Immune Sensitization to Mycobacterium tuberculosis Among Young Children with and without Tuberculosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
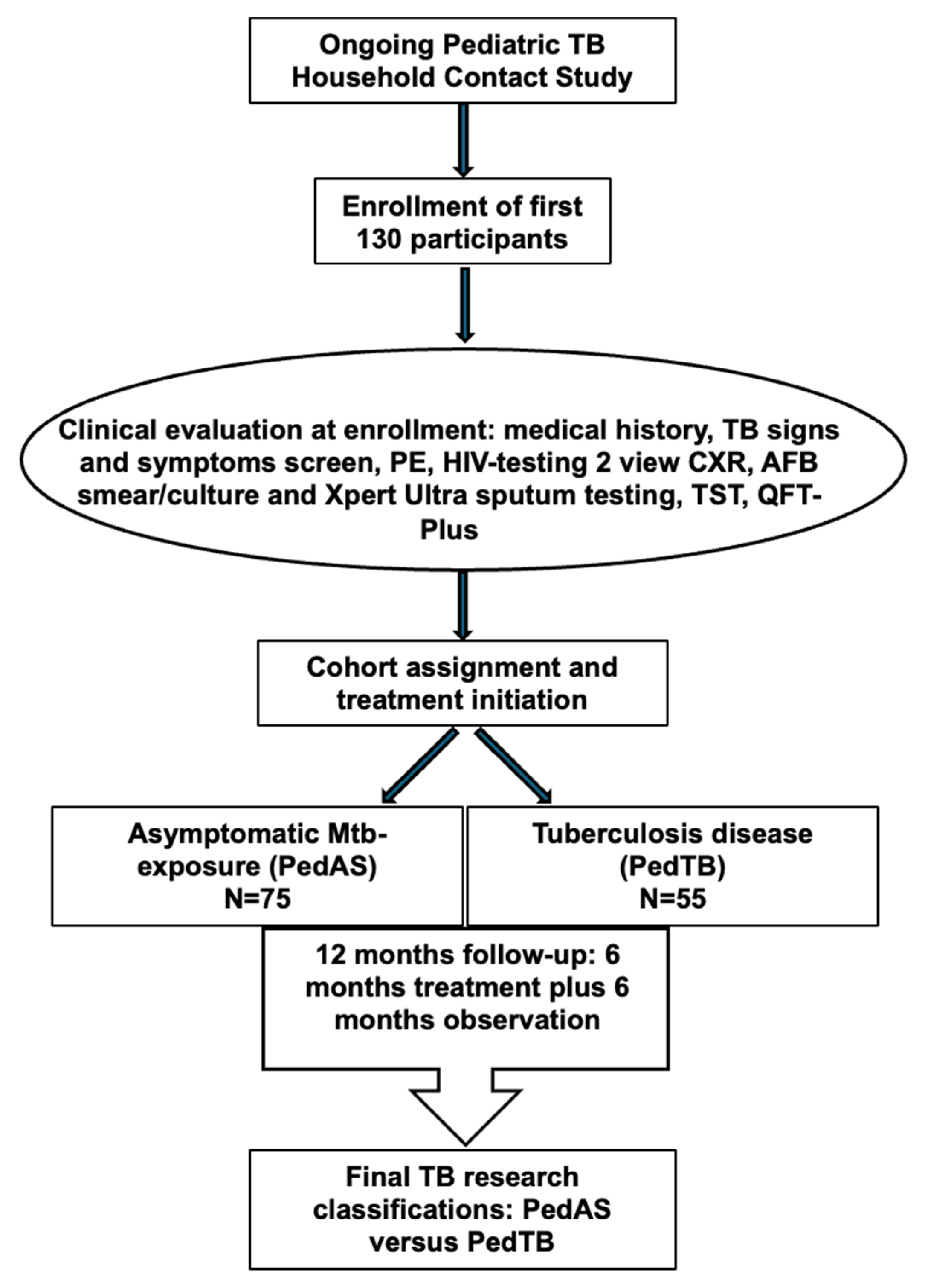
2.2. Study Population, Diagnostic Evaluation, and Cohort Assignment
2.3. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics, Diagnostic Results, and TB Classification Among Study Participants
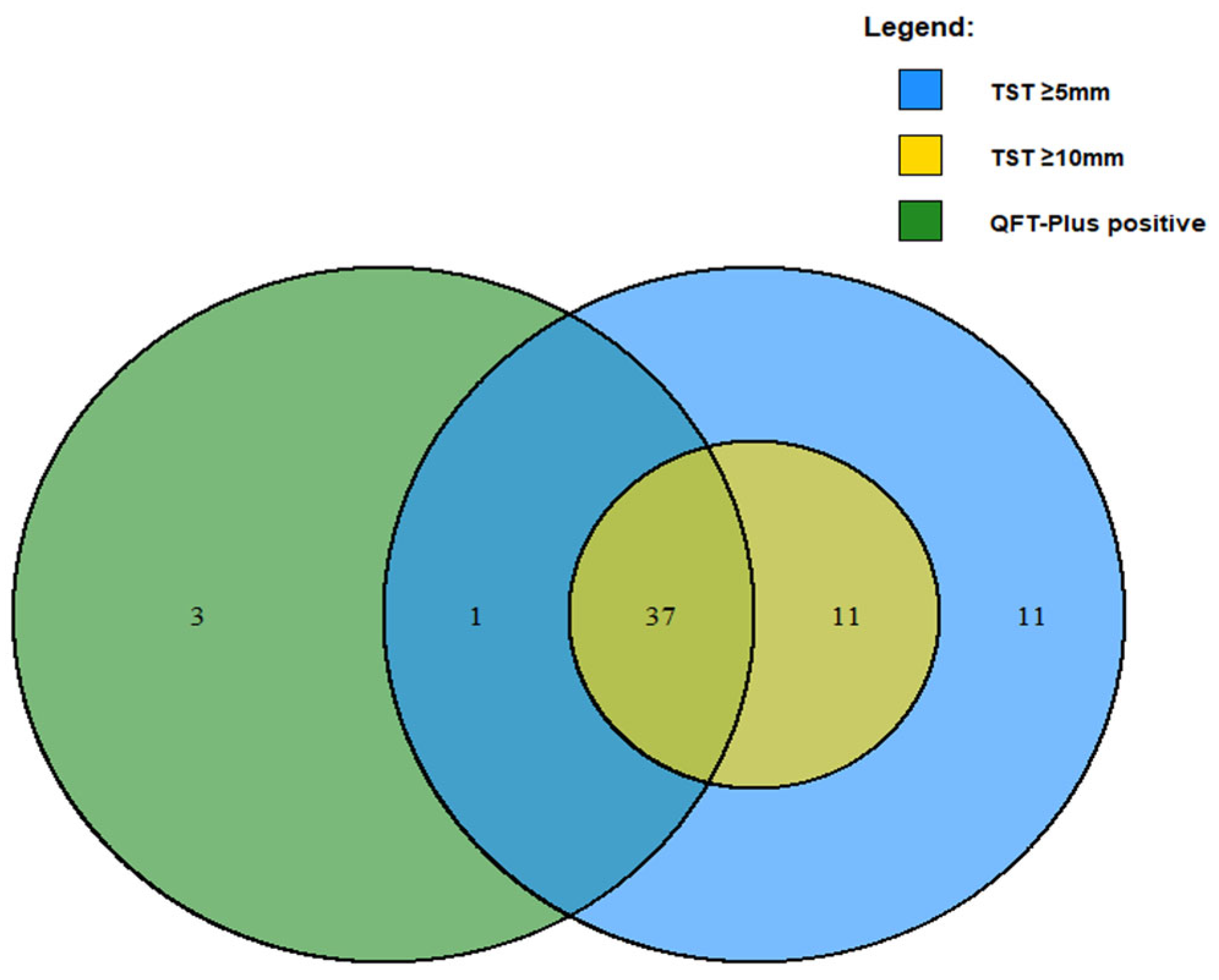
3.2. Concordance Between TST and QFT-Plus Among Study Participants
3.3. Diagnostic Accuracy of TST and QFT-Plus for Pediatric TB
3.4. Relationship Between Age and Assessments for Mtb Sensitization Among Children with and Without TB
3.5. Role of TST in Predicting TB
3.6. Contribution of QFT-Plus Tube 1 Versus Tube 2 in Identification of Children with Mtb Immune Sensitization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Mtb | Mycobacterium tuberculosis |
| TB | Tuberculosis |
| HHC | Household contact |
| TST | Tuberculin skin test |
| IGRA | Interferon gamma release essay |
| QFT-Plus | QuantiFERON Gold Plus |
| WHO | World Health Organization |
| BCG | Bacilli Calmete–Guérin |
| HUU | HIV-unexposed/uninfected |
| HEU | HIV-exposed/uninfected |
| CLWH | A child living-with-HIV |
| PedTB | Diagnosed with TB |
| PedAS | Not diagnosed with TB |
| ERS | Epidemiologic risk score |
| FFM | Free fat mass |
| BMI | Body mass index |
References
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Reuter, A.; Hughes, J.; Furin, J. Challenges and controversies in childhood tuberculosis. Lancet 2019, 394, 967–978. [Google Scholar] [CrossRef]
