Chemical Treatments Tested Against Xylella fastidiosa: Strategies, Successes and Limitations
Abstract
1. Introduction
2. Organic Molecules
2.1. N-Acetylcysteine (NAC)
2.2. Phenolic Compounds
2.3. Oxylipins
2.4. NuovOlivo®
3. Synthetic Molecules
3.1. Menadione and Benzethonium Chloride
3.2. Nanoparticles
3.2.1. Silver (Ag) NPs
3.2.2. Thymol Nanoparticles
3.2.3. Fosetyl–Al Nanocrystals
3.2.4. Zinkicide®
3.2.5. Calcium Carbonate Nanocarriers
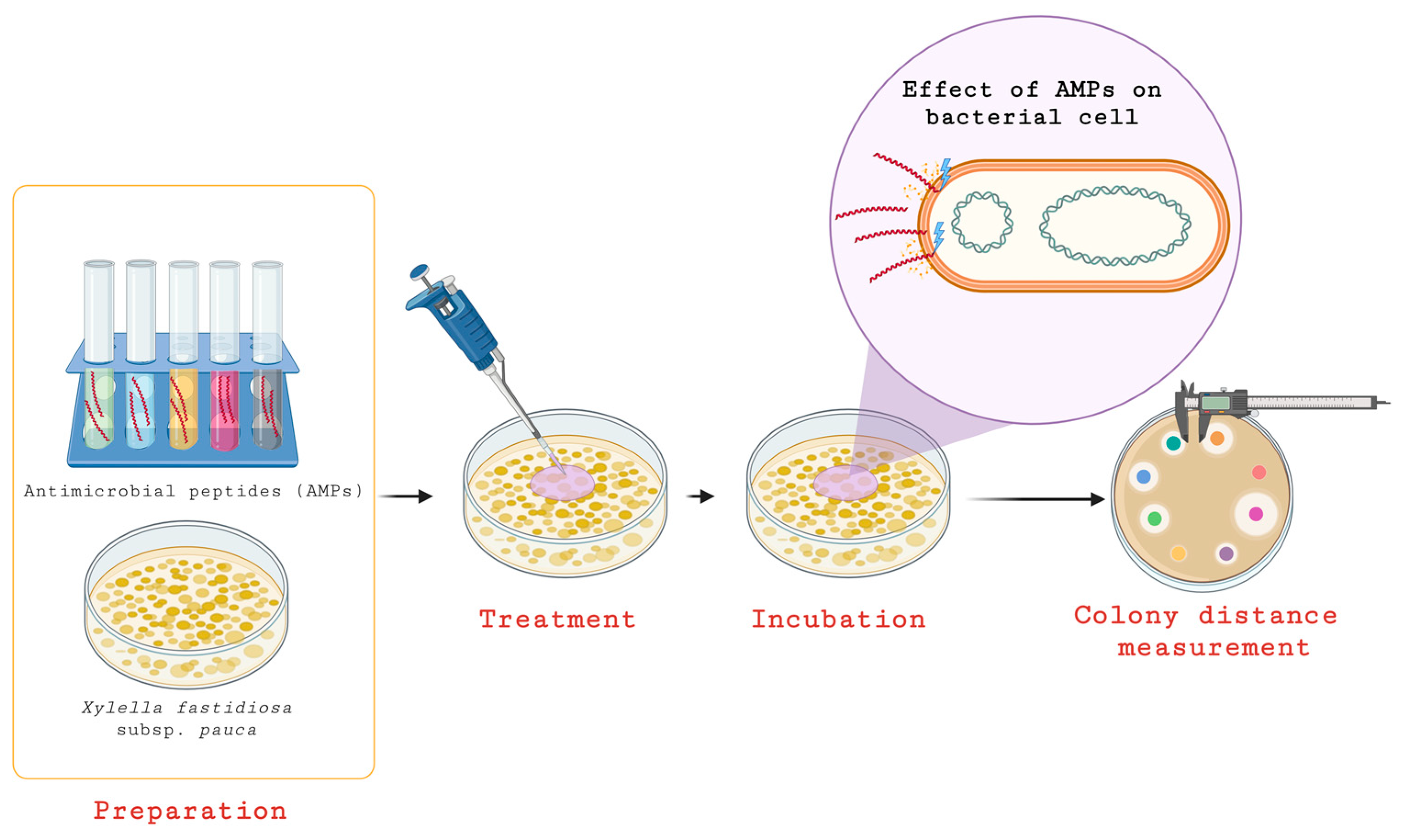
3.3. Antimicrobial Peptides (AMPs)
3.4. Siliforce®, Kalex Zn® and Kalex Cu®
3.5. Antibiotics
4. Salt and Metal Compounds
4.1. Ammonium Chloride
4.2. Dentamet®
5. Alternative Approaches for Managing Xf
6. Chemical Control of Xf Vector Insects
7. The Fight Against Xf: Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Xf | Xylella fastidiosa |
| NAC | N-acetylcysteine |
| ROS | Reactive oxygen species |
| CVC | Citrus Variegated Chlorosis |
| EPS | Exopolysaccharide |
| MIC | Minimum inhibitory concentration |
| NP | Nanoparticles |
| NanoFos | Fosetyl–Al nanocrystals |
| CH-nanofos | Chitosan–Fosetyl–Al nanocrystals |
| ZnO | Zinc oxide |
| ZnK | Zinkicide® |
| PBS | Phosphate-Buffered Saline |
| AMPs | Antimicrobial peptides |
| AUDPC | Area Under the Disease Progress Curve |
| OD | Optical density |
References
- Uceda-Campos, G.; Feitosa-Junior, O.R.; Santiago, C.R.N.; Pierry, P.M.; Zaini, P.A.; de Santana, W.O.; Martins-Junior, J.; Barbosa, D.; Digiampietri, L.A.; Setubal, J.C.; et al. Comparative Genomics of Xylella fastidiosa Explores Candidate Host-Specificity Determinants and Expands the Known Repertoire of Mobile Genetic Elements and Immunity Systems. Microorganisms 2022, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A.H.; Hopkins, D.L. Fastidious Xylem-Limited Bacterial Plant Pathogens. Phytopathol 1996, 34, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Almeida, R.P.P.; Lindow, S. Living in Two Worlds: The Plant and Insect Lifestyles of Xylella fastidiosa. Annu. Rev. Phytopathol. 2008, 46, 243–271. [Google Scholar] [CrossRef] [PubMed]
- Janse, J.D.; Obradovic, A. Xylella fastidiosa: Its Biology, Diagnosis, Control and Risk. Plant Pathol. J. 2010, 92, S35–S48. [Google Scholar]
- Weng, L.W.; Lin, Y.C.; Su, C.C.; Huang, C.T.; Cho, S.T.; Chen, A.P.; Chou, S.J.; Tsai, C.W.; Kuo, C.H. Complete Genome Sequence of Xylella taiwanensis and Comparative Analysis of Virulence Gene Content With Xylella fastidiosa. Front. Microbiol. 2021, 12, 684092. [Google Scholar] [CrossRef]
- Niza, B.; Coletta-Filho, H.D.; Merfa, M.V.; Takita, M.A.; de Souza, A.A. Differential Colonization Patterns of Xylella fastidiosa Infecting Citrus Genotypes. Plant Pathol. 2015, 64, 1259–1269. [Google Scholar] [CrossRef]
- Vanhove, M.; Retchless, A.C.; Sicard, A.; Rieux, A.; Coletta-filho, H.D.; La Fuente, L.D.; Stenger, D.C.; Almeida, R.P.P. Genomic Diversity and Recombination among Xylella fastidiosa Subspecies. Appl Environ Microbiol. 2019, 85, 02972-18. [Google Scholar] [CrossRef]
- Pérez-Giraldo, C.; Rodriguez-Benito, A.; Morán, F.J.; Hurtado, C.; Blanco, M.T.; Gómez-Garcí, A.C. Influence of N-Acetylcysteine on the Formation of Biofilm by Staphylococcus epidermidis. J. Antimicrob. Chemother. 1997, 39, 643–646. [Google Scholar] [CrossRef]
- Hafez, M.M.; Aboulwafa, M.M.; Yassien, M.A.; Hassouna, N.A. Activity of Some Mucolytics against Bacterial Adherence to Mammalian Cells. Appl. Biochem. Biotechnol. 2009, 158, 97–112. [Google Scholar] [CrossRef]
- Giampreti, A.; Lonati, D.; Ragghianti, B.; Ronchi, A.; Petrolini, V.M.; Vecchio, S.; Locatelli, C.A. N-Acetyl-Cysteine as Effective and Safe Chelating Agent in Metal-on-Metal Hip-Implanted Patients: Two Cases. Case Rep. Orthop. 2016, 2016, 8682737. [Google Scholar] [CrossRef]
