Repellent Activity of the Botanical Compounds Thymol, Carvacrol, Nootkatone, and Eugenol Against Amblyomma sculptum Nymphs
Abstract
1. Introduction
2. Material and Methods
2.1. Ticks
2.2. Test Compounds
2.3. Laboratory Bioassays
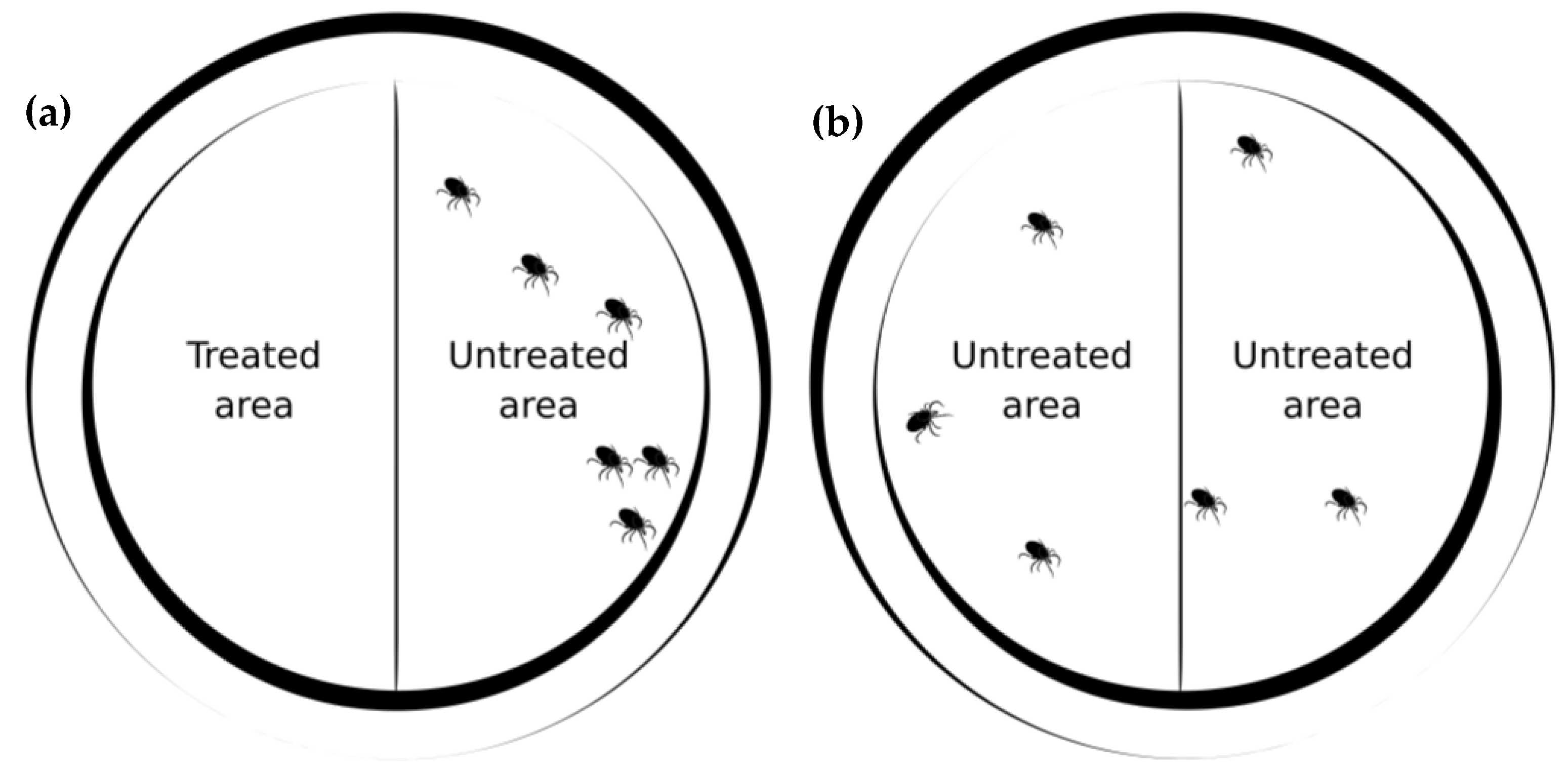
2.3.1. Contact Repellency in One-Choice Petri Dish Tests
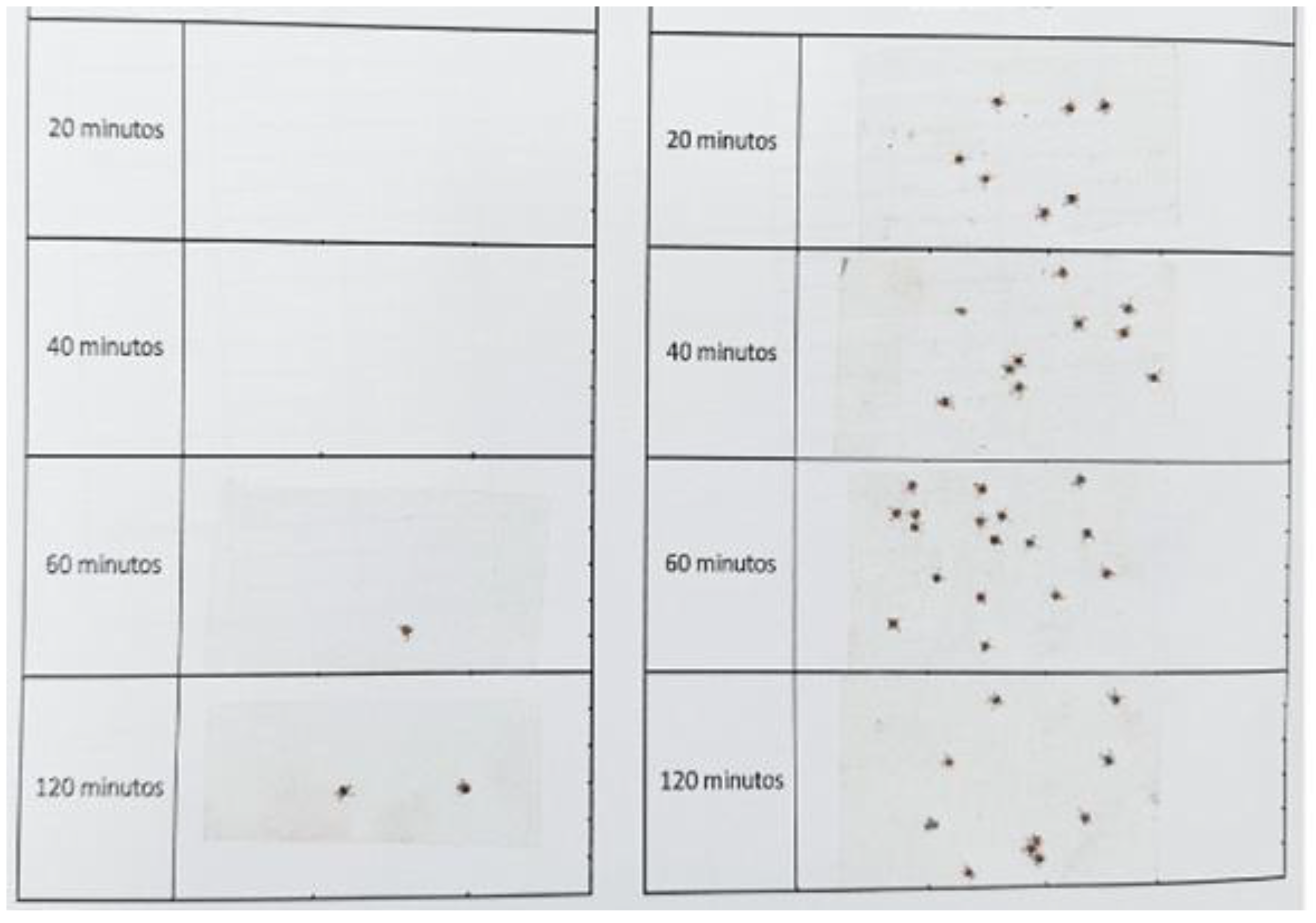
2.3.2. Y-Tube Olfactometer Bioassay: Spatial Repellency
2.4. Field Bioassays (Sock Assay)
2.5. Cost Evaluation
2.6. Data Analysis
3. Results
3.1. Contact Repellency in Petri Dish Tests
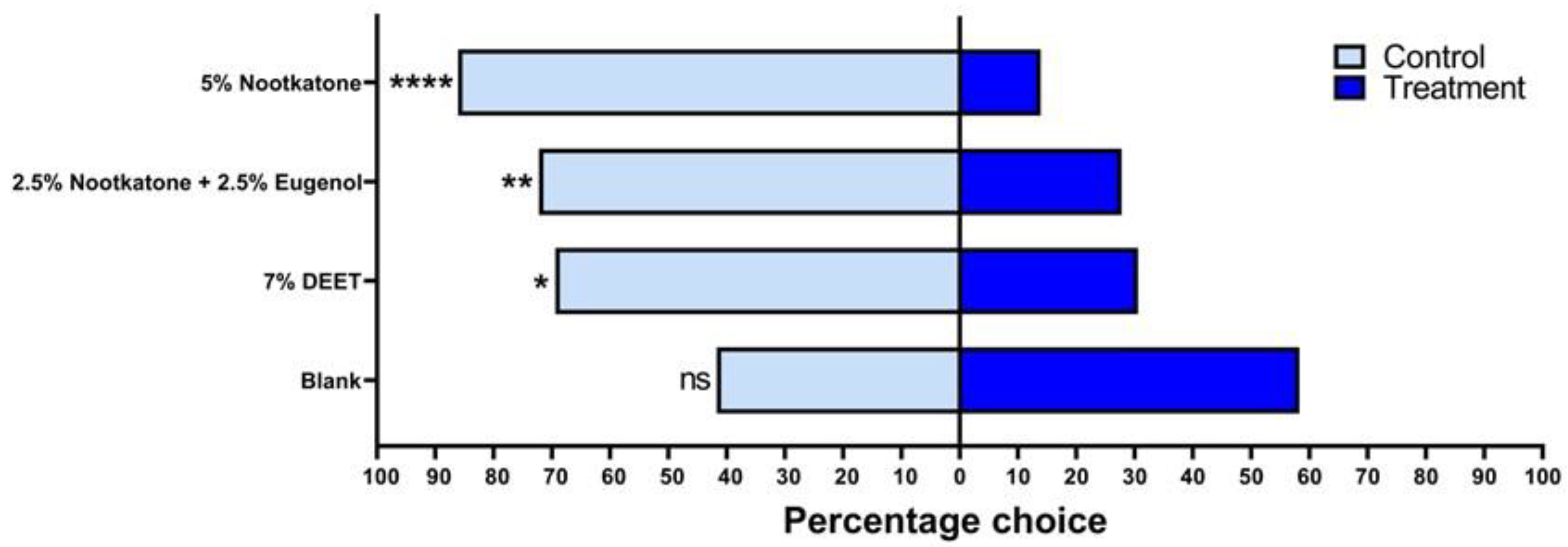
3.2. Spatial Repellency in Y-Tube Olfactometer
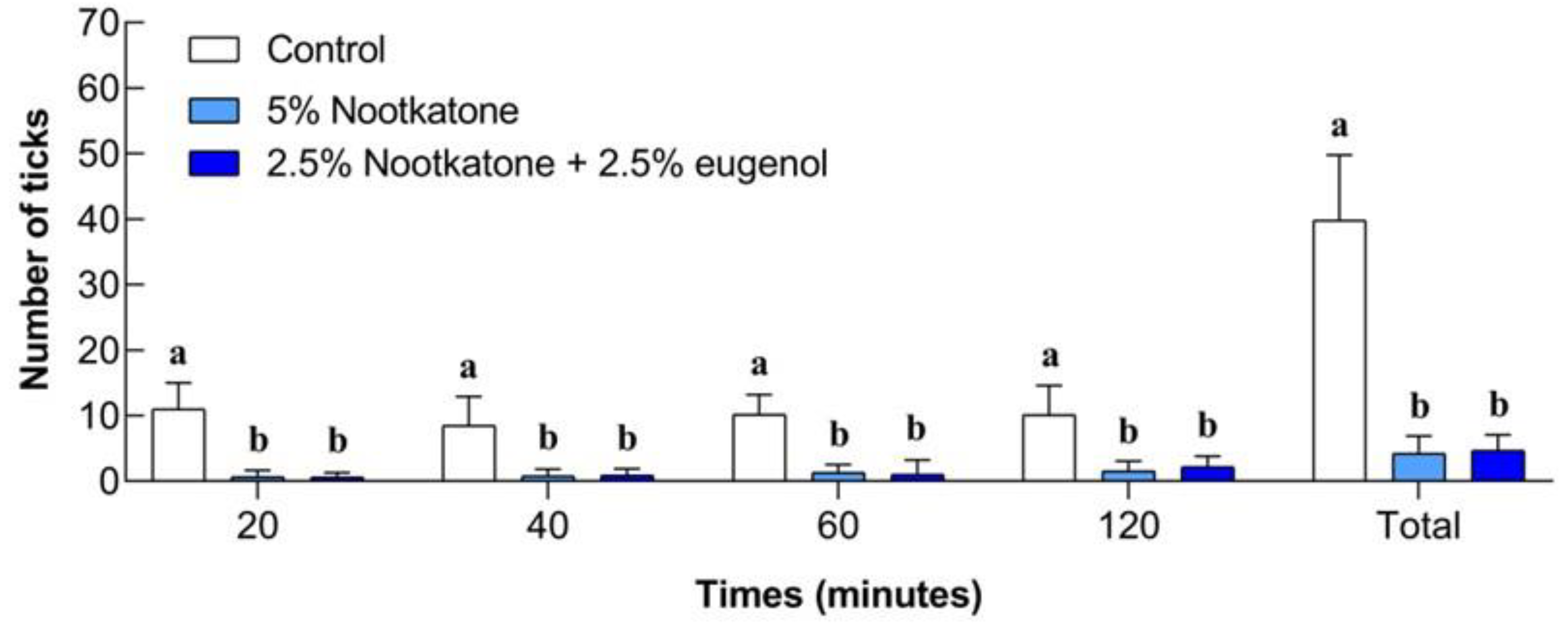
3.3. Field Bioassays
3.4. Cost Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CEP | Research ethics committee (Comitê de Ética em Pesquisa) |
| CEUA | Animal use ethics committee (Comissão de Ética no Uso de Animais) |
| DEET | N, N-diethyl-3-methylbenzamide |
| EO(s) | Essential oil(s) |