- Dodd, P.J.; Yuen, C.M.; Sismanidis, C.; Seddon, J.A.; Jenkins, H.E. The global burden of tuberculosis mortality in children: A mathematical modelling study. Lancet Glob. Health 2017, 5, e898–e906. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, H.E.; Yuen, C.M.; Rodriguez, C.A.; Nathavitharana, R.R.; McLaughlin, M.M.; Donald, P.; Marais, B.J.; Becerra, M.C. Mortality in children diagnosed with tuberculosis: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Smieja, M.J.; Marchetti, C.A.; Cook, D.J.; Smaill, F.M. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst. Rev. 2000, 1999, CD001363. [Google Scholar] [CrossRef]
- van Halsema, C.L.; Fielding, K.L.; Chihota, V.N.; Russell, E.C.; Lewis, J.J.; Churchyard, G.J.; Grant, A.D. Tuberculosis outcomes and drug susceptibility in individuals exposed to isoniazid preventive therapy in a high HIV prevalence setting. AIDS 2010, 24, 1051–1055. [Google Scholar] [CrossRef]
- Lalvani, A.; Pareek, M. Interferon gamma release assays: Principles and practice. Enferm. Infecc. Microbiol. Clin. 2010, 28, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Menzies, D.; Pai, M.; Comstock, G. Meta-analysis: New tests for the diagnosis of latent tuberculosis infection: Areas of uncertainty and recommendations for research. Ann. Intern. Med. 2007, 146, 340–354. [Google Scholar] [CrossRef]
- Nolt, D.; Starke, J.R. Tuberculosis Infection in Children and Adolescents: Testing and Treatment. Pediatrics 2021, 148, e2021054663. [Google Scholar] [CrossRef]
- Shafeque, A.; Bigio, J.; Hogan, C.A.; Pai, M.; Banaei, N. Fourth-Generation QuantiFERON-TB Gold Plus: What Is the Evidence? J. Clin. Microbiol. 2020, 58, e01950-19. [Google Scholar] [CrossRef]