- D’Ambrosi, R.; Ursino, N. N-Acetyl-Cysteine Reduces Blood Chromium and Cobalt Levels in Metal-on-Metal Hip Arthroplasty. Arthroplast. Today 2020, 6, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an Antioxidant and Disulphide Breaking Agent: The Reasons Why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The Chemistry and Biological Activities of N-Acetylcysteine. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Picchi, S.C.; De Souza e Silva, M.; Saldanha, L.L.; Ferreira, H.; Takita, M.A.; Caldana, C.; de Souza, A.A. GC-TOF/MS-Based Metabolomics Analysis to Investigate the Changes Driven by N-Acetylcysteine in the Plant-Pathogen Xanthomonas citri subsp. citri. Sci. Rep. 2021, 11, 15558. [Google Scholar] [CrossRef]
- Dinicola, S.; De Grazia, S.; Carlomagno, G.; Pintucci, J.P. N-Acetylcysteine as Powerful Molecule to Destroy Bacterial Biofilms. A Systematic Review. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2942–2948. [Google Scholar] [PubMed]
- Muranaka, L.S.; Giorgiano, T.E.; Takita, M.A.; Forim, M.R.; Silva, L.F.C.; Coletta-Filho, H.D.; Machado, M.A.; de Souza, A.A. N-Acetylcysteine in Agriculture, a Novel Use for an Old Molecule: Focus on Controlling the Plant-Pathogen Xylella fastidiosa. PLoS ONE 2013, 8, 72937. [Google Scholar] [CrossRef]
- Da Silva, A.M.; Murillo, D.M.; Anbumani, S.; von Zuben, A.A.; Cavalli, A.; Obata, H.T.; Fischer, E.R.; de Souza e Silva, M.; Bakkers, E.; Souza, A.A.; et al. N-Acetylcysteine Effects on Extracellular Polymeric Substances of Xylella fastidiosa: A Spatiotemporal Investigation with Implications for Biofilm Disruption. Int. J. Antimicrob. Agents 2024, 64, 107340. [Google Scholar] [CrossRef]
- Helenius, J.; Heisenberg, C.P.; Gaub, H.E.; Muller, D.J. Single-Cell Force Spectroscopy. J. Cell Sci. 2008, 121, 1785–1791. [Google Scholar] [CrossRef]
- Cattò, C.; De Vincenti, L.; Cappitelli, F.; D’attoma, G.; Saponari, M.; Villa, F.; Forlani, F. Non-Lethal Effects of N-Acetylcysteine on Xylella fastidiosa Strain de Donno Biofilm Formation and Detachment. Microorganisms 2019, 7, 656. [Google Scholar] [CrossRef]
- Wise, J.C.; VanWoerkom, A.H.; Aćimović, S.G.; Sundin, G.W.; Cregg, B.M.; Vandervoort, C. Trunk Injection: A Discriminating Delivering System for Horticulture Crop IPM. Entomol. Ornithol. Herpetol. Curr. Res. 2014, 3, 3–9. [Google Scholar] [CrossRef]
- Archer, L.; Crane, J.H.; Albrecht, U. Correction: Archer et al. Trunk Injection as a Tool to Deliver Plant Protection Materials—An Overview of Basic Principles and Practical Considerations. Horticulturae 2022, 8, 552. Horticulturae 2024, 10, 351. [Google Scholar] [CrossRef]
- Picchi, S.C.; Rebelatto, D.; Martins, P.M.M.; Blumer, S.; Mesquita, G.L.; Hippler, F.W.R.; Mattos, D.; Boaretto, R.M.; Machado, M.A.; Takita, M.A.; et al. N-Acetylcysteine Absorption and Its Potential Dual Effect Improve Fitness and Fruit Yield in Xylella fastidiosa Infected Plants. Pest Manag. Sci. 2024, 80, 4333–4343. [Google Scholar] [CrossRef] [PubMed]
- Boscia, D.; Saponari, M. Recent advances on the control of Xylella fastidiosa and its vectors in olive groves: State of the art from the ongoing Europe’s Horizon 2020 Research Program. J. Plant Pathol. 2019, 101, 849–883. [Google Scholar] [CrossRef]
- Boudet, A.M. Evolution and Current Status of Research in Phenolic Compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and Analysis of Biophenols Recovered from Olive Mill Waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef]
- Azaizeh, H.; Tafesh, A.; Najami, N.; Jadoun, J.; Halahlih, F.; Riepl, H. Synergistic Antibacterial Effects of Polyphenolic Compounds from Olive Mill Wastewater. Evid.-Based Complement. Altern. Med. 2011, 2011, 431021. [Google Scholar] [CrossRef]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial Activity of Phenolic Compounds against the Phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial Spectrum of Plant Polyphenols and Extracts Depending upon Hydroxyphenyl Structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef]