| EVZ | School of veterinary medicine and animal science (Escola de Veterinária e Zootecnia) |
| PPE | Personal protective equipment |
| RH | Relative humidity |
| SBP | Brazilian society of parasitology (Sociedade Brasileira de Parasitologia) |
| SD | Standard deviation |
| UFG | Federal University of Goiás |
References
- Szabó, M.P.J.; Pinter, A.; Labruna, M.B. Ecology, biology and distribution of spotted-fever tick vectors in Brazil. Front. Cell. Infect. Microbiol. 2013, 4, 27. [Google Scholar] [CrossRef]
- Krawczak, F.S.; Nieri-Bastos, F.A.; Nunes, F.P.; Soares, J.F.; Moraes-Filho, J.; Labruna, M.B. Rickettsial infection in Amblyomma cajennense ticks and capybaras (Hydrochoerus hydrochaeris) in a Brazilian spotted fever-endemic area. Parasites Vectors 2014, 7, 7. [Google Scholar] [CrossRef]
- Martins, T.F.; Barbieri, A.R.M.; Costa, F.B.; Terassini, F.A.; Camargo, L.M.A.; Peterka, C.R.L.; Pacheco, R.C.; Dias, R.A.; Nunes, P.H.; Marcili, A.; et al. Geographical distribution of Amblyomma cajennense (sensu lato) ticks (Parasitiformes: Ixodidae) in Brazil. Parasites Vectors 2016, 9, 186. [Google Scholar] [CrossRef]
- Nava, S.; Venzal, J.M.; González-Acuña, D.; Martins, T.; Guglielmone, A.A. Ticks of the Southern Cone of America: Diagnosis, Distribution, and Hosts with Taxonomy, Ecology and Sanitary Importance; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- de Paula, L.G.F.; do Nascimento, R.M.; Franco, A.d.; Szabó, M.P.J.; Labruna, M.B.; Monteiro, C.; Krawczak, F.S. Seasonal dynamics of Amblyomma sculptum: A review. Parasites Vectors 2022, 15, 193. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Repellence of essential oils and selected compounds against ticks—A systematic review. Acta Trop. 2018, 179, 47–54. [Google Scholar] [CrossRef]
- CDC—Centers for Disease Control and Prevention. How to Prevent Tick Bites. 2023. Available online: https://www.cdc.gov/ticks/prevention/?CDC_AAref_Val=https://www.cdc.gov/ticks/avoid/on_people.html (accessed on 21 March 2025).