- Bozaykut, A.; Ipek, I.O.; Ozkars, M.Y.; Seren, L.P.; Atay, E.; Atay, Z. Effect of BCG vaccine on tuberculin skin tests in 1–6-year-old children. Acta Paediatr. 2002, 91, 235–238. [Google Scholar] [CrossRef]
- Mancuso, J.D.; Mody, R.M.; Olsen, C.H.; Harrison, L.H.; Santosham, M.; Aronson, N.E. The Long-term Effect of Bacille Calmette-Guerin Vaccination on Tuberculin Skin Testing: A 55-Year Follow-Up Study. Chest 2017, 152, 282–294. [Google Scholar] [CrossRef]
- Farhat, M.; Greenaway, C.; Pai, M.; Menzies, D. False-positive tuberculin skin tests: What is the absolute effect of BCG and non-tuberculous mycobacteria? Int. J. Tuberc. Lung Dis. 2006, 10, 1192–1204. [Google Scholar]
- Buonsenso, D.; Noguera-Julian, A.; Moroni, R.; Hernandez-Bartolome, A.; Fritschi, N.; Lancella, L.; Cursi, L.; Soler-Garcia, A.; Krüger, R.; Feiterna-Sperling, C.; et al. Performance of QuantiFERON-TB Gold Plus assays in paediatric tuberculosis: A multicentre PTBNET study. Thorax 2023, 78, 288–296. [Google Scholar] [CrossRef]
- Soler-Garcia, A.; Gamell, A.; Monsonis, M.; Korta-Murua, J.J.; Espiau, M.; Rincon-Lopez, E.; Rodríguez-Molino, P.; Pérez-Porcuna, T.; Bustillo-Alonso, M.; Santiago, B.; et al. The Value of the Second QuantiFERON-TB Gold-Plus Antigen Tube at Diagnosis and at Treatment Completion in Spanish Children With Tuberculosis. Pediatr. Infect. Dis. J. 2023, 42, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Module 3: Diagnosis: Tests for tuberculosis infection. In WHO Operational Handbook on Tuberculosis; World Health Organization: Geneva, Switzerland, 30 September 2022; Available online: https://www.who.int/publications/i/item/9789240056084 (accessed on 10 September 2025).
