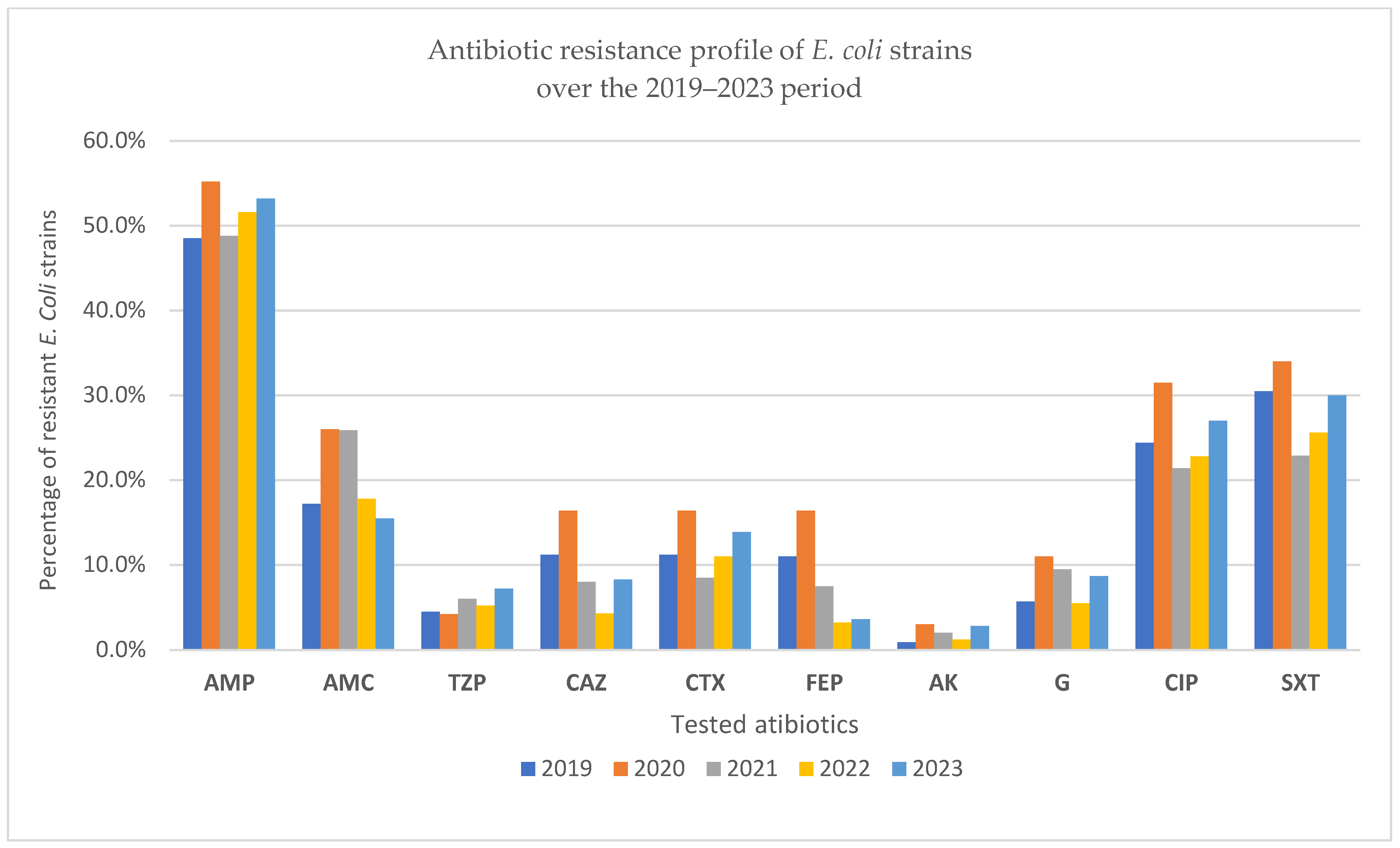
| Year | AMP | AMC | TZP | CAZ | CTX | FEP | AK | G | CIP | SXT |
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 161 48.5% | 57 17.2% | 15 4.5% | 37 11.2% | 37 11.2% | 36 11% | 3 0.9% | 19 5.7% | 81 24.4% | 101 30.5% |
| 2020 | 91 55.2% | 43 26% | 7 4.2% | 27 16.4% | 27 16.4% | 27 16.4% | 5 3% | 18 11% | 52 31.5% | 56 34% |
| 2021 | 98 48.8% | 52 25.9% | 12 6% | 16 8% | 17 8.5% | 15 7.5% | 4 2% | 19 9.5% | 43 21.4% | 46 22.9% |
| 2022 | 131 51.6% | 45 17.8% | 13 5.2% | 11 4.3% | 28 11% | 8 3.2% | 3 1.2% | 14 5.5% | 58 22.8% | 65 25.6% |
| 2023 | 134 53.2% | 39 15.5% | 18 7.2% | 21 8.3% | 35 13.9% | 9 3.6% | 7 2.8% | 22 8.7% | 68 27% | 75 30% |
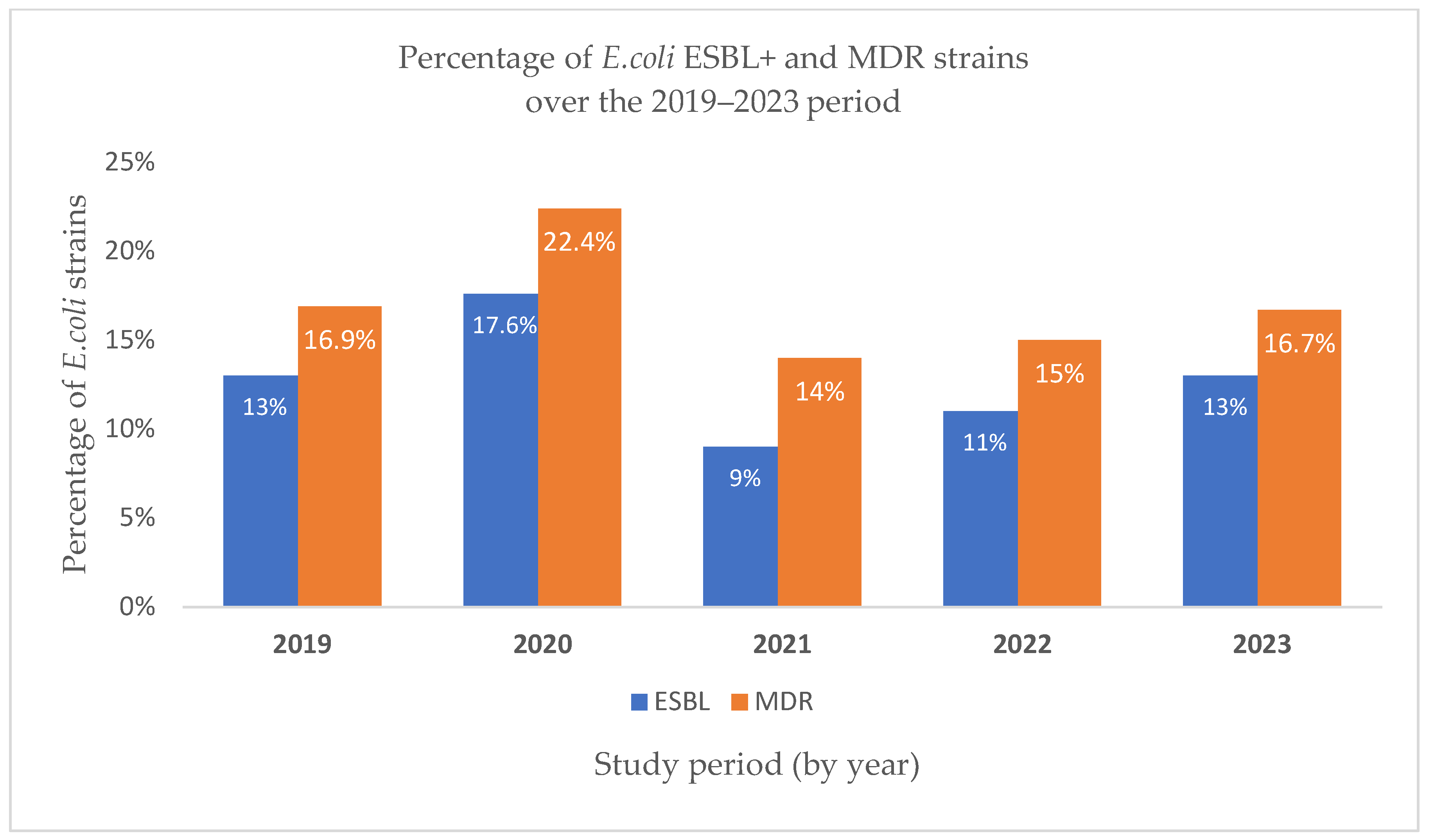
| Year | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|
| ESBL | 43 13% | 29 17.6% | 18 9% | 28 11% | 33 13% |
| MDR | 56 16.9% | 37 22.4% | 28 14% | 38 15% | 42 16.7% |
| Total E. coli strains isolated | 332 | 165 | 201 | 254 | 252 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goleanu, C.D.; Vrancianu, C.O.; Goleanu, D.A.; Tantu, M.M.; Csutak, O. Trends in Antibiotic Resistance