- Evans, S.M.; Cowan, M.M. Plant Products as Antimicrobial Agents. Cosmet. Drug Microbiol. 2016, 12, 205–231. [Google Scholar] [CrossRef]
- Lee, S.A.; Wallis, C.M.; Rogers, E.E.; Burbank, L.P. Grapevine Phenolic Compounds Influence Cell Surface Adhesion of Xylella fastidiosa and Bind to Lipopolysaccharide. PLoS ONE 2020, 15, 0240101. [Google Scholar] [CrossRef]
- Vizzarri, V.; Ienco, A.; Benincasa, C.; Perri, E.; Pucci, N.; Cesari, E.; Novellis, C.; Rizzo, P.; Pellegrino, M.; Zaffina, F.; et al. Phenolic Extract from Olive Leaves as a Promising Endotherapeutic Treatment against Xylella fastidiosa in Naturally Infected Olea Europaea (Var. Europaea) Trees. Biology 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Balzano, A.; Wheeler, E.A.; Baas, P. Tyloses and Gums: A Review of Structure, Function and Occurrence of Vessel Occlusions. IAWA J. 2016, 37, 186–205. [Google Scholar] [CrossRef]
- Mumtaz, F.; Zubair, M.; Khan, F.; Niaz, K. Chapter 22. Analysis of Plants Lipids. In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 677–705. [Google Scholar] [CrossRef]
- Scala, V.; Pucci, N.; Salustri, M.; Modesti, V.; L’Aurora, A.; Scortichini, M.; Zaccaria, M.; Momeni, B.; Reverberi, M.; Loreti, S. Xylella fastidiosa subsp. pauca and Olive Produced Lipids Moderate the Switch Adhesive versus Non-Adhesive State and Viceversa. PLoS ONE 2020, 15, 0240101. [Google Scholar] [CrossRef]
- Scala, V.; Reverberi, M.; Salustri, M.; Pucci, N.; Modesti, V.; Lucchesi, S.; Loreti, S. Lipid Profile of Xylella fastidiosa subsp. pauca Associated with the Olive Quick Decline Syndrome. Front. Microbiol. 2018, 9, 1839. [Google Scholar] [CrossRef]
- Scala, V.; Pucci, N.; Salustri, M.; Modesti, V.; L’Aurora, V.; Scortichini, M.; Zaccaria, M.; Momeni, B.; Reverberi, M.; Loreti, S. Bacterial and Plant Produced Lipids Can Exacerbate the Olive Quick Decline Syndrome Caused by Xylella. Sustainability 2019, 11, 867523. [Google Scholar] [CrossRef]
- Scala, V.; Salustri, M.; Merfa, M.V.; Beccaccioli, M.; Lascala, L.; De La Fuente, L.; Reverberi, M. XadA-like Adhesin XADA2 Regulates Biofilm Formation in X. fastidiosa subsp. fastidiosa Putatively by Engaging Oleic-Acid Derived Oxylipins. Mol. Biol. Rep. 2025, 52, 263. [Google Scholar] [CrossRef]
- Bruno, G.L.; Cariddi, C.; Botrugno, L. Exploring a Sustainable Solution to Control Xylella fastidiosa subsp. pauca on Olive in the Salento Peninsula, Southern Italy. Crop Prot. 2021, 139, 105288. [Google Scholar] [CrossRef]
- Bruno, G.L. Coexistence between Xylella fastidiosa subsp. pauca and Susceptible Olive Plants in the Salento Peninsula (Southern Italy). Agronomy 2024, 14, 2119. [Google Scholar] [CrossRef]
- Corey, E.J. Stereospecific Total Synthesis of the dl-C18 Cecropia Juvenile Hormone. J. Am. Chem. Soc. 1968, 90, 5618–5620. [Google Scholar] [CrossRef]
- Robin, D.C.; Marchand, P.A. Evolution of the Biocontrol Active Substances in the Framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest Manag. Sci. 2019, 75, 950–958. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on the Safety and Efficacy of Vitamin K3 (Menadione Sodium Bisulphite and Menadione Nicotinamide Bisulphite) as a Feed Additive for All Animal Species. EFSA J. 2014, 12, 3532. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Y.; Jin, Z.; Bai, R.; Wang, J.; Wu, L.; He, Y. Benzalkonium Chloride and Benzethonium Chloride Effectively Reduce Spore Germination of Ginger Soft Rot Pathogens: Fusarium solani and Fusarium oxysporum. J. Fungi 2024, 10, 8. [Google Scholar] [CrossRef]