- Surve, S.; Bhor, V.; Naukariya, K.; Begum, S.; Munne, K.; Tipre, P.; Sutar, N.; Jaiswal, A.; Bhonde, G.; Chauhan, S.; et al. Discordance between TST and QFT-TBGold Plus for Latent Tuberculosis Screening among Under-Five Children: An Interim Analysis. J. Trop. Pediatr. 2021, 67, fmab103. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Seddon, J.A.; Esposito, S.; Barcellini, L. QuantiFERON-TB Gold Plus performance in children: A narrative review. Pediatr. Infect. Dis. J. 2023, 42, e158–e165. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Cords, O.; Horsburgh, C.R.; Andrews, J.R.; Pediatric TB Contact Studies Consortium. The risk of tuberculosis in children after close exposure: A systematic review and individual-participant meta-analysis. Lancet 2020, 395, 973–984. [Google Scholar] [CrossRef]
- Graham, S.M.; Cuevas, L.E.; Jean-Philippe, P.; Browning, R.; Casenghi, M.; Detjen, A.K.; Gnanashanmugam, D.; Hesseling, A.C.; Kampmann, B.; Mandalakas, A.; et al. Clinical Case Definitions for Classification of Intrathoracic Tuberculosis in Children: An Update. Clin. Infect. Dis. 2015, 61 (Suppl. S3), S179–S187. [Google Scholar] [CrossRef]
- Aceng, J.R. The Uganda National Tuberculosis Prevalence Survey, 2014–2015; Ministry of Health: Kampala, Uganda, 2016. [Google Scholar]
- Mandalakas, A.M.; Kirchner, H.L.; Lombard, C.; Walzl, G.; Grewal, H.M.; Gie, R.P.; Hesseling, A.C. Well-quantified tuberculosis exposure is a reliable surrogate measure of tuberculosis infection. Int. J. Tuberc. Lung Dis. 2012, 16, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- QIAGEN GmbH. QuantiFERON®-TB Gold Plus (QFT®-Plus) ELISA Package Insert (Rev. 09). Available online: https://www.qiagen.com/us/resources/download.aspx?id=ac068fc7-a994-4443-ac7c-dda43ce2bc5e&lang=en (accessed on 3 September 2025).
- World Health Organization. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children, 2nd ed.; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 11 November 2006; Available online: https://www.who.int/publications/i/item/924154693X (accessed on 10 September 2025).
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 5: Management of Tuberculosis in Children and Adolescents; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Martinez, L.; Gray, D.M.; Botha, M.; Nel, M.; Chaya, S.; Jacobs, C.; Workman, L.; Nicol, M.; Zar, H. The Long-Term Impact of Early-Life Tuberculosis Disease on Child Health: A Prospective Birth Cohort Study. Am. J. Respir. Crit. Care Med. 2023, 207, 1080–1088. [Google Scholar] [CrossRef]
- Marais, B.J.; Gie, R.P.; Schaaf, H.S.; Hesseling, A.C.; Obihara, C.C.; Starke, J.J.; Enarsonet, D.A.; Donald, P.R.; Beyers, N. The natural history of childhood intra-thoracic tuberculosis: A critical review of literature from the pre-chemotherapy era. Int. J. Tuberc. Lung Dis. 2004, 8, 392–402. [Google Scholar]
- Chiabi, A.; Wirngo, T.; Yves Bassong, P.; Ngoufo, F.N.; Ngum, E.N.; Angwafor, S.; Nforniwe, D.N. The Spectrum of Childhood Tuberculosis in an African Setting: A Hospital-Based Experience in Bamenda, Cameroon. Turk. Arch. Pediatr. 2023, 58, 154–158. [Google Scholar] [CrossRef]
- Mandalakas, A.M.; Detjen, A.K.; Hesseling, A.C.; Benedetti, A.; Menzies, D. Interferon-gamma release assays and childhood tuberculosis: Systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2011, 15, 1018–1032. [Google Scholar] [CrossRef]