of Escherichia coli Strains Isolated from Clinical Samples (2019–2023): A Hospital-Based Retrospective Analysis. Pathogens 2025, 14, 927. https://doi.org/10.3390/pathogens14090927
Goleanu CD, Vrancianu CO, Goleanu DA, Tantu MM, Csutak O. Trends in Antibiotic Resistance of Escherichia coli Strains Isolated from Clinical Samples (2019–2023): A Hospital-Based Retrospective Analysis. Pathogens. 2025; 14(9):927. https://doi.org/10.3390/pathogens14090927
Chicago/Turabian StyleGoleanu (Vasiloiu), Claudia Daniela, Corneliu Ovidiu Vrancianu, Daria Adelina Goleanu, Monica Marilena Tantu, and Ortansa Csutak. 2025. "Trends in Antibiotic Resistance of Escherichia coli Strains Isolated from Clinical Samples (2019–2023): A Hospital-Based Retrospective Analysis" Pathogens 14, no. 9: 927. https://doi.org/10.3390/pathogens14090927
APA StyleGoleanu, C. D., Vrancianu, C. O., Goleanu, D. A., Tantu, M. M., & Csutak, O. (2025). Trends in Antibiotic Resistance of Escherichia coli Strains Isolated from Clinical Samples (2019–2023): A Hospital-Based Retrospective Analysis. Pathogens, 14(9), 927. https://doi.org/10.3390/pathogens14090927