- Zhang, S.; Jain, M.; Fleites, L.A.; Rayside, P.A.; Gabriel, D.W. Identification and Characterization of Menadione and Benzethonium Chloride as Potential Treatments of Pierce’s Disease of Grapevines. Phytopathology 2019, 109, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Singh Sekhon, B. Nanotechnology in Agri-Food Production: An Overview. Nanotechnol. Sci. Appl. 2014, 7, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Kookana, R.S.; Boxall, A.B.A.; Reeves, P.T.; Ashauer, R.; Beulke, S.; Chaudhry, Q.; Cornelis, G.; Fernandes, T.F.; Gan, J.; Kah, M.; et al. Nanopesticides: Guiding Principles for Regulatory Evaluation of Environmental Risks. J. Agric. Food Chem. 2014, 62, 4227–4240. [Google Scholar] [CrossRef]
- Sundin, G.W.; Castiblanco, L.F.; Yuan, X.; Zeng, Q.; Yang, C.H. Bacterial Disease Management: Challenges, Experience, Innovation and Future Prospects: Challenges in Bacterial Molecular Plant Pathology. Mol. Plant Pathol. 2016, 17, 1506–1518. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, P.; Li, M.; Shakoor, N.; Adeel, M.; Zhou, P.; Guo, M.; Jiang, Y.; Zhao, W.; Lou, B.Z.; et al. Application and Mechanisms of Metal-Based Nanoparticles in the Control of Bacterial and Fungal Crop Diseases. Pest Manag. Sci. 2023, 79, 21–36. [Google Scholar] [CrossRef]
- Angelini, G.; Scotti, L.; Aceto, A.; Gasbarri, C. Silver Nanoparticles as Interactive Media for the Azobenzenes Isomerization in Aqueous Solution: From Linear to Stretched Kinetics. J. Mol. Liq. 2019, 284, 592–598. [Google Scholar] [CrossRef]
- Chavan, P.S.; Tupe, S.G. Antifungal Activity and Mechanism of Action of Carvacrol and Thymol against Vineyard and Wine Spoilage Yeasts. Food Control 2014, 46, 115–120. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Zikeli, F.; Vinciguerra, V.; Sennato, S.; Scarascia Mugnozza, G.; Romagnoli, M. Preparation of Lignin Nanoparticles with Entrapped Essential Oil as a Bio-Based Biocide Delivery System. ACS Omega 2020, 5, 358–368. [Google Scholar] [CrossRef]
- Mattos, B.D.; Tardy, B.L.; Pezhman, M.; Kämäräinen, T.; Linder, M.; Schreiner, W.H.; Magalhães, W.L.E.; Rojas, O.J. Controlled Biocide Release from Hierarchically-Structured Biogenic Silica: Surface Chemistry to Tune Release Rate and Responsiveness. Sci. Rep. 2018, 8, 5555. [Google Scholar] [CrossRef]
- Medina, E.; Caro, N.; Abugoch, L.; Gamboa, A.; Díaz-Dosque, M.; Tapia, C. Chitosan Thymol Nanoparticles Improve the Antimicrobial Effect and the Water Vapour Barrier of Chitosan-Quinoa Protein Films. J. Food Eng. 2019, 240, 191–198. [Google Scholar] [CrossRef]
- Suresh Kumar, R.S.; Shiny, P.J.; Anjali, C.H.; Jerobin, J.; Goshen, K.M.; Magdassi, S.; Mukherjee, A.; Chandrasekaran, N. Distinctive Effects of Nano-Sized Permethrin in the Environment. Environ. Sci. Pollut. Res. 2013, 20, 2593–2602. [Google Scholar] [CrossRef]
- Yearla, S.R.; Padmasree, K. Exploitation of Subabul Stem Lignin as a Matrix in Controlled Release Agrochemical Nanoformulations: A Case Study with Herbicide Diuron. Environ. Sci. Pollut. Res. 2016, 23, 18085–18098. [Google Scholar] [CrossRef]
- Koshani, R.; Jafari, S.M. Ultrasound-Assisted Preparation of Different Nanocarriers Loaded with Food Bioactive Ingredients. Adv. Colloid Interface Sci. 2019, 270, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Abdolmaleki, A.; Tabesh, F. Ultrasonic-Assisted Manufacturing of New Hydrogel Nanocomposite Biosorbent Containing Calcium Carbonate Nanoparticles and Tragacanth Gum for Removal of Heavy Metal. Ultrason. Sonochem. 2018, 41, 572–581. [Google Scholar] [CrossRef]
- Naranjo, E.; Merfa, V.M.; Santra, S.; Ozcan, A.; Johnson, E.; Cobine, A.P.; De La Fuente, L. Crossm Activity against Liberibacter crescens in Batch Cultures and In Batch Cultures and in Microfluidic Chambers Simulating Plant Vascular Systems. Appl. Environ. Microbiol. 2020, 86, 00788-20. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Johnson, E.G.; Myers, M.E.; Young, M.; Rajasekaran, P.; Das, S.; Santra, S. Potential of Nano-Formulated Zinc Oxide for Control of Citrus Canker on Grapefruit Trees. Plant Dis. 2016, 100, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Peters, R.J.B.; Bouwmeester, H.; Gottardo, S.; Amenta, V.; Arena, M.; Brandhoff, P.; Marvin, H.J.P.; Mech, A.; Moniz, F.B.; Pesudo, L.Q.; et al. Nanomaterials for Products and Application in Agriculture, Feed and Food. Trends Food Sci. Technol. 2016, 54, 155–164. [Google Scholar] [CrossRef]