- Barcelos, B.R.; Coelho, N.G.S.S.; Barrozo, M.M.; Vale, F.L.; Teixeira, A.L.C.; Souza, L.M.P.; Zeringóta, V.; Monteiro, C.; Eugenio, C.U.O.; Obara, M.T. Do commercial insect repellents provide protection against the tick Amblyomma sculptum (Acari: Ixodidae)? Pathogens 2024, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.; Peters, P.W.J. Intrauterine diethyltoluamide exposure and fetal outcome. Reprod. Toxicol. 1992, 6, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Dugas, J.; Nieuwenhuijsen, M.J.; Martinez, D.; Iszatt, N.; Nelson, P.; Elliott, P. Use of biocides and insect repellents and risk of hypospadias. Occup. Environ. Med. 2010, 67, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Canale, A.; Mehlhorn, H. Tick repellents and acaricides of botanical origin: A green roadmap to control tick-borne diseases? Parasitol. Res. 2016, 115, 2545–2560. [Google Scholar] [CrossRef]
- Ferreira, L.L.; Oliveira Filho, J.G.; Mascarin, G.M.; León, A.A.P.; Borges, L.M.F. In vitro repellency of DEET and β-citronellol against the ticks Rhipicephalus sanguineus sensu lato and Amblyomma sculptum. Vet. Parasitol. 2017, 239, 42–45. [Google Scholar] [CrossRef]
- Gonzaga, B.C.F.; Barrozo, M.M.; Coutinho, A.L.; Pereira E Sousa, L.J.M.; Vale, F.L.; Marreto, L.; Marchesini, P.; de Castro Rodrigues, D.; de Souza, E.D.F.; Sabatini, G.A.; et al. Essential oils and isolated compounds for tick control: Advances beyond the laboratory. Parasites Vectors 2023, 16, 415. [Google Scholar] [CrossRef] [PubMed]
- Novelino, A.M.S.; Daemon, E.; Soares, G.L.G. Evaluation of the acaricide effect of thymol, menthol, salicylic acid, and methyl salicylate on Boophilus microplus (Canestrini 1887) (Acari: Ixodidae) larvae. Parasitol. Res. 2007, 101, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.A.; Schulze, T.L.; Dolan, M.C. Efficacy of plant-derived and synthetic compounds on clothing as repellents against Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 2012, 49, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Jordan, R.A.; Dolan, M.C. Experimental use of two standard tick collection methods to evaluate the relative effectiveness of several plant-derived and synthetic repellents against Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J. Econ. Entomol. 2011, 104, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
- Tabari, M.A.; Youssefi, M.R.; Maggi, F.; Benelli, G. Toxic and repellent activity of selected monoterpenoids (thymol, carvacrol and linalool) against the castor bean tick, Ixodes ricinus (Acari: Ixodidae). Vet. Parasitol. 2017, 245, 86–91. [Google Scholar] [CrossRef]
- Rezende, D.A.d.S.; Cardoso, M.d.; Konig, I.F.M.; Lunguinho, A.S.; Ferreira, V.R.F.; Brandão, R.M.; Gonçalves, R.R.P.; Caetano, A.R.S.; Nelson, D.L.; Remedio, R.N. Repellent effect on Rhipicephalus sanguineus and inhibition of acetylcholinesterase by volatile oils. Rev. Bras. Farmacogn. 2021, 31, 470–476. [Google Scholar] [CrossRef]
- O’Neil, M.J.; Heckelman, P.E.; Dobbelaar, P.H.; Roman, K.J. (Eds.) The Merck Index, an Encyclopedia of Chemicals, Drugs, and Biologicals, 15th ed.; The Royal Society of Chemistry: Cambridge, UK, 2013. [Google Scholar]
- Da Silva Costa, J.R.; do Vale, T.L.; da Silva, G.F.; da Silva, N.C.S.; Lima, A.S.; Costa-Júnior, L.M.; Luz, H.R. Encapsulation of carvacrol and thymol with yeast cell wall and its repellent activity against Amblyomma sculptum and Rhipicephalus sanguineus. Exp. Appl. Acarol. 2024, 92, 555–565. [Google Scholar] [CrossRef]
- Alibeigi, Z.; Rakhshandehroo, E.; Saharkhiz, M.J.; Alavi, A.M. The acaricidal and repellent activity of the essential and nano essential oil of Thymus vulgaris against the larval and engorged adult stages of the brown dog tick, Rhipicephalus sanguineus (Acari: Ixodidae). BMC Vet. Res. 2025, 21, 135. [Google Scholar] [CrossRef]
- EPA—Environmental Protection Agency. Product Performance Test Guidelines. OPPTS 810.3700: Insect Repellents to Be Applied to Human Skin; EPA: Washington, DC, USA, 2020. Available online: https://nepis.epa.gov (accessed on 21 March 2025).
