Epidemiology and Diversity of Paratuberculosis in the Arabian Peninsula: A Systematic Review and Meta-Analysis with Implications for One Health
Abstract
1. Introduction
2. Materials and Methods
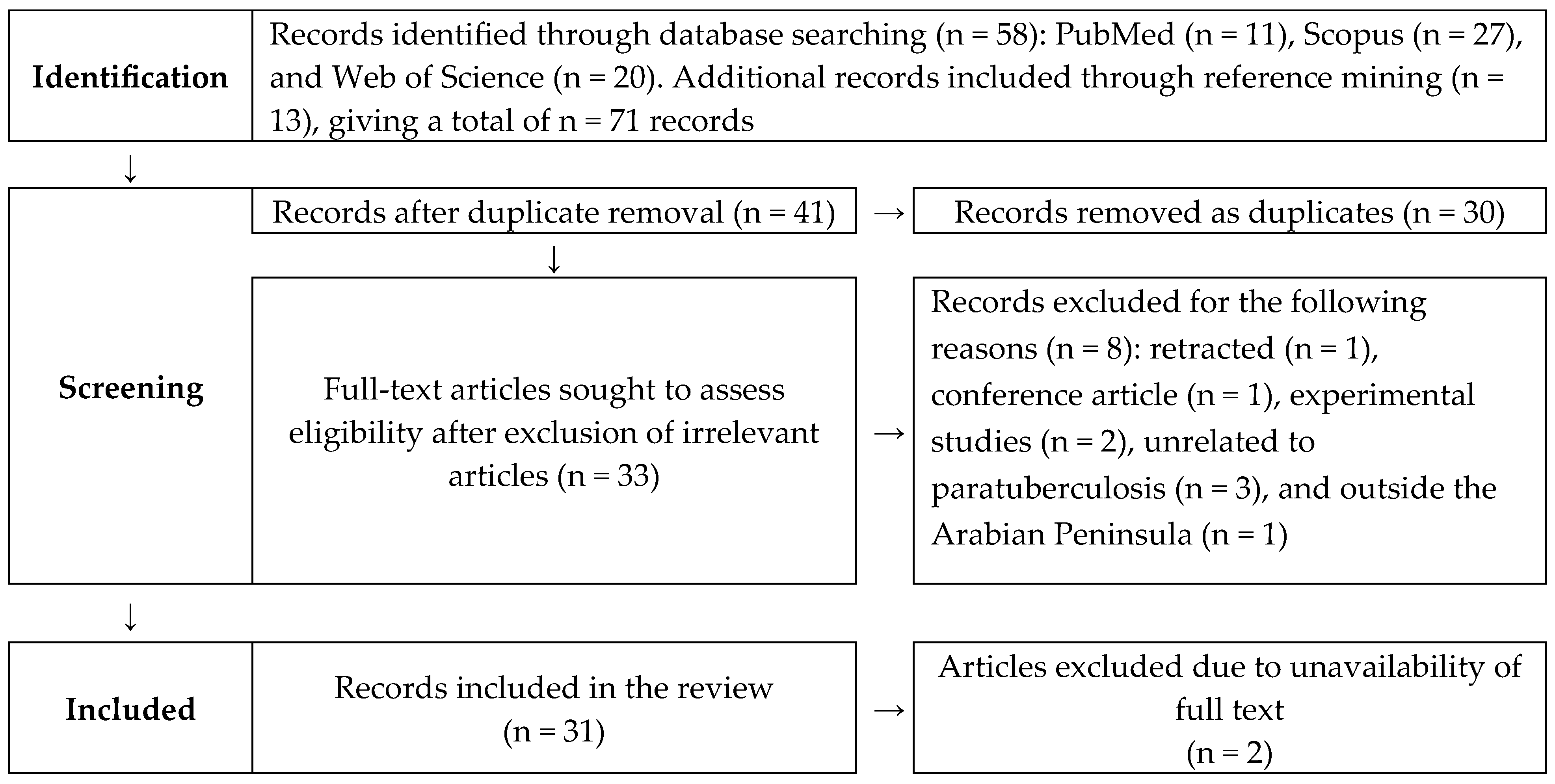
2.1. Systematic Search
2.2. Data Extraction
2.3. Quality Appraisal
2.4. Data Analysis
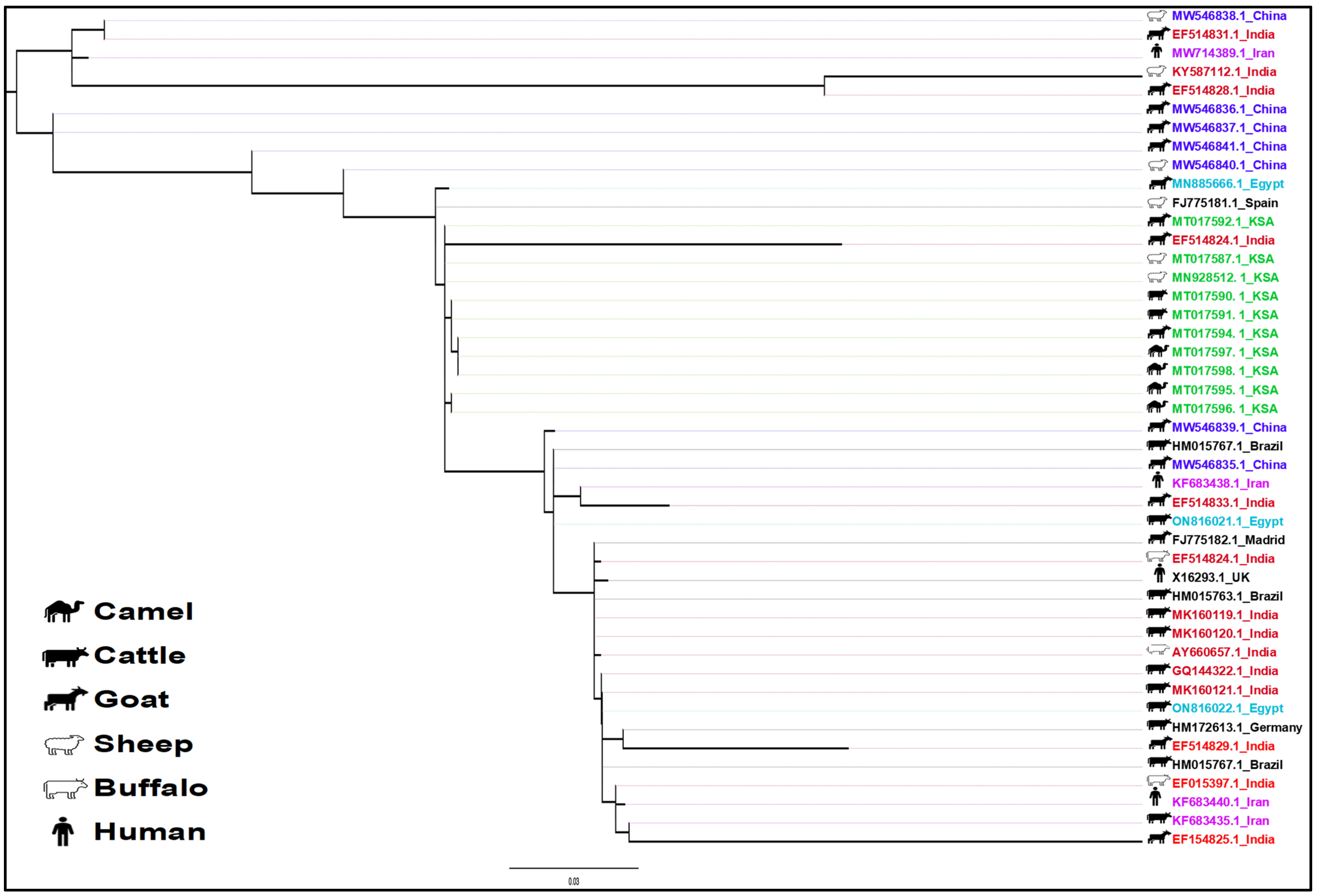
2.5. Phylogenetic Analysis
3. Results
3.1. Research on Paratuberculosis in the Arabian Peninsula
3.2. Distribution and Evolutionary Diversity
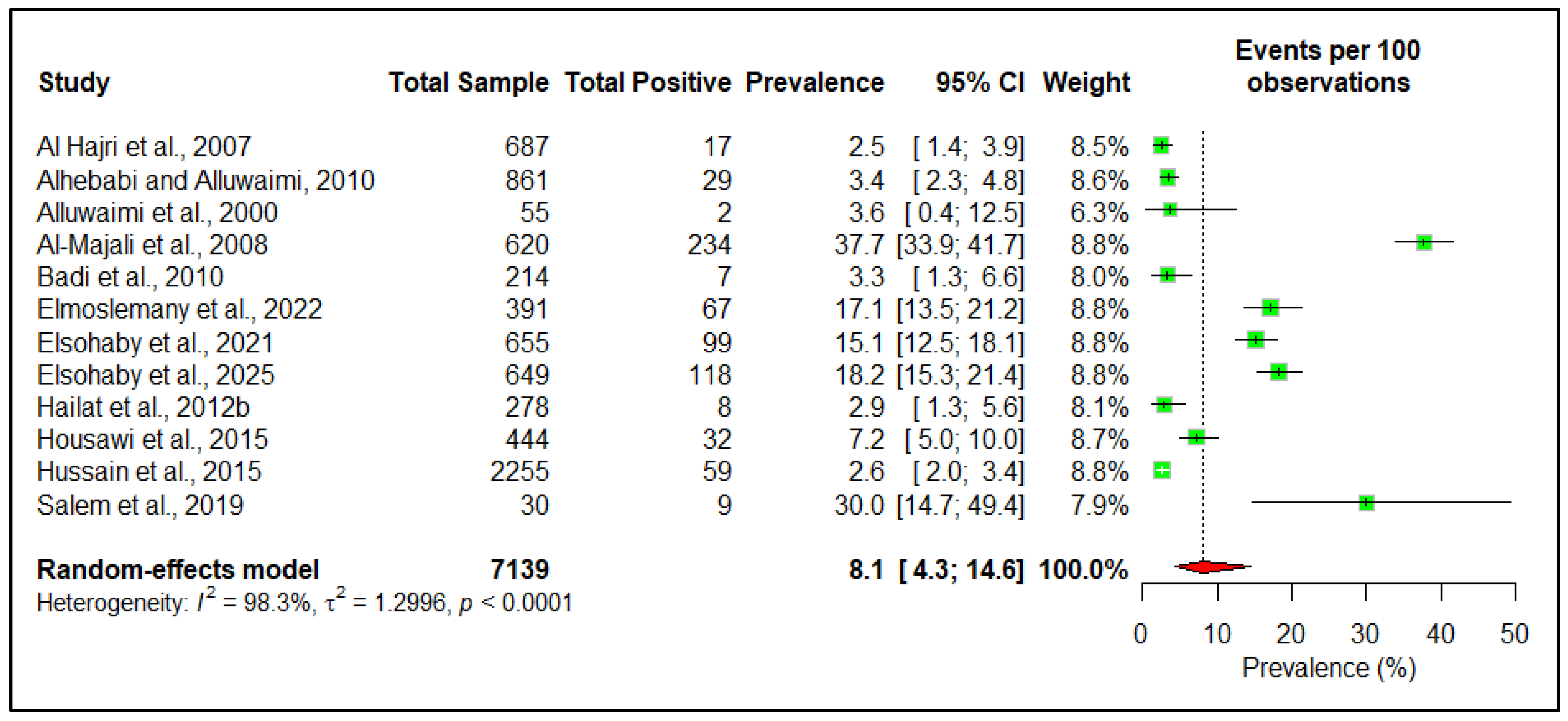
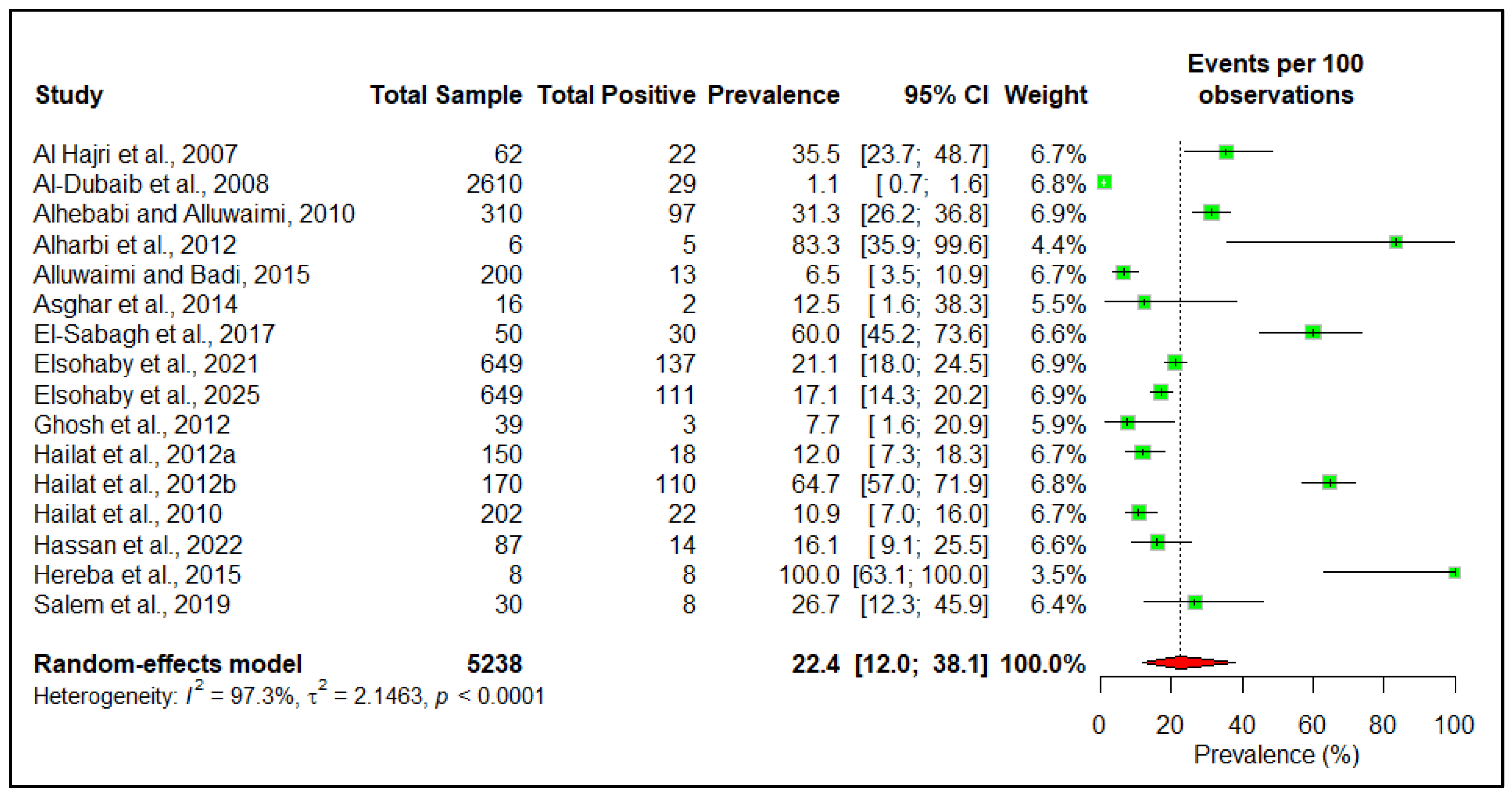
3.3. Prevalence
3.4. Clinical Features
3.5. Pathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cocito, C.; Gilot, P.; Coene, M.; Kesel, M.d.; Poupart, P.; Vannuffel, P. Paratuberculosis. Clin. Microbiol. Rev. 1994, 7, 328–345. [Google Scholar] [CrossRef]
- Underwood, W.J.; Blauwiekel, R.; Delano, M.L.; Gillesby, R.; Mischler, S.A.; Schoell, A. Chapter 15—Biology and Diseases of Ruminants (Sheep, Goats, and Cattle). In Laboratory Animal Medicine, 3rd ed.; Fox, J.G., Anderson, L.C., Otto, G.M., Pritchett-Corning, K.R., Whary, M.T., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 623–694. [Google Scholar]
- Radostits, O.; Gay, C.; Hinchcliff, K.; Constable, P. Veterinary Medicine. A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats; Elsevier: St. Louis, MO, USA, 2007. [Google Scholar]