- Nicol, M.P.; Davies, M.A.; Wood, K.; Hatherill, M.; Workman, L.; Hawkridge, A.; Eley, B.; Wilkinson, K.A.; Wilkinson, R.J.; Hanekom, W.A.; et al. Comparison of T-SPOT.TB assay and tuberculin skin test for the evaluation of young children at high risk for tuberculosis in a community setting. Pediatrics 2009, 123, 38–43. [Google Scholar] [CrossRef]
- Debord, C.; De Lauzanne, A.; Gourgouillon, N.; Guerin-El Khourouj, V.; Pedron, B.; Gaudelus, J.; Faye, A.; Sterkers, G. Interferon-gamma release assay performance for diagnosing tuberculosis disease in 0- to 5-year-old children. Pediatr. Infect. Dis. J. 2011, 30, 995–997. [Google Scholar] [CrossRef]
- Moyo, S.; Isaacs, F.; Gelderbloem, S.; Verver, S.; Hawkridge, A.J.; Hatherill, M.; Tameris, M.; Geldenhuys, H.; Workman, L.; Pai, M.; et al. Tuberculin skin test and QuantiFERON(R) assay in young children investigated for tuberculosis in South Africa. Int. J. Tuberc. Lung Dis. 2011, 15, 1176–1181. [Google Scholar] [CrossRef]
- Machingaidze, S.; Wiysonge, C.S.; Gonzalez-Angulo, Y.; Hatherill, M.; Moyo, S.; Hanekom, W.; Moyo, S.; Hanekom, W.; Mahomed, H. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: A systematic review and meta-analysis. Pediatr. Infect. Dis. J. 2011, 30, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Pavic, I.; Topic, R.Z.; Raos, M.; Aberle, N.; Dodig, S. Interferon-gamma release assay for the diagnosis of latent tuberculosis in children younger than 5 years of age. Pediatr. Infect. Dis. J. 2011, 30, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Lewinsohn, D.M.; Leonard, M.K.; LoBue, P.A.; Cohn, D.L.; Daley, C.L.; Desmond, E.; Keane, J.; Lewinsohn, D.A.; Loeffler, A.M.; Mazurek, G.H.; et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin. Infect. Dis. 2017, 64, e1–e33. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.E.; Wu, Y.; Ho, C.S.; Pettit, A.C.; Feng, P.J.; Katz, D.J.; Ghosh, S.; Venkatappa, T.; Luo, R. Evaluating latent tuberculosis infection diagnostics using latent class analysis. Thorax 2018, 73, 1062–1070. [Google Scholar] [CrossRef]
- Ho, C.S.; Feng, P.I.; Narita, M.; Stout, J.E.; Chen, M.; Pascopella, L.; Garfein, R.; Reves, R.; Katz, D.J.; Flood, J.; et al. Comparison of three tests for latent tuberculosis infection in high-risk people in the USA: An observational cohort study. Lancet Infect. Dis. 2022, 22, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.T.; Starke, J.R. Relationship between tuberculin skin test (TST) size and interferon gamma release assay (IGRA) result: When should clinicians obtain IGRAs in children with positive TSTs? Clin. Pediatr. 2014, 53, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.A.; Paton, J.; Nademi, Z.; Keane, D.; Williams, B.; Williams, A.; Welch, S.B.; Liebeschutz, S.; Riddell, A.; Bernatoniene, J.; et al. The impact of BCG vaccination on tuberculin skin test responses in children is age dependent: Evidence to be considered when screening children for tuberculosis infection. Thorax 2016, 71, 932–939. [Google Scholar] [CrossRef]
- Menzies, D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am. J. Respir. Crit. Care Med. 1999, 159, 15–21. [Google Scholar] [CrossRef]
- Sepulveda, R.L.; Burr, C.; Ferrer, X.; Sorensen, R.U. Booster effect of tuberculin testing in healthy 6-year-old school children vaccinated with Bacillus Calmette-Guerin at birth in Santiago, Chile. Pediatr. Infect. Dis. J. 1988, 7, 578–581. [Google Scholar]