- Fan, J.; Liu, Z.; Xu, S.; Yan, X.; Cheng, W.; Yang, R.; Guo, Y. Non-food bioactive product (+)-nootkatone: Chemistry and biological activities. Ind. Crops Prod. 2022, 177, 114490. [Google Scholar] [CrossRef]
- Zhang, L.L.; Chen, Y.; Li, Z.J.; Fan, G.; Li, X. Production, function, and applications of the sesquiterpenes valencene and nootkatone: A comprehensive review. J. Agric. Food Chem. 2023, 71, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Ramachanderan, R.; Schaefer, B. (+)-Nootkatone, the flavor of grapefruit. ChemTexts 2024, 10, 11. [Google Scholar] [CrossRef]
- Kim, E.-H.; Kim, H.-K.; Ahn, Y.J. Acaricidal activity of clove bud oil compounds against Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J. Agric. Food Chem. 2003, 51, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, S.; Nazzi, F. Repellent effect of sweet basil compounds on Ixodes ricinus ticks. Exp. Appl. Acarol. 2008, 45, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Alimi, D.; Hajri, A.; Jallouli, S.; Sebai, H. Toxicity, repellency, and anti-cholinesterase activities of bioactive molecules from clove buds Syzygium aromaticum L. as an ecological alternative in the search for control of Hyalomma scupense (Acari: Ixodidae). Heliyon 2023, 9, e18899. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.V.; De Lima, A.C.A.; Silva, M.G.; Caetano, V.F.; Andrade, M.F.; da Silva, R.G.C.; Moraes-Filho, L.E.P.T.; Silva, I.D.L.; Vinhas, G.M. Clove essential oil and eugenol: A review of their significance and uses. Food Biosci. 2024, 62, 105112. [Google Scholar] [CrossRef]
- Vale, F.L.; Paula, L.G.F.; Vieira, M.; Alves, S.G.A.; Moraes Junior, N.R.; Filgueiras, M.D.; Teixeira, W.F.P.; Rizzo, P.V.; Freitas, F.M.C.; Ferreira, L.L.; et al. Binary combinations of thymol, carvacrol and eugenol for Amblyomma sculptum control: Evaluation of in vitro synergism and effectiveness under semi-field conditions. Ticks Tick Borne Dis. 2021, 12, 101816. [Google Scholar] [CrossRef]
- Waltz, E. A biotech insect repellent, safe enough to eat. Nat. Biotechnol. 2020, 38, 1368–1369. [Google Scholar] [CrossRef]
- Soares, J.F.; Soares, H.S.; Barbieri, A.M.; Labruna, M.B. Experimental infection of the tick Amblyomma cajennense, Cayenne tick, with Rickettsia rickettsii, the agent of Rocky Mountain spotted fever. Med. Vet. Entomol. 2012, 26, 139–151. [Google Scholar] [CrossRef]
- Resende, J.D.S.A.; Daemon, E.; Monteiro, C.M.O.; Maturano, R.; Azevedo Prata, M.C.; Rodrigues, A.F.S.F. Toxicity of solvents and surfactants to Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae) and Dermacentor nitens (Neumann, 1897) (Acari: Ixodidae) larvae. Exp. Parasitol. 2012, 131, 139–142. [Google Scholar] [CrossRef]
- Barrozo, M.M.; Zeringóta, V.; Borges, L.M.F.; Moraes, N.; Benz, K.; Farr, A.; Zhu, J.J. Repellent and acaricidal activity of coconut oil fatty acids and their derivative compounds and catnip oil against Amblyomma sculptum. Vet. Parasitol. 2021, 300, 109591. [Google Scholar] [CrossRef]
- Ferreira, L.L.; Sarria, A.L.F.; de Oliveira Filho, J.G.; de Silva, F.O.; Powers, S.J.; Caulfield, J.C.; Pickett, J.A.; Birkett, M.A.; Borges, L.M.F. Identification of a non-host semiochemical from tick-resistant donkeys (Equus asinus) against Amblyomma sculptum ticks. Ticks Tick Borne Dis. 2019, 10, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.L.; Sarria, A.L.F.; de Oliveira Filho, J.G.; de Silva, F.O.; Ferraz, A.L.L.; Mascarin, G.M. Attract or repel Amblyomma sculptum ticks: Screening of semiochemicals. Vet. Parasitol. 2020, 278, 109036. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2014, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Barrozo, M.M.; Chagas, H.D.F.; Damaceno, G.B.R.; Santos, E.F.; Carvalho, R.A.; Silva, I.S.; Vale, F.L.; Sousa, L.J.M.P.; Luz, H.R.; Ferreira, L.L.; et al. Repellent activity of DEET combined with botanical compounds against Amblyomma sculptum nymphs: Laboratory and field evaluations. Pathogens 2025, 14, 495. [Google Scholar] [CrossRef]
- Paula, L.G.F.; Zeringóta, V.; Sampaio, A.L.N.; Bezerra, G.P.; Barreto, A.L.G.; Santos, A.A.; Miranda, V.C.; Paula, W.V.F.; Neves, L.C.; Secchis, M.V.; et al. Seasonal dynamics of Amblyomma sculptum in two areas of the Cerrado biome, midwestern Brazil, where human cases of rickettsiosis have been reported. Exp. Appl. Acarol. 2021, 84, 215–225. [Google Scholar] [CrossRef]
- Neves, L.C.; Paula, W.V.F.; Paula, L.G.F.; Silva, B.B.F.; Dias, S.A.; Pereira, B.G.; Silva, B.S.A.; Sevá, A.P.; Dantas-Torres, F.; Labruna, M.B.; et al. Detection of Rickettsia spp. in animals and ticks in midwestern Brazil, where human cases of rickettsiosis were reported. Animals 2023, 13, 1288. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, B.W.; Apperson, C.S.; Watson, D.W.; Arellano, C.; Sonenshine, D.E.; Roe, R.M. Novel field assays and the comparative repellency of BioUD®, DEET and permethrin against Amblyomma americanum. Med. Vet. Entomol. 2011, 25, 217–226. [Google Scholar] [CrossRef]
- Martins, T.F.; Onofrio, V.C.; Barros-Battesti, D.M.; Labruna, M.B. Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: Descriptions, redescriptions, and identification key. Ticks Tick Borne Dis. 2010, 1, 75–99. [Google Scholar] [CrossRef]
- Suzin, A.; Rodrigues, V.S.; Ramos, V.N.; Szabó, M.P.J. Comparing scapular morphology of Amblyomma sculptum and Amblyomma dubitatum nymphs allows a fast and practical differential diagnosis of ticks in highly infested areas with dominance of these two species. Exp. Appl. Acarol. 2022, 86, 455–463. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Eugenol. Available online: https://www.sigmaaldrich.com/BR/pt/product/aldrich/e51791 (accessed on 19 March 2025).