- Marcé, C.; Ezanno, P.; Weber, M.F.; Seegers, H.; Pfeiffer, D.U.; Fourichon, C. Invited review: Modeling within-herd transmission of Mycobacterium avium subspecies paratuberculosis in dairy cattle: A review. J. Dairy Sci. 2010, 93, 4455–4470. [Google Scholar] [CrossRef]
- Münster, P.; Völkel, I.; Wemheuer, W.; Schwarz, D.; Döring, S.; Czerny, C.P. A longitudinal study to characterize the distribution patterns of Mycobacterium avium ssp. paratuberculosis in semen, blood and faeces of a naturally infected bull by IS 900 semi-nested and quantitative real-time PCR. Transbound. Emerg. Dis. 2013, 60, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Judge, J.; Kyriazakis, I.; Greig, A.; Davidson, R.S.; Hutchings, M.R. Routes of intraspecies transmission of Mycobacterium avium subsp. paratuberculosis in rabbits (Oryctolagus cuniculus): A field study. Appl. Environ. Microbiol. 2006, 72, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Whittington, R.J.; Marshall, D.J.; Nicholls, P.J.; Marsh, I.B.; Reddacliff, L.A. Survival and dormancy of Mycobacterium avium subsp. paratuberculosis in the environment. Appl. Environ. Microbiol. 2004, 70, 2989–3004. [Google Scholar] [CrossRef]
- Samba-Louaka, A.; Robino, E.; Cochard, T.; Branger, M.; Delafont, V.; Aucher, W.; Wambeke, W.; Bannantine, J.P.; Biet, F.; Héchard, Y. Environmental Mycobacterium avium subsp. paratuberculosis Hosted by Free-Living Amoebae. Front. Cell. Infect. Microbiol. 2018, 8, 28. [Google Scholar] [CrossRef]