- Mandalakas, A.M.; Kirchner, H.L.; Walzl, G.; Gie, R.P.; Schaaf, H.S.; Cotton, M.F.; Grewal, H.M.S.; Hesseling, A.C. Optimizing the detection of recent tuberculosis infection in children in a high tuberculosis-HIV burden setting. Am. J. Respir. Crit. Care Med. 2015, 191, 820–830. [Google Scholar] [CrossRef]
- Ronge, L.; Sloot, R.; Du Preez, K.; Kay, A.W.; Kirchner, H.L.; Grewal, H.M.S.; Mandalakas, A.M.; Hesseling, A.C. The Magnitude of Interferon Gamma Release Assay Responses in Children With Household Tuberculosis Contact Is Associated With Tuberculosis Exposure and Disease Status. Pediatr. Infect. Dis. J. 2021, 40, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Arnaiz, E.; Batllori, M.; Monsonis, M.; Valls, A.; Rios-Barnes, M.; Simo-Nebot, S.; Gamell, A.; Fortuny, C.; Tebruegge, M.; Noguera-Julian, A. Host, technical, and environmental factors affecting QuantiFERON-TB Gold In-Tube performance in children below 5 years of age. Sci. Rep. 2022, 12, 19908. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, L.H.; Juarez, E.; Carranza, C.; Carreto-Binaghi, L.E.; Alejandre, A.; Cabello-Gutierrez, C.; Gonzalez, Y. Immunological aspects of diagnosis and management of childhood tuberculosis. Infect. Drug Resist. 2021, 14, 929–946. [Google Scholar] [CrossRef]
- Lundtoft, C.; Awuah, A.A.; Nausch, N.; Enimil, A.; Mayatepek, E.; Owusu-Dabo, E.; Jacobsen, M. Alternative Quantiferon cytokines for diagnosis of children with active tuberculosis and HIV co-infection in Ghana. Med. Microbiol. Immunol. 2017, 206, 259–265. [Google Scholar] [CrossRef]
- Chegou, N.N.; Detjen, A.K.; Thiart, L.; Walters, E.; Mandalakas, A.M.; Hesseling, A.C.; Walzl, G.; Pai, M. Utility of host markers detected in Quantiferon supernatants for the diagnosis of tuberculosis in children in a high-burden setting. PLoS ONE 2013, 8, e64226. [Google Scholar] [CrossRef] [PubMed]
- Tebruegge, M.; Dutta, B.; Donath, S.; Ritz, N.; Forbes, B.; Camacho-Badilla, K.; Clifford, V.; Zufferey, C.; Robins-Browne, R.; Hanekom, W.; et al. Mycobacteria-Specific Cytokine Responses Detect Tuberculosis Infection and Distinguish Latent from Active Tuberculosis. Am. J. Respir. Crit. Care Med. 2015, 192, 485–499. [Google Scholar] [CrossRef]
- Delgado, J.C.; Tsai, E.Y.; Thim, S.; Baena, A.; Boussiotis, V.A.; Reynes, J.M.; Sath, S.; Grosjean, P.; Yunis, E.J.; Goldfeld, A.E. Antigen-specific and persistent tuberculin anergy in a cohort of pulmonary tuberculosis patients from rural Cambodia. Proc. Natl. Acad. Sci. USA 2002, 99, 7576–7581. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.P.; Zar, H.J. New specimens and laboratory diagnostics for childhood pulmonary TB: Progress and prospects. Paediatr. Respir. Rev. 2011, 12, 16–21. [Google Scholar] [CrossRef]
- Swaminathan, S.; Rekha, B. Pediatric tuberculosis: Global overview and challenges. Clin. Infect. Dis. 2010, 50 (Suppl. S3), S184–S194. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.M.; Ahmed, T.; Amanullah, F.; Browning, R.; Cardenas, V.; Casenghi, M.; Cuevas, L.E.; Gale, M.; Gie, R.P.; Grzemska, M.; et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J. Infect. Dis. 2012, 205 (Suppl. S2), S199–S208. [Google Scholar] [CrossRef]
- Zar, H.J.; Hanslo, D.; Apolles, P.; Swingler, G.; Hussey, G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: A prospective study. Lancet 2005, 365, 130–134. [Google Scholar] [CrossRef]