- Chen, H.; Yada, R. Nanotechnologies in Agriculture: New Tools for Sustainable Development. Trends Food Sci. Technol. 2011, 22, 585–594. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Torres-Chávez, P.I.; Ramírez-Wong, B.; Rascón-Chu, A.; Plascencia-Jatomea, M.; Barreras-Urbina, C.G.; Rangel-Vázquez, N.A.; Rodríguez-Félix, F. Micro- and Nanoparticles by Electrospray: Advances and Applications in Foods. J. Agric. Food Chem. 2015, 63, 4699–4707. [Google Scholar] [CrossRef] [PubMed]
- Orfei, B.; Moretti, C.; Loreti, S.; Tatulli, G.; Onofri, A.; Scotti, L.; Aceto, A.; Buonaurio, R. Silver Nanoclusters with Ag2+/3+ Oxidative States Are a New Highly Effective Tool against Phytopathogenic Bacteria. Appl. Microbiol. Biotechnol. 2023, 107, 4519–4531. [Google Scholar] [CrossRef]
- Baldassarre, F.; Schiavi, D.; Ciarroni, S.; Tagliavento, V.; De Stradis, A.; Vergaro, V.; Suranna, G.P.; Balestra, G.M.; Ciccarella, G. Thymol-Nanoparticles as Effective Biocides against the Quarantine Pathogen Xylella fastidiosa. Nanomaterials 2023, 13, 1285. [Google Scholar] [CrossRef]
- Cagnarini, C.; De Angelis, P.; Liberati, D.; Valentini, R.; Falanga, V.; Valentini, F.; Dongiovanni, C.; Carrieri, M.; Chiriacò, M.V. Physiological Response of Olive Trees Under Xylella fastidiosa Infection and Thymol Therapy Monitored Through Advanced IoT Sensors. Plants 2025, 14, 1380. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, F.; Tatulli, G.; Vergaro, V.; Mariano, S.; Scala, V.; Nobile, C.; Pucci, N.; Dini, L.; Loreti, S.; Ciccarella, G. Sonication-Assisted Production of Fosetyl-Al Nanocrystals: Investigation of Human Toxicity and in vitro Antibacterial Efficacy against Xylella fastidiosa. Nanomaterials 2020, 10, 1174. [Google Scholar] [CrossRef] [PubMed]
- Tatulli, G.; Baldassarre, F.; Schiavi, D.; Tacconi, S.; Cognigni, F.; Costantini, F.; Balestra, G.M.; Dini, L.; Pucci, N.; Rossi, M.; et al. Chitosan-Coated Fosetyl-Al Nanocrystals’ Efficacy on Nicotiana Tabacum Colonized by Xylella Fastidiosa. Phytopathology 2024, 114, 1466–1479. [Google Scholar] [CrossRef]
- Hernández-Montelongo, J.; Nascimento, V.F.; Murillo, D.; Taketa, T.B.; Sahoo, P.; De Souza, A.A.; Beppu, M.M.; Cotta, M.A. Nanofilms of Hyaluronan/Chitosan Assembled Layer-by-Layer: An Antibacterial Surface for Xylella Fastidiosa. Carbohydr. Polym. 2016, 136, 0144–8617. [Google Scholar] [CrossRef]
- Shantharaj, D.; Naranjo, E.; Merfa, M.V.; Cobine, P.A.; Santra, S.; De La Fuente, L. Zinc Oxide-Based Nanoformulation Zinkicide Mitigates the Xylem-Limited Pathogen Xylella fastidiosa in Tobacco and Southern Highbush Blueberry. Plant Dis. 2023, 107, 1096–1106. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of Micro and Nano-Sized Calcium Carbonate Particles and Their Applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Baldassarre, F.; De Stradis, A.; Altamura, G.; Vergaro, V.; Citti, C.; Cannazza, G.; Capodilupo, A.L.; Dini, L.; Ciccarella, G. Application of Calcium Carbonate Nanocarriers for Controlled Release of Phytodrugs against Xylella fastidiosa Pathogen. Pure Appl. Chem. 2020, 92, 429–444. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Sullivan, C.J.; Kuehn, M.J. Envelope Control of Outer Membrane Vesicle Production in Gram-Negative Bacteria. Biochemistry 2013, 52, 3031–3040. [Google Scholar] [CrossRef]