- Idris, S.M.; Eltom, K.H.; Okuni, J.B.; Ojok, L.; Elmagzoub, W.A.; El Wahed, A.A.; Eltayeb, E.; Gameel, A.A. Paratuberculosis: The Hidden Killer of Small Ruminants. Animals 2021, 12, 12. [Google Scholar] [CrossRef]
- World Organization for Animal Health. Paratuberculosis. Available online: https://www.woah.org/en/disease/paratuberculosis/ (accessed on 14 May 2025).
- The Center for Food Security and Public Health. Paratuberculosis. Available online: https://www.cfsph.iastate.edu/Factsheets/pdfs/paratuberculosis.pdf (accessed on 30 July 2023).
- Al-Dubaib, M.; Mahmoud, O. Paratuberculosis of goats at Qassim region of Central Saudi Arabia. Bulgharian J. Vet. Med. 2008, 11, 65–69. [Google Scholar]
- Lumen. World Regional Geography, Chapter 8: North Africa and Southwest Asia. Available online: https://courses.lumenlearning.com/atd-herkimer-worldgeography/chapter/8-5-arabs-islam-and-oil/ (accessed on 20 January 2024).
- Kodzhakova, Z. Food security in the GCC: Specific characteristics and solutions. RUDN J. Econ. 2021, 29, 126–136. [Google Scholar] [CrossRef]
- Farag, E.; Sikkema, R.S.; Vinks, T.; Islam, M.M.; Nour, M.; Al-Romaihi, H.; Al Thani, M.; Atta, M.; Alhajri, F.H.; Al-Marri, S.; et al. Drivers of MERS-CoV Emergence in Qatar. Viruses 2018, 11, 22. [Google Scholar] [CrossRef]