- Ruiz Jimenez, M.; Guillen Martin, S.; Prieto Tato, L.M.; Cacho Calvo, J.B.; Alvarez Garcia, A.; Soto Sanchez, B.; Ramos Amador, J.T. Induced sputum versus gastric lavage for the diagnosis of pulmonary tuberculosis in children. BMC Infect. Dis. 2013, 13, 222. [Google Scholar] [CrossRef]
- Drancourt, M. Please, No More Gastric Aspirate to Diagnose Pulmonary Tuberculosis in Children. Clin. Infect. Dis. 2017, 65, 2158. [Google Scholar] [CrossRef]

| Full Cohort | PedAS | PedTB | p-Value (Test) | |
|---|---|---|---|---|
| n | 130 | 75 | 55 | |
| Age (months) | 27.5 [±6.2] | 25.9 [±16.2] | 29.6 [±16.2] | 0.20 (t-test) |
| Sex = male | 73 (56.2) | 42 (56.0) | 31 (56.4) | 1.00 (χ2) |
| Epidemiologic Risk Score (range: 0–10) | 7 [6.0–8.0] | 7 [6.0–9.0] | 7 [6.0–8.0] | 0.12 (MWU) |
| HIV classification | 0.86 (Fisher’s) | |||
| HIV unexposed uninfected (HUU) | 87 (66.9) | 48 (64.0) | 39 (70.9) | |
| HIV exposed uninfected (HEU) | 19 (14.6) | 12 (16.0) | 7 (12.7) | |
| Child living with HIV | 4 (3.1) | 2 (2.7) | 2 (3.7) | |
| HIV exposure unknown HIV result pending a | 19 (14.6) 1 (0.8) | 12 (16.0) 1 (1.3) | 7 (12.7) 0 (0) | |
| IGRA (QFT-Plus) = positive b | 41 (31.5) | 21 (28.0) | 20 (36.4) | 0.34 (χ2) |
| Mean quantitative IGRA result c | 0.01 [0–2.47] | 0.02 [0–1.77] | 0.03 [0–4.96] | 0.75 (MWU) |
| Mean quantitative IGRA result c in children with positive IGRA (n = 41) | 6.59 [2.72–9.22] | 5.34 [1.98–9.51] | 6.89 [4.63–7.97] | 0.82 (MWU) |
| TST = positive (10 mm) | 51 (39.2) | 25 (33.3) | 26 (47.3) | 0.15 (χ2) |
| TST = positive (5 mm) | 64 (49.2) | 31 (39.7) | 33 (61.1) | 0.02 * (χ2) |
| Quantitative TST result | 3.4 [0–14.9] | 0 [0–13.5] | 8.6 [0–16.3] | 0.02 * (MWU) |
| BCG scar present | 112 (86.2) | 64 (85.3) | 48 (87.3) | 0.80 (Fisher’s) |
| PedAS | PedTB | |
|---|---|---|
| n | 75 | 55 |
| Chest X-ray (CXR) | ||
| Normal | 70 (93.3%) | 5 (9.1%) |
| Moderately advanced disease a Unknown | 3 (4.0%) 2 (2.7%) | 50 (90.9%) 0 (0%) |
| TB symptoms | ||
| No symptoms | 62 (82.7%) | 36 (65.5%) |
| At least one symptom | 13 (17.3%) | 19 (34.5%) |
| Extra-pulmonary TB symptoms | ||
| No symptoms | 75 (100%) | 55 (100%) |
| At least one symptom | 0 (0%) | 0 (0%) |
| Xpert Ultra result | ||
| Negative | 75 (100%) | 51 (92.7%) |
| Positive Unknown | 0 (0%) 0 (0%) | 3 (5.5%) 1 (1.8%) |
| Smear Microscopy result | ||
| Negative | 74 (98.7%) | 53 (96.4%) |
| Positive Unknown | 0 (0%) 1 (1.3%) | 2 (3.6%) 0 (0%) |
| Culture result | ||
| Negative | 75 (100%) | 50 (90.9%) |
| Positive | 0 (0%) | 5 (9.1%) |
| TST Results (10 mm) | |||||
|---|---|---|---|---|---|
| Full Cohort | QFT-Plus Results | N = 125 * | Positive | Negative | |
| Positive | 37 (29.6%) | 4 (3.2%) | McNemar χ2 = 0.12 | ||
| Negative | 11 (8.8%) | 73 (58.4%) | Cohen’s Kappa a = 0.74 | ||
| Children <2 years of age | QFT-Plus Results | N = 53 | Positive | Negative | |
| Positive | 11 (20.7%) | 1 (1.9%) | McNemar χ2 = 0.04 | ||
| Negative | 8 (15.1%) | 33 (62.3%) | Cohen’s Kappa a = 0.60 | ||
| Children 2–5 years of age | QFT-Plus Results | N = 72 | Positive | Negative | |
| Positive | 26 (36.0%) | 3 (4.2%) | McNemar χ2 = 1.0 | ||
| Negative | 3 (4.2%) | 40 (55.6%) | Cohen’s Kappa a = 0.83 | ||
| TST Results (5 mm) | |||||
|---|---|---|---|---|---|
| Full Cohort | QFT-Plus Results | N = 125 * | Positive | Negative | |
| Positive | 38 (30.4%) | 3 (2.4%) | McNemar χ2 = 0.0003 | ||
| Negative | 22 (17.6%) | 62 (49.6%) | Cohen’s Kappa a = 0.59 | ||
| Children <2 years of age | QFT-Plus Results | N = 53 | Positive | Negative | |