- Seo, M.D.; Won, H.S.; Kim, J.H.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial Peptides for Therapeutic Applications: A Review. Molecules 2012, 17, 12276–12286. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.E.; Hammami, R. Recent Insights into Structure–Function Relationships of Antimicrobial Peptides. J. Food Biochem. 2019, 43, e12546. [Google Scholar] [CrossRef]
- Zhu, S.; Sani, M.A.; Separovic, F. Interaction of Cationic Antimicrobial Peptides from Australian Frogs with Lipid Membranes. Pept. Sci. 2018, 110, e24016. [Google Scholar] [CrossRef]
- Alves, D.; Olívia Pereira, M. Mini-Review: Antimicrobial Peptides and Enzymes as Promising Candidates to Functionalize Biomaterial Surfaces. Biofouling 2014, 30, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Altman, H.; Steinberg, D.; Porat, Y.; Mor, A.; Fridman, D.; Friedman, M.; Bachrach, G. In Vitro Assessment of Antimicrobial Peptides as Potential Agents against Several Oral Bacteria. J. Antimicrob. Chemother. 2006, 58, 198–201. [Google Scholar] [CrossRef] [PubMed]
- El Handi, K.; Sabri, M.; Valentini, F.; De Stradis, A.; Achbani, E.H.; Hafidi, M.; El Moujabber, M.; Elbeaino, T. Exploring Active Peptides with Antimicrobial Activity In Planta against Xylella fastidiosa. Biology 2022, 11, 1685. [Google Scholar] [CrossRef]
- Baró, A.; Badosa, E.; Montesinos, L.; Feliu, L.; Planas, M.; Montesinos, E.; Bonaterra, A. Screening and Identification of BP100 Peptide Conjugates Active against Xylella fastidiosa Using a Viability-QPCR Method. BMC Microbiol. 2020, 20, 229. [Google Scholar] [CrossRef]
- Moll, L.; Badosa, E.; Planas, M.; Feliu, L.; Montesinos, E.; Bonaterra, A. Antimicrobial Peptides With Antibiofilm Activity Against Xylella fastidiosa. Front. Microbiol. 2021, 12, 753874. [Google Scholar] [CrossRef]
- Li, Z.T.; Gray, D.J. Effect of Five Antimicrobial Peptides on the Growth of Agrobacterium Tumefaciens, Escherichia Coli and Xylella Fastidiosa. Vitis 2003, 42, 95–97. [Google Scholar]
- Fogaça, A.C.; Zaini, P.A.; Wulff, N.A.; Da Silva, P.I.P.; Fázio, M.A.; Miranda, A.; Daffre, S.; Da Silva, A.M. Effects of the Antimicrobial Peptide Gomesin on the Global Gene Expression Profile, Virulence and Biofilm Formation of Xylella fastidiosa. FEMS Microbiol. Lett. 2010, 306, 152–159. [Google Scholar] [CrossRef]
- Baró, A.; Saldarelli, P.; Saponari, M.; Montesinos, E.; Montesinos, L. Nicotiana Benthamiana as a Model Plant Host for Xylella fastidiosa: Control of Infections by Transient Expression and Endotherapy with a Bifunctional Peptide. Front. Plant Sci. 2022, 13, 1061463. [Google Scholar] [CrossRef]
- Del Grosso, C.; Saponari, M.; Saldarelli, P.; Palmieri, D.; Altamura, G.; Kubaa, R.A.; De Curtis, F.; Lima, G. Use of commercial fertilizers in an IPDM protocol to mitigate Olive Quick Decline Syndrome caused by Xylella fastidiosa subsp. pauca in Southern Italy. Plant Dis. 2025. [Google Scholar] [CrossRef]
- Lacava, P.T.; Araújo, W.L.; Maccheroni, W.; Azevedo, J.L. RAPD Profile and Antibiotic Susceptibility of Xylella fastidiosa, Causal Agent of Citrus Variegated Chlorosis. Lett. Appl. Microbiol. 2001, 33, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Bleve, G.; Gallo, A.; Altomare, C.; Vurro, M.; Maiorano, G.; Cardinali, A.; D’Antuono, I.; Marchi, G.; Mita, G. In vitro Activity of Antimicrobial Compounds against Xylella fastidiosa, the Causal Agent of the Olive Quick Decline Syndrome in Apulia (Italy). FEMS Microbiol. Lett. 2018, 365, fnx281. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, C.; Fumarola, G.; Zicca, S.; Surano, A.; Di Carolo, M.; Datome, G. In vitro and in vivo Effects of Ammonium Chloride on Xylella fastidiosa subsp. pauca Infecting Olives. In Proceedings of the 3rd European Conference on Xylella fastidiosa and XF-ACTORS final meeting, Online, 26–28 April 2021; pp. 26–30. [Google Scholar] [CrossRef]