- Islam, M.M.; Farag, E.; Hassan, M.M.; Enan, K.A.; Mohammad Sabeel, K.V.; Alhaddad, M.M.; Smatti, M.K.; Al-Marri, A.M.; Al-Zeyara, A.A.; Al-Romaihi, H.; et al. Diversity of bacterial pathogens and their antimicrobial resistance profile among commensal rodents in Qatar. Vet. Res. Commun. 2022, 46, 487–498. [Google Scholar] [CrossRef]
- Salem, M.A.; El-Deeb, W.M.; Zaghawa, A.A.; Housawi, F.M.; Alluwaimi, A.M. Investigation of Mycobacterium paratuberculosis in Arabian dromedary camels (Camelus dromedarius). Vet. World 2019, 12, 219–223. [Google Scholar] [CrossRef]
- Tigani, E.; Tigani-Asil, A.; El, G.; Abdelwahab, D.; Hadi, E.; Abdu, A.; Mohammed, A.; Terab, A.; Altaib, N.; Khalil, H.; et al. Pathological, microscopic, and molecular diagnosis of paratuberculosis/John’s disease in naturally infected dromedary camel (Camelus dromedarius). Vet. World 2023, 16, 1277–1283. [Google Scholar] [CrossRef]
- PRISMA. PRISMA 2020 Statement Paper. Available online: https://www.prisma-statement.org/prisma-2020-statement (accessed on 7 June 2025).
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; Blunt, H.; Brigham, T.; Chang, S.; et al. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Elfadl, A.K.; Islam, M.M. Prevalence and Molecular Pattern of Paratuberculosis in the Arabian Peninsula: A systematic review and meta-analysis. Available online: http://osf.io/qn7gs (accessed on 10 August 2025).