| Positive | 11 (20.8%) | 1 (1.9%) | McNemar χ2 = 0.002 | ||
| Negative | 14 (26.4%) | 27 (50.9%) | Cohen’s Kappa a = 0.42 | ||
| Children 2–5 years of age | QFT-Plus Results | N = 72 | Positive | Negative | |
| Positive | 27 (37.5%) | 2 (2.8%) | McNemar χ2 = 0.11 | ||
| Negative | 8 (11.1%) | 35 (48.6%) | Cohen’s Kappa a = 0.72 | ||
| Tuberculosis (n = 55) | Asymptomatic Exposure (n = 75) | Sensitivity | Specificity | |
|---|---|---|---|---|
| TST positive (5 mm) | 33 | 31 | 0.6 | 0.59 |
| TST negative (5 mm) | 22 | 44 | ||
| TST positive (10 mm) | 26 | 25 | 0.47 | 0.67 |
| TST negative (10 mm) | 29 | 50 | ||
| QFT-Plus positive | 20 | 21 | 0.36 | 0.72 |
| QFT-Plus negative | 35 | 54 |
| Under 2 Years of Age (n = 57) | 2 to 5 Years of Age (n = 73) | ||||||
|---|---|---|---|---|---|---|---|
| PedAS | PedTB | PedAS | PedTB | ||||
| IGRA a (QFT-Plus) | Positive | 8 (24.2%) | 4 (20%) | p = 0.98 | 13 (35.6%) | 16 (50%) | p = 0.21 |
| Negative | 25 (75.8%) | 16 (80%) | 27 (67.5%) | 16 (50%) | |||
| TST (10 mm) | Positive | 13 (38.2%) | 8 (34.8%) | p = 1.00 | 12 (29.3%) | 18 (56.3%) | p = 0.04 * |
| Negative | 21 (61.8%) | 15 (65.2%) | 29 (70.7%) | 14 (43.8%) | |||
| TST (5 mm) | Positive | 16 (47.1%) | 12 (52.2%) | p = 0.91 | 15 (35.7%) | 21 (67.7%) | p = 0.01 * |
| Negative | 18 (52.9%) | 11 (47.8%) | 27 (64.3%) | 10 (32.3%) | |||
| Covariates | Adjusted OR | 95% CI | p-Value |
|---|---|---|---|
| TST results (5 mm) | |||
| Negative | (Ref.) | (Ref.) | (Ref.) |
| Positive | 2.09 | 1.02–4.37 | 0.04 * |
| Sex = male | 0.85 | 0.40–1.82 | 0.68 |
| Age (months) | 1.02 | 0.99–1.04 | 0.15 |
| HIV = positive | 0.80 | 0.04–9.22 | 0.86 |
| BCG scar = present | 1.19 | 0.42–3.58 | 0.75 |
| TST results (10 mm) | |||
| Negative | (Ref.) | (Ref.) | (Ref.) |
| Positive | 1.71 | 0.83–3.55 | 0.15 |
| Sex = male | 0.87 | 0.41–1.85 | 0.73 |
| Age (months) | 1.02 | 0.99–1.04 | 0.20 |
| HIV = positive | 0.72 | 0.03–8.16 | 0.79 |
| BCG scar = present | 1.29 | 0.46–3.85 | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez, J.; Malone, L.L.; Mohammadi, M.; Mukisa, J.; Atuhairwe, M.; Mwesigwa, S.P.G.; Athieno, S.; Buwule, S.; Ameda, F.; Nalukwago, S.; et al. Immune Sensitization to Mycobacterium tuberculosis Among Young Children with and without Tuberculosis. Pathogens 2025, 14, 924. https://doi.org/10.3390/pathogens14090924
Gutierrez J, Malone LL, Mohammadi M, Mukisa J, Atuhairwe M, Mwesigwa SPG, Athieno S, Buwule S, Ameda F, Nalukwago S, et al. Immune Sensitization to Mycobacterium tuberculosis Among Young Children with and without Tuberculosis. Pathogens. 2025; 14(9):924. https://doi.org/10.3390/pathogens14090924
Chicago/Turabian StyleGutierrez, Jesús, LaShaunda L. Malone, Mitchka Mohammadi, John Mukisa, Michael Atuhairwe, Simon Peter G. Mwesigwa, Salome Athieno, Sharon Buwule, Faith Ameda, Sophie Nalukwago, and et al. 2025. "Immune Sensitization to Mycobacterium tuberculosis Among Young Children with and without Tuberculosis" Pathogens 14, no. 9: 924. https://doi.org/10.3390/pathogens14090924
APA StyleGutierrez, J., Malone, L. L., Mohammadi, M., Mukisa, J., Atuhairwe, M., Mwesigwa, S. P. G., Athieno, S., Buwule, S., Ameda, F., Nalukwago, S., Mupere, E., Stein, C. M., & Lancioni, C. L. (2025). Immune Sensitization to Mycobacterium tuberculosis Among Young Children with and without Tuberculosis. Pathogens, 14(9), 924. https://doi.org/10.3390/pathogens14090924