- Sigma-Aldrich. Nootkatone. Available online: https://www.sigmaaldrich.com/BR/pt/product/aldrich/w316620 (accessed on 19 March 2025).
- Arafa, W.M.; Aboelhadid, S.M.; Moawad, K.; Shokeir, K.M.; Ahmed, O. Toxicity, repellency and anti-cholinesterase activities of thymol-eucalyptus combinations against phenotypically resistant Rhipicephalus annulatus ticks. Exp. Appl. Acarol. 2020, 81, 265–277. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Int. J. Mol. Sci. 2022, 23, 1938. [Google Scholar] [CrossRef]
- Teixeira, A.L.C.; Marreto, L.C.N.L.; Vale, F.L.; Sousa, L.J.M.P.; Gonzaga, B.C.F.; Silva, I.S.; Santos, E.F.; Lopes, F.F.S.; Morais, S.M.M.; Lopes, W.D.Z.; et al. Combinations of amitraz with essential oils from Lippia sidoides and Thymus vulgaris, thymol and thymol acetate for Rhipicephalus microplus control: Studies under laboratory and field conditions. Vet. Parasitol. 2023, 321, 109997. [Google Scholar] [CrossRef]
- Behle, R.W.; Flor-Weiler, L.B.; Bharadwaj, A.; Stafford, K.C. A formulation to encapsulate nootkatone for tick control. J. Med. Entomol. 2011, 48, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Zeringóta, V.; Senra, T.O.S.; Calmon, F.; Maturano, R.; Faza, A.P.; Catunda-Junior, F.E.A.; Monteiro, C.M.O.; Carvalho, M.G.; Daemon, E. Repellent activity of eugenol on larvae of Rhipicephalus microplus and Dermacentor nitens (Acari: Ixodidae). Parasitol. Res. 2013, 112, 2675–2679. [Google Scholar] [CrossRef]
- HSDB—Hazardous Substances Data Bank. Eugenol. Banco de Desenvolvimento Econômico e Social. 2019. Available online: https://toxnet-nlm-nih-gov.ez49.periodicos.capes.gov.br/cgibin/sis/search/a?dbs+hsdb:@term+@DOCNO+210 (accessed on 6 March 2025).
- Siegel, E.L.; Xu, G.; Li, A.Y.; Pearson, P.; D’hers, S.; Elman, N.; Mather, T.N.; Rich, S.M. Ixodes scapularis is the most susceptible of the three canonical human-biting tick species of North America to repellent and acaricidal effects of the natural sesquiterpene, (+)-nootkatone. Insects 2024, 15, 8. [Google Scholar] [CrossRef]
- Andreev, S.V.; Sakharov, K.A.; Zverev, S.A.; Lapina, E.A.; Savraeva, D.V.; Akhmetshina, M.B.; Ushakova, E.V.; Kuzovlev, A.S. The effects of natural and synthetic attractants and repellents on Ixodes persulcatus. J. Acarina 2024, 32, 59–68. [Google Scholar] [CrossRef]
- Le Mauff, A.; Norris, E.J.; Li, A.Y.; Swale, D.R. Repellent activity of essential oils to the lone star tick, Amblyomma americanum. Parasites Vectors 2024, 17, 202. [Google Scholar] [CrossRef]