- Munn, Z.; Moola, S.; Riitano, D.; Lisy, K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int. J. Health Policy Manag. 2014, 3, 123–128. [Google Scholar] [CrossRef]
- Islam, M.M.; Khanom, H.; Farag, E.; Mim, Z.T.; Naidoo, P.; Mkhize-Kwitshana, Z.L.; Tibbo, M.; Islam, A.; Soares Magalhaes, R.J.; Hassan, M.M. Global patterns of Middle East respiratory syndrome coronavirus (MERS-CoV) prevalence and seroprevalence in camels: A systematic review and meta-analysis. One Health 2023, 16, 100561. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Elsohaby, I.; Fayez, M.; Alkafafy, M.; Refaat, M.; Al-Marri, T.; Alaql, F.A.; Al Amer, A.S.; Abdallah, A.; Elmoslemany, A. Serological and molecular characterization of Mycobacterium avium subsp. paratuberculosis (MAP) from sheep, goats, cattle and camels in the Eastern province, Saudi Arabia. Animals 2021, 11, 323. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Al-Shamali, M.; Khan, I.; Al-Nakib, B.; Al-Hassan, F.; Mustafa, A.S. A multiplex polymerase chain reaction assay for the detection of Mycobacterium paratuberculosis DNA in Crohn’s disease tissue. Scand. J. Gastroenterol. 1997, 32, 819–823. [Google Scholar] [CrossRef]
- Alluwaimi, A.; Hatem, M.; Almousa, J. The efficacy of gel immunodiffusion and fecal smear tests for diagnosis of ovine paratuberculosis in sheep in Saudi Arabia. Egyp. J. Immunol. 2000, 7, 29–32. [Google Scholar]
- Al Hajri, S.M.; Alluwaimi, A.M. ELISA and PCR for evaluation of subclinical paratuberculosis in the Saudi dairy herds. Vet. Microbiol. 2007, 121, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Al-Majali, A.M.; Jawasreh, K.; Al Nsour, A. Epidemiological studies on foot and mouth disease and paratuberculosis in small ruminants in Tafelah and Ma’ an, Jordan. Small Rumin. Res. 2008, 78, 197–201. [Google Scholar] [CrossRef]
- Alhebabi, A.; Alluwaimi, A. Paratuberculosis in Camel (Camelus dromedarius): The Diagnostic Efficiency of ELISA and PCR. Open Vet. Sci. J. 2010, 4, 41–44. [Google Scholar] [CrossRef]
- Alluwaimi, A.M. The efficiency of bovine ELISA in detection of the Mycobacterium avium subspecies paratuberculosis (MAP) infection in Camel (Camelus dromedaries) at different ages. J. Camel Pract. Res. 2008, 15, 163–165. [Google Scholar]
- Badi, F.A.; Haroon, A.I.A.; Alluwaimi, A.M. The γδ cells as marker of non-seroconverted cattle naturally infected with Mycobacterium avium subspecies paratuberculosis. Res. Vet. Sci. 2010, 88, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Hailat, N.Q.; Hananeh, W.; Metekia, A.S.; Stabel, J.R.; Al-Majali, A.; Lafi, S. Pathology of subclinical paratuberculosis (Johne’s Disease) in Awassi sheep with reference to its occurrence in Jordan. Vet. Med. 2010, 55, 590–602. [Google Scholar] [CrossRef]
- Al Hajri, S.M.; Alluwaimi, A.M. The efficiency of ELISA and PCR in detecting subclinical paratuberculosis in the Saudi dairy herds. Pak. J. Biol. Sci. 2007, 10, 1906–1909. [Google Scholar] [CrossRef]
- Alharbi, K.B.; Al-Swailem, A.; Al-Dubaib, M.A.; Al-Yamani, E.; Al-Naeem, A.; Shehata, M.; Hashad, M.E.; Albusadah, K.A.; Mahmoud, O.M. Pathology and molecular diagnosis of paratuberculosis of camels. Trop. Anim. Health Prod. 2012, 44, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Alluwaimi, A.; Badi, F. Mycobacterium avium subspecies paratuberculosis infection in naturally infected cattle is associated with an upregulation of lipid metabolism gene expression. Int. J. Adv. Life Sci. 2015, 8, 377–397. [Google Scholar]
- Almujalli, A.M.; Ghamdi, G.M. Clinicopathological Findings of Partuberclosis in Camels Possible Steps for Control Strategy. Res. J. Biol. Sci. 2012, 7, 128–131. [Google Scholar] [CrossRef]
- Asghar, A.; Abd El-Rahim, I.; Mohamed, A.; Ahmed, O. Clinical and molecular investigations of Johne’s disease among small ruminants in Makkah, Saudi Arabia. Int. J. Bioassays 2014, 3, 3445–3451. [Google Scholar]
- El-Deeb, W.M.; Fouda, T.A.; El-Bahr, S.M. Clinico-biochemical investigation of paratuberculosis of dromedary camels in Saudi Arabia: Proinflammatory cytokines, acute phase proteins and oxidative stress biomarkers. Pak. Vet. J. 2014, 34, 484–488. [Google Scholar]
- Ghosh, P.; Hsu, C.; Alyamani, E.J.; Shehata, M.M.; Al-Dubaib, M.A.; Al-Naeem, A.; Hashad, M.; Mahmoud, O.M.; Alharbi, K.B.J.; Al-Busadah, K.; et al. Genome-wide analysis of the emerging infection with Mycobacterium avium subspecies paratuberculosis in the arabian camels (Camelus dromedarius). PLoS ONE 2012, 7, e31947. [Google Scholar] [CrossRef]
- Hailat, N.; Fayyad, A.; Ababneh, M.; Hananeh, W.; Rezig, F.E.; Jaradat, S. PCR-restriction endonuclease analysis of Mycobacterium avium subsp. paratuberculosis isolates from goats, sheep, and cattle in Jordan. Comp. Clin. Pathol. 2012, 21, 755–760. [Google Scholar] [CrossRef]