- Stefan-Kharicha, M.; Kharicha, A.; Mogeritsch, J.; Wu, M.; Ludwig, A. Review of Ammonium Chloride-Water Solution Properties. J. Chem. Eng. Data 2018, 63, 3170–3183. [Google Scholar] [CrossRef]
- Scortichini, M.; Loreti, S.; Pucci, N.; Scala, V.; Tatulli, G.; Verweire, D.; Oehl, M.; Widmer, U.; Codina, J.M.; Hertl, P.; et al. Progress towards Sustainable Control of Xylella fastidiosa subsp. pauca in Olive Groves of Salento (Apulia, Italy). Pathogens 2021, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Scortichini, M.; Chen, J.; De Caroli, M.; Dalessandro, G.; Pucci, N.; Modesti, V.; L’Aurora, A.; Petriccione, M.; Zampella, L.; Mastrobuoni, F.; et al. A Zinc, Copper and Citric Acid Biocomplex Shows Promise for Control of Xylella fastidiosa subsp. pauca in Olive Trees in Apulia Region (Southern Italy). Phytopathol. Mediterr. 2018, 57, 48–72. [Google Scholar] [CrossRef]
- Girelli, C.R.; Angilè, F.; Del Coco, L.; Migoni, D.; Zampella, L.; Marcelletti, S.; Cristella, N.; Marangi, P.; Scortichini, M.; Fanizzi, F.P. 1H-NMR Metabolite Fingerprinting Analysis Reveals a Disease Biomarker and a Field Treatment Response in Xylella fastidiosa subsp. pauca-Infected Olive Trees. Plants 2019, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Tatulli, G.; Modesti, V.; Pucci, N.; Scala, V.; L’aurora, A.; Lucchesi, S.; Salustri, M.; Scortichini, M.; Loreti, S. Further in vitro Assessment and Mid-Term Evaluation of Control Strategy of Xylella fastidiosa subsp. pauca in Olive Groves of Salento (Apulia, Italy). Pathogens 2021, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Girelli, C.R.; Del Coco, L.; Angilè, F.; Scortichini, M.; Fanizzi, F.P. Olive Cultivars Susceptible or Tolerant to Xylella fastidiosa subsp. pauca Exhibit Mid-Term Different Metabolomes upon Natural Infection or a Curative Treatment. Plants 2021, 10, 772. [Google Scholar] [CrossRef]
- Hussain, M.; Girelli, C.R.; Verweire, D.; Oehl, M.C.; Avendaño, M.S.; Scortichini, M.; Fanizzi, F.P. 1H-NMR Metabolomics Study after Foliar and Endo-Therapy Treatments Of Xylella fastidiosa subsp. pauca Infected Olive Trees: Medium Time Monitoring of Field Experiments. Plants 2023, 12, 1946. [Google Scholar] [CrossRef]
- Raffini, F.; Bertorelle, G.; Biello, R.; D’Urso, G.; Russo, D.; Bosso, L. From Nucleotides to Satellite Imagery: Approaches to Identify and Manage the Invasive Pathogen Xylella Fastidiosa and Its Insect Vectors in Europe. Sustainability 2020, 12, 4508. [Google Scholar] [CrossRef]
- Chartois, M.; Mesmin, X.; Quiquerez, I.; Borgomano, S.; Farigoule, P.; Pierre, É.; Thuillier, J.M.; Streito, J.C.; Casabianca, F.; Hugot, L.; et al. Environmental Factors Driving the Abundance of Philaenus Spumarius in Mesomediterranean Habitats of Corsica (France). Sci. Rep. 2023, 13, 1901. [Google Scholar] [CrossRef]
- Aprile, A.; Negro, C.; Sabella, E.; Luvisi, A.; Nicolì, F.; Nutricati, E.; Vergine, M.; Miceli, A.; Blando, F.; De Bellis, L. Antioxidant Activity and Anthocyanin Contents in Olives (CV Cellina Di Nardò) during Ripening and after Fermentation. Antioxidants 2019, 8, 138. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K. Phenolic Compounds in Olives. Analyst 1998, 123, 31–44. [Google Scholar] [CrossRef]
- Proietti, P.; Nasini, L.; Reale, L.; Caruso, T.; Ferranti, F. Productive and Vegetative Behavior of Olive Cultivars in Super High-Density Olive Grove. Sci. Agric. 2015, 72, 20–27. [Google Scholar] [CrossRef]
- Sabella, E.; Luvisi, A.; Aprile, A.; Negro, C.; Vergine, M.; Nicolì, F.; Miceli, A.; De Bellis, L. Xylella Fastidiosa Induces Differential Expression of Lignification Related-Genes and Lignin Accumulation in Tolerant Olive Trees Cv. Leccino. J. Plant Physiol. 2018, 220, 60–68. [Google Scholar] [CrossRef] [PubMed]