- Carr, A.L.; Salgado, V.L. Ticks home in on body heat: A new understanding of Haller’s organ and repellent action. PLoS ONE 2019, 14, e0221659. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.C.; Janich, A.J.; Sanchez-Vargas, I.; Markle, E.D.; Gray, M.; Foster, J.R.; Black IV, W.C.; Foy, B.D.; Olson, K.E. Nootkatone is an effective repellent against Aedes aegypti and Aedes albopictus. Insects 2021, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wu, T.; Liu, P.; Chen, D.; Cai, S.; Chen, H.; Zhou, J.; Zhu, C.; Li, S. Green production of a high-value mosquito insecticide of nootkatone from seaweed hydrolysates. J. Agric. Food Chem. 2023, 71, 18919–18927. [Google Scholar] [CrossRef]
- Legeay, S.; Clere, N.; Apaire-Marchais, V.; Faure, S.; Lapied, B. Unusual modes of action of the repellent DEET in insects highlight some human side effects. Eur. J. Pharmacol. 2018, 825, 92–98. [Google Scholar] [CrossRef]
- Abel-Nwachukwu, J.U.; Matthews, B.J. Insect chemosensation: A grapefruit squeeze on mosquito-borne disease. Curr. Biol. 2025, 35, R113–R115. [Google Scholar] [CrossRef] [PubMed]
- Del-Fabbro, S.D.; Nazzi, F. From chemistry to behavior: Molecular structure and bioactivity of repellents against Ixodes ricinus ticks. PLoS ONE 2013, 8, e67832. [Google Scholar] [CrossRef]
- Kfoury, M.; Landy, D.; Ruellan, S.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Nootkatone encapsulation by cyclodextrins: Effect on water solubility and photostability. Food Chem. 2017, 236, 41–48. [Google Scholar] [CrossRef] [PubMed]


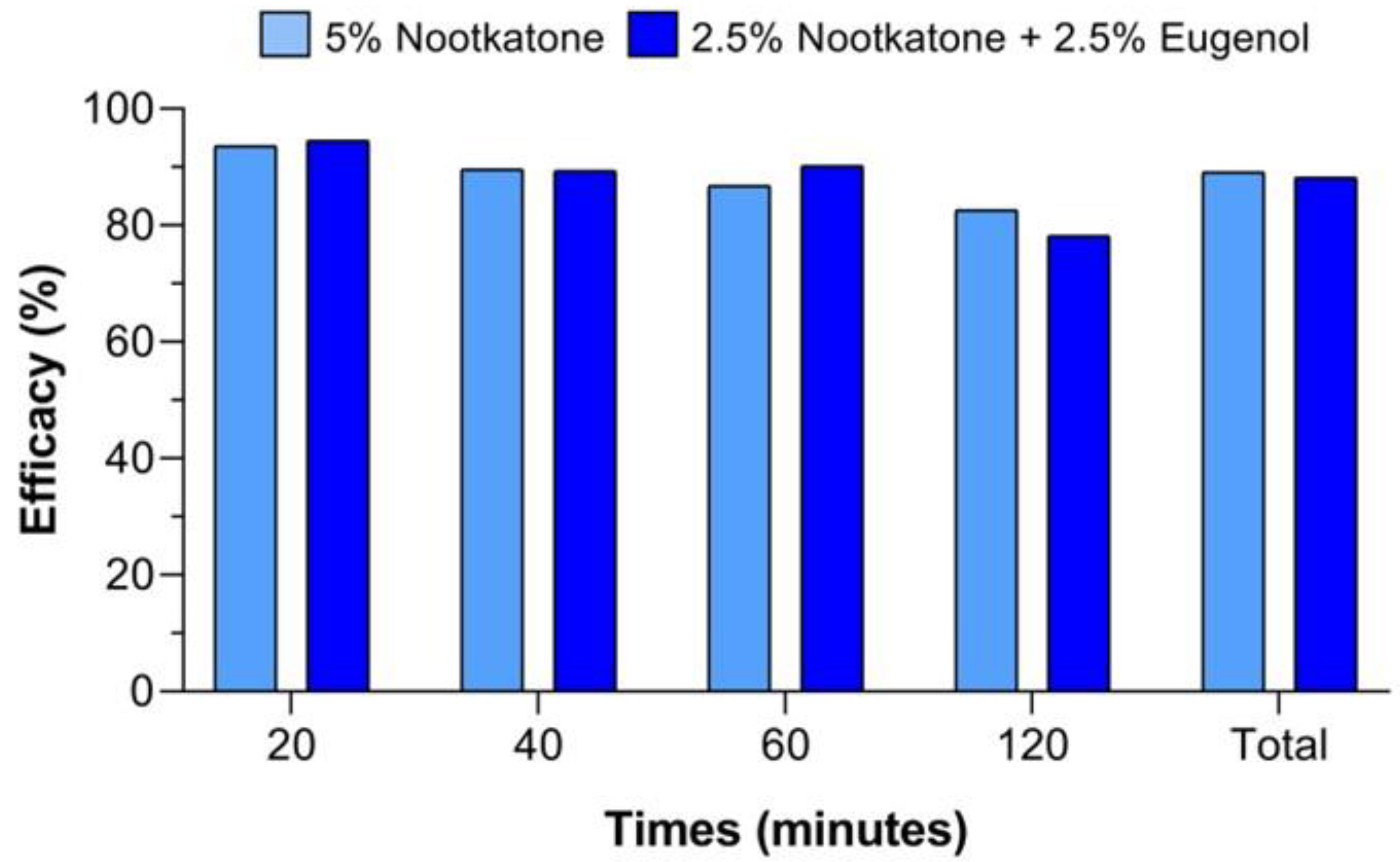
| Evaluation Time | Control (Ethanol) | Control (Blank) | 7% DEET | 5% Thymol | 5% Carvacrol | 5% Nootkatone | 5% Eugenol | 2.5% Nootkatone + 2.5% Eugenol | |
|---|---|---|---|---|---|---|---|---|---|
| Minutes | 1 | 38.3 ± 19.3 | 46.7 ± 20.5 | 83.3 * ± 17.6 | 93.3 * ± 11.7 | 100.0 * ± 0.0 | 88.3 * ± 11.2 | 90.0 * ± 16.1 | 86.7 * ± 13.1 |
| 15 | 45.0 ± 17.7 | 48.3 ± 12.3 | 80.0 * ± 17.2 | 93.3 * ± 11.7 | 91.7 * ± 8.8 | 95.0 * ± 8.1 | 86.7 * ± 13.1 | 90.0 * ± 8.6 | |
| 30 | 56.7 ± 11.7 | 46.7 ± 15.3 | 93.3 * ± 11.7 | 96.7 * ± 7.0 | 100.0 * ± 0.0 | 90.0 * ± 11.7 | 90.0 * ± 11.7 | 90.0 * ± 11.7 | |
| Hours | 1 | 55.0 ± 15.8 | 53.3 ± 17.2 | 91.7 * ± 8.8 | 100.0 * ± 0.0 | 100.0 * ± 0.0 | 98.3 * ± 5.3 | 96.7 * ± 7.0 | 98.3 * ± 5.3 |
| 2 | 55.0 ± 19.3 | 61.7 ± 11.2 | 90.0 * ± 11.7 | 100.0 * ± 0.0 | 98.3 * ± 5.3 | 93.3 * ± 8.6 | 93.3 * ± 11.7 | 100.0 * ± 0.0 | |
| 3 | 55.0 ± 19.3 | 48.3 ± 14.6 | 90.0 * ± 11.7 | 98.3 * ± 5.3 | 100.0 * ± 0.0 | 96.7 * ± 7.0 | 91.7 * ± 8.8 | 100.0 * ± 0.0 | |