- Hailat, N.; Hemida, H.; Hananeh, W.; Stabel, J.; Rezig, F.E.; Jaradat, S.; Al-Saleh, A.A. Investigation on the occurrence and pathology of paratuberculosis (Johne’s disease) in apparently healthy cattle in Jordan. Comp. Clin. Pathol. 2012, 21, 879–888. [Google Scholar] [CrossRef]
- Hereba, A.M.; Hamouda, M.A.; Al-Hizab, F.A. Pathological and molecular diagnosis of paratuberculosis among dromedary camels in Saudi Arabia. J. Anim. Plant Sci. 2015, 25, 997–1002. [Google Scholar]
- Housawi, F.M.T.; Zaghawa, A.A.; Al-Naeem, A. Seroprevalence of paratuberculosis among camels in Al-Ahsa and Riyadh Regions, Kingdom of Saudi Arabia. Pak. Vet. J. 2015, 35, 375–378. [Google Scholar]
- Hussain, M.H.; Saqib, M.; Al-Maawali, M.G.; Al-Makhladi, S.; Al-Zadjali, M.S.; Al-Sidairi, T.; Asubaihi, S.; Al-Rawahi, A.; Mansoor, M.K. Seroprevalence of Mycobacterium avium subspecies paratuberculosis (MAP) and evaluation of risk factors in camels of the Sultanate of Oman. Trop. Anim. Health Prod. 2015, 47, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Radad, K.; Khalil, S. Coccidiosis, paratuberculosis and enterotoxaemia in Saudi goats. Braz. J. Vet. Pathol. 2011, 4, 219–224. [Google Scholar]
- El-Sabagh, I.M.; Al-Ali, A.M.; Al-Naeem, A.A. Group A rotavirus and Mycobacterium avium subspecies paratuberculosis associated with diarrhea in dromedary camels in Eastern province, Saudi Arabia. Thai J. Vet. Med. 2017, 47, 383–388. [Google Scholar] [CrossRef]
- Ahmad, S.; Mokaddas, E. Diversity of nontuberculous mycobacteria in Kuwait: Rapid identification and differentiation of Mycobacterium species by multiplex PCR, INNO-LiPA Mycobacteria v2 Assay and PCR Sequencing of rDNA. Med. Princ. Pract. 2019, 28, 208–215. [Google Scholar] [CrossRef]
- Al-Ramadan, S.; Al-Mohammed Salem, K.; Alshubaith, I.; Alluwaimi, A. CD markers of camel (Camelus dromedarius) intestine naturally infected with Mycobacterium avium subsp. paratuberculosis: Distinct expression of Madcam-1 and CX3CR1. Turk. J. Vet. Anim. Sci. 2020, 44, 1010–1023. [Google Scholar] [CrossRef]
- Elmoslemany, A.; Alanazi, F.; Elsohaby, I.; Fayez, M.; Alnaeem, A. Associations between management factors and seroprevalence of Mycobacterium avium subsp. paratuberculosis in dromedary camels. Comp. Immunol. Microbiol. Infect. Dis. 2022, 83, 101780. [Google Scholar] [CrossRef]
- Elsohaby, I.; Kostoulas, P.; Fayez, M.; Elmoslemany, A.; Alkafafy, M.E.; Bahhary, A.M.; Alzahrani, R.; Morsi, A.; Arango-Sabogal, J.C. Bayesian estimation of diagnostic accuracy of fecal smears, fecal PCR and serum ELISA for detecting Mycobacterium avium subsp. paratuberculosis infections in four domestic ruminant species in Saudi Arabia. Vet. Microbiol. 2025, 301, 110377. [Google Scholar] [CrossRef]
- Hassan, A.A.; Rahawy, M.; Alkattan, L.M.; Khan, I.U.H.; Abdulmawjood, A.; Bülte, M. First report of paratuberculosis (Johne’s disease) in livestock farms of river buffaloes (Bubalus Bubalis) in Nineveh, Iraq. Vet. Ital. 2022, 58, 231–236. [Google Scholar] [CrossRef]
- Hussein, M.F.; Al-Khalifa, I.M.; Aljumaah, R.S.; Elnabi, A.G.; Mohammed, O.B.; Omer, S.A.; Macasero, W.V. Serological prevalence of Coxiella burnetii in captive wild ruminants in Saudi Arabia. Comp. Clin. Pathol. 2012, 21, 33–38. [Google Scholar] [CrossRef]
- Schuler, Y.; Hammer, C.; Clauss, M.; Hammer, S. Birth Seasonality in Captive Bovids at Al Wabra Wildlife Preservation (AWWP), Qatar; Leibniz Institute for Zoo and Wildlife Research: Berlin, Germany, 2009. [Google Scholar] [CrossRef]
- Waddell, L.A.; Rajić, A.; Stärk, K.D.; Mc, E.S. The zoonotic potential of Mycobacterium avium ssp. paratuberculosis: A systematic review and meta-analyses of the evidence. Epidemiol. Infect. 2015, 143, 3135–3157. [Google Scholar] [CrossRef] [PubMed]
- Amirizadehfard, S.; Mahzounieh, M.; Safarpour, A.; Nejabat, M.; Nazari, N. Genomic Detection of Mycobacterium avium subspecies paratuberculosis in Blood Samples of Patients with Inflammatory Bowel Disease in Southern Iran. Iran. J. Med. Sci. 2020, 45, 214–219. [Google Scholar] [CrossRef]
- Okuni, J.B.; Hansen, S.; Eltom, K.H.; Eltayeb, E.; Amanzada, A.; Omega, J.A.; Czerny, C.P.; Abd El Wahed, A.; Ojok, L. Paratuberculosis: A Potential Zoonosis and a Neglected Disease in Africa. Microorganisms 2020, 8, 1007. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M. Mycobacterium Avium Paratuberculosis: A Disease Burden on the Dairy Industry. Animals 2020, 10, 1773. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health. Disease Data Collection Before 2005. Available online: https://www.woah.org/en/what-we-do/animal-health-and-welfare/disease-data-collection/data-before-2005/ (accessed on 14 May 2025).
- Fèvre, E.M.; Bronsvoort, B.M.; Hamilton, K.A.; Cleaveland, S. Animal movements and the spread of infectious diseases. Trends Microbiol. 2006, 14, 125–131. [Google Scholar] [CrossRef]
- Boshra, H.; Truong, T.; Babiuk, S.; Hemida, M.G. Seroprevalence of Sheep and Goat Pox, Peste Des Petits Ruminants and Rift Valley Fever in Saudi Arabia. PLoS ONE 2015, 10, e0140328. [Google Scholar] [CrossRef]
- Galila, E.; Arnaout, F. Seroprevalence and associated risk factors for bovine paratuberculosis in dairy cattle. J. Hell. Vet. Med. Soc. 2021, 72, 2647–2652. [Google Scholar] [CrossRef]
- Haghkhah, M.; Derakhshandeh, A.; Jamshidi, R.; Moghiseh, A.; Karimaghaei, N.; Ayaseh, M.; Mostafaei, M. Detection of Mycobacterium avium subspecies paratuberculosis infection in two different camel species by conventional and molecular techniques. Vet. Res. Forum 2015, 6, 337–341. [Google Scholar]
- Islam, M.M.; Dutta, P.; Bansal, D.; Gongal, G.; Farag, E.; Magalhaes, R.J.S.; Alawneh, J.I.; Heller, J.; Hassan, M.M. Prevalence and Risk Factors of Coxiellosis at the Human–Animal–Environment Interface in the South Asian Countries: A Systematic Review and Meta-Analysis. Transbound. Emerg. Dis. 2025, 2025, 2890693. [Google Scholar] [CrossRef]
- Khiav, L.A.; Abyaneh, H.K.; Mehrabadi, M.H.F.; Mosavari, N.; Tadayon, K. Meta-analysis of Johne’s disease in the Iranian animal population (1999–2020). Arch. Razi Inst. 2024, 79, 168–179. [Google Scholar]
- Sonawane, G.G.; Narnaware, S.D.; Tripathi, B.N. Molecular epidemiology of Mycobacterium avium subspecies paratuberculosis in ruminants in different parts of India. Int. J. Mycobacteriology 2016, 5, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, I.; Singh, S.V.; Garrido, J.M.; Aduriz, G.; Rodríguez, S.; Geijo, M.V.; Whittington, R.J.; Saunders, V.; Whitlock, R.H.; Juste, R.A. Molecular typing of Mycobacterium avium subspecies paratuberculosis strains from different hosts and regions. Rev. Sci. Tech. 2005, 24, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Windsor, P.; Whittington, R. Ovine Paratuberculosis Control in Australia Revisited. Animals 2020, 10, 1623. [Google Scholar] [CrossRef] [PubMed]
- Links, I.J.; Denholm, L.J.; Evers, M.; Kingham, L.J.; Greenstein, R.J. Is vaccination a viable method to control Johne’s disease caused by Mycobacterium avium subsp. paratuberculosis? Data from 12 million ovine vaccinations and 7.6 million carcass examinations in New South Wales, Australia from 1999–2009. PLoS ONE 2021, 16, e0246411. [Google Scholar] [CrossRef]
- Farag, E.; Nour, M.; Islam, M.M.; Mustafa, A.; Khalid, M.; Sikkema, R.S.; Alhajri, F.; Bu-Sayaa, A.; Haroun, M.; Van Kerkhove, M.D.; et al. Qatar experience on One Health approach for middle-east respiratory syndrome coronavirus, 2012–2017: A viewpoint. One Health 2019, 7, 100090. [Google Scholar] [CrossRef]
- Bansal, D.; Jaffrey, S.; Al-Emadi, N.A.; Hassan, M.; Islam, M.M.; Al-Baker, W.A.A.; Radwan, E.; Hamdani, D.; Haroun, M.I.; Enan, K.; et al. A new One Health Framework in Qatar for future emerging and re-emerging zoonotic diseases preparedness and response. One Health 2023, 16, 100487. [Google Scholar] [CrossRef]


| Characteristics | Number of Articles (%, 95% CI) | References |
|---|---|---|
| Publication year | ||
| 1996–2000 | 2, 6.45 (1.12–22.84) | [29,30] |
| 2006–2010 | 8, 25.54 (12.54–44.93) | [12,31,32,33,34,35,36,37] |
| 2011–2015 | 12, 38.71 (22.42–57.71) | [38,39,40,41,42,43,44,45,46,47,48,49] |
| 2016–2020 | 4, 12.90 (4.21–30.76) | [17,50,51,52] |
| 2021–2025 | 5, 16.12 (6.09–34.47) | [18,26,53,54,55] |
| Animal species | ||
| Buffalo | 1, 3.23 (0.16 – 18.51) | [55] |
| Camel | 16, 41.94 (25.07 – 60.74) | [17,18,26,33,34,38,40,42,43,46,47,48,50,52,53,54] |
| Cattle | 9, 29.03 (14.89 – 48.24) | [26,31,35,37,39,44,45,51,54] |