| 4 | 56.7 ± 11.7 | 51.7 ± 12.3 | 88.3 * ± 13.7 | 100.0 * ± 0.0 | 98.3 * ± 5.3 | 95.0 * ± 8.1 | 86.7 * ± 13.1 | 100.0 * ± 0.0 | |
| 24 | 45.0 ± 24.9 | 45.0 ± 22.3 | 77.1 * ± 15.8 | 81.7 * ± 18.3 | 95.0 * ± 8.1 | 95.0 * ± 8.1 | 81.7 * ± 12.3 | 88.3 * ± 11.2 | |
| 48 | 41.7 ± 11.8 | 58.3 ± 22.6 | 65.0 * ± 12.3 | 58.3 ± 29.7 | 80.0 * ± 20.5 | 93.3 * ± 8.6 | 81.7 * ± 12.3 | 91.7 * ± 8.8 | |
| 72 | 55.0 ± 23.6 | 45.0 ± 11.2 | 63.3 * ± 13.1 | 80.0 * ± 15.3 | 81.7 * ± 14.6 | 91.7 * ± 8.8 | 73.3 * ± 19.6 | 83.3 * ± 15.7 | |
| Mean (%) ± standard deviation | 50.3 ± 7.0 | 50.5 ± 5.7 | 82.2 ± 12.1 | 90.2 ± 13.4 | 94.5 ± 7.7 | 93.7 ± 3.0 | 87.2 ± 6.8 | 92.8 ± 6.2 | |
| Compound/Combination | Concentration (% m/v) | Amount (g) in 100 mL | Cost per 100 mg (USD) | Estimated Cost (USD) |
|---|---|---|---|---|
| Nootkatone | 5.0 | 5.0 | $1017.00 | $50.8 |
| Nootkatone + Eugenol (1:1) | 2.5 + 2.5 | 2.5 + 2.5 | $1017.00 + $87.00 | $28.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrozo, M.M.; Santos, E.F.; Chagas, H.D.F.; Carvalho, R.A.; Silva, I.S.; de Souza Oliveira, A.; Faria, L.C.F.; Teixeira, A.L.C.; Zeringota, V.; Luz, H.R.; et al. Repellent Activity of the Botanical Compounds Thymol, Carvacrol, Nootkatone, and Eugenol Against Amblyomma sculptum Nymphs. Pathogens 2025, 14, 926. https://doi.org/10.3390/pathogens14090926
Barrozo MM, Santos EF, Chagas HDF, Carvalho RA, Silva IS, de Souza Oliveira A, Faria LCF, Teixeira ALC, Zeringota V, Luz HR, et al. Repellent Activity of the Botanical Compounds Thymol, Carvacrol, Nootkatone, and Eugenol Against Amblyomma sculptum Nymphs. Pathogens. 2025; 14(9):926. https://doi.org/10.3390/pathogens14090926
Chicago/Turabian StyleBarrozo, Mayara Macêdo, Emilly Faria Santos, Haile Dean Figueiredo Chagas, Rafael Assunção Carvalho, Isabela Santos Silva, Ariel de Souza Oliveira, Laura Cristina Ferreira Faria, Ana Lúcia Coutinho Teixeira, Viviane Zeringota, Hermes Ribeiro Luz, and et al. 2025. "Repellent Activity of the Botanical Compounds Thymol, Carvacrol, Nootkatone, and Eugenol Against Amblyomma sculptum Nymphs" Pathogens 14, no. 9: 926. https://doi.org/10.3390/pathogens14090926
APA StyleBarrozo, M. M., Santos, E. F., Chagas, H. D. F., Carvalho, R. A., Silva, I. S., de Souza Oliveira, A., Faria, L. C. F., Teixeira, A. L. C., Zeringota, V., Luz, H. R., Ferreira, L. L., & Monteiro, C. (2025). Repellent Activity of the Botanical Compounds Thymol, Carvacrol, Nootkatone, and Eugenol Against Amblyomma sculptum Nymphs. Pathogens, 14(9), 926. https://doi.org/10.3390/pathogens14090926