| Goat | 7, 22.58 (10.28–41.54) | [12,26,32,41,44,49,54] |
| Sheep | 16, 41.94 (25.07 – 60.74) | [17,18,26,33,34,38,40,42,43,46,47,48,50,52,53,54] |
| Human | 2, 6.45 (1.12–22.84) | [29,51] |
| Study detail | ||
| Clinical history | 20, 64.51 (45.38 – 70.17) | [12,17,18,26,29,30,31,34,37,38,40,41,42,43,46,47,48,50,51,53] |
| Pathological study | 14, 48.28 (29.89–67.10) | [12,18,26,33,34,36,38,40,42,43,45,46,49,52] |
| Serology | 15, 48.39 (30.56 – 66.60) | [17,26,30,31,32,33,34,35,39,45,47,48,51,53,54] |
| Pathogen detection | 20, 64.51 (45.38 – 70.17) | [12,17,18,26,29,30,31,33,36,37,38,39,41,43,44,45,46,50,51,55] |
| Study type | ||
| Cross-sectional | 29, 93.35 (77.15–98.87) | [12,17,26,29,30,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] |
| Case report | 1, 3.45 (0.1–19.63) | [18] |
| Editorial | 1, 3.45 (0.1–19.63) | [31] |
| Sl No. | Risk Factor | Variable | Number of Studies | Estimated Pooled Prevalence | 95% Confidence Interval | Heterogeneity I2 (%) | p-Value |
|---|---|---|---|---|---|---|---|
| 1 | Country | Saudi Arabia | 9 | 8.3 | 4.4–15.1 | 98 | 0.9 |
| Other countries * | 3 | 7.4 | 1.0–37.5 | ||||
| 2 | Type | Large ruminant | 4 | 7.4 | 4.4–12.2 | 97 | 0.05 |
| Small ruminant | 10 | 19.3 | 8.5–38.3 | ||||
| 3 | Species | Camel | 7 | 9.8 | 6.0–15.7 | 97 | <0.01 |
| Cattle | 5 | 6.6 | 2.4–16.7 | ||||
| Goat | 3 | 28.7 | 11.9–54.7 | ||||
| Sheep | 4 | 21.5 | 17.6–26.0 | ||||
| 4 | Sex | Female | 4 | 7.4 | 2.0–24.1 | 89 | 0.90 |
| Male | 2 | 8.2 | 3.1–17.9 | ||||
| 5 | Age | Adult | 5 | 7.0 | 3.0–15.6 | 91 | 0.32 |
| Female | 4 | 3.3 | 0.9–11.1 |
| Sl No. | Risk Factor | Variable | Number of Studies | Estimated Pooled Prevalence | 95% Confidence Interval | Heterogeneity I2 (%) | p-Value |
|---|---|---|---|---|---|---|---|
| 1 | Country | Saudi Arabia | 12 | 23.1 | 10.2–44.3 | 97 | 0.68 |
| Jordan | 3 | 24.0 | 5.2–64.3 | ||||
| 2 | Ruminant type | Large ruminant | 14 | 25.1 | 13.0–42.8 | 96 | 0.19 |
| Small ruminant | 10 | 13.9 | 7.1–25.3 | ||||
| 3 | Species | Camel | 7 | 35.6 | 17.5–59.0 | 95 | 0.11 |
| Cattle | 4 | 10.4 | 3.7–25.6 | ||||
| Goat | 4 | 11.8 | 3.8–31.3 | ||||
| Sheep | 5 | 11.2 | 3.1–33.2 | ||||
| 4 | Sex | Female | 4 | 32.4 | 16.2–54.3 | 71 | 0.72 |
| Male | 2 | 48.3 | 2.9–96.7 | ||||
| 5 | Age | Adult | 6 | 23.5 | 6.8–56.6 | 97 | 0.33 |
| Young | 6 | 45.2 | 17.4–57.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.M.; Elfadl, A.K.; Naeem, A.; Abdeen, R.; Al-Hajri, H.M.; Sayeed, M.A.; Dejene, H.; Alawneh, J.I.; Hassan, M.M. Epidemiology and Diversity of Paratuberculosis in the Arabian Peninsula: A Systematic Review and Meta-Analysis with Implications for One Health. Pathogens 2025, 14, 841. https://doi.org/10.3390/pathogens14090841
Islam MM, Elfadl AK, Naeem A, Abdeen R, Al-Hajri HM, Sayeed MA, Dejene H, Alawneh JI, Hassan MM. Epidemiology and Diversity of Paratuberculosis in the Arabian Peninsula: A Systematic Review and Meta-Analysis with Implications for One Health. Pathogens. 2025; 14(9):841. https://doi.org/10.3390/pathogens14090841
Chicago/Turabian StyleIslam, Md Mazharul, Ahmed K. Elfadl, Aisha Naeem, Randa Abdeen, Haya M. Al-Hajri, Md Abu Sayeed, Haileyesus Dejene, John I. Alawneh, and Mohammad Mahmudul Hassan. 2025. "Epidemiology and Diversity of Paratuberculosis in the Arabian Peninsula: A Systematic Review and Meta-Analysis with Implications for One Health" Pathogens 14, no. 9: 841. https://doi.org/10.3390/pathogens14090841
APA StyleIslam, M. M., Elfadl, A. K., Naeem, A., Abdeen, R., Al-Hajri, H. M., Sayeed, M. A., Dejene, H., Alawneh, J. I., & Hassan, M. M. (2025). Epidemiology and Diversity of Paratuberculosis in the Arabian Peninsula: A Systematic Review and Meta-Analysis with Implications for One Health. Pathogens, 14(9), 841. https://doi.org/10.3390/pathogens14090841