- De Pascali, M.; Vergine, M.; Sabella, E.; Aprile, A.; Nutricati, E.; Nicolì, F.; Buja, I.; Negro, C.; Miceli, A.; Rampino, P.; et al. Molecular Effects of Xylella Fastidiosa and Drought Combined Stress in Olive Trees. Plants 2019, 8, 437. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cruz, A.; Aguirre-Liguori, J.; Massonnet, M.; Minio, A.; Zaccheo, M.; Cochetel, N.; Walker, A.; Riaz, S.; Zhou, Y.; Cantu, D.; et al. Multigenic Resistance to Xylella Fastidiosa in Wild Grapes (Vitis Sps.) and Its Implications within a Changing Climate. Commun. Biol. 2023, 6, 580. [Google Scholar] [CrossRef]
- Hopkins, D.L.; Purcell, A.H. Xylella fastidiosa: Cause of Pierce’s Disease of Grapevine and Other Emergent Diseases. Plant Dis. 2002, 86, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Mårtensson, A. Need for Protective Measures to Combat Potential Outbreaks of Homalodisca coagulata and Pierce’s Disease in European Viticulture. Acta Agric. Scand. Sect. B Soil Plant Sci. 2007, 57, 1–9. [Google Scholar] [CrossRef]
- Cornara, D.; Bosco, D.; Fereres, A. Philaenus Spumarius: When an Old Acquaintance Becomes a New Threat to European Agriculture. J. Pest Sci. 2018, 91, 957–972. [Google Scholar] [CrossRef]
- Purcell, A.; Feil, H. Glassy-Winged Sharpshooter. Pestic. Outlook 2001, 12, 199–203. [Google Scholar] [CrossRef]
- Bethke, J.A.; Blua, M.J.; Redak, R.A. Effect of Selected Insecticides on Homalodisca coagulata (Homoptera: Cicadellidae) and Transmission of Oleander Leaf Scorch in a Greenhouse Study. J. Econ. Entomol. 2001, 94, 1031–1036. [Google Scholar] [CrossRef]
- Rathé, A.A.; Pilkington, L.J.; Gurr, G.M.; Hoddle, M.S.; Daugherty, M.P.; Constable, F.E.; Luck, J.E.; Powell, K.S.; Fletcher, M.J.; Edwards, O.R. Incursion Preparedness: Anticipating the Arrival of an Economically Important Plant Pathogen Xylella fastidiosa Wells (Proteobacteria: Xanthomonadaceae) and the Insect Vector Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae) in Australia. Aust. J. Entomol. 2012, 51, 209–220. [Google Scholar] [CrossRef]
- Prabhaker, N.; Castle, S.J.; Toscano, N.C. Susceptibility of Immature Stages of Homalodisca coagulata (Hemiptera: Cicadellidae) to Selected Insecticides. J. Econ. Entomol. 2006, 99, 1805–1812. [Google Scholar] [CrossRef]
- Mamta, B.; Rajam, M.V. RNAi Technology: A New Platform for Crop Pest Control. Physiol. Mol. Biol. Plants 2017, 23, 487–501. [Google Scholar] [CrossRef]
- Willow, J.; Smagghe, G. RNAi Applications toward Environmentally Sustainable Food Security. Curr. Opin. Environ. Sci. Health 2025, 45, 100612. [Google Scholar] [CrossRef]
- Dáder, B.; Viñuela, E.; Moreno, A.; Plaza, M.; Garzo, E.; Del Estal, P.; Fereres, A. Sulfoxaflor and Natural Pyrethrin with Piperonyl Butoxide Are Effective Alternatives to Neonicotinoids against Juveniles of Philaenus spumarius, the European Vector of Xylella fastidiosa. Insects 2019, 10, 225. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Altamura, G.; Di Carolo, M.; Fumarola, G.; Saponari, M.; Cavalieri, V. Evaluation of Efficacy of Different Insecticides Against Philaenus spumarius L., Vector of Xylella fastidiosa in Olive Orchards in Southern Italy, 2015–17. Arthropod Manag. Tests 2018, 43, tsy034. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Di Carolo, M.; Fumarola, G.; Tauro, D.; Altamura, G.; Cavalieri, V. Evaluation of Insecticides for the Control of Juveniles of Philaenus Spumarius L., 2015–2017. Arthropod Manag. Tests 2018, 43, tsy073. [Google Scholar] [CrossRef]
- Germinara, G.S.; Ganassi, S.; Pistillo, M.O.; Di Domenico, C.; De Cristofaro, A.; Di Palma, A.M. Antennal Olfactory Responses of Adult Meadow Spittlebug, Philaenus spumarius, to Volatile Organic Compounds (VOCs). PLoS ONE 2017, 12, 0190454. [Google Scholar] [CrossRef] [PubMed]
| Type of Nanoparticle | Mechanism of Action | Experimental Conditions | Characteristics | Ref. |
|---|---|---|---|---|
| Silver (Ag) NPs (ARGIRIUM-SUNCs®) | Antibacterial activity | In vitro | -Small size (1.79 nm) -Diagonal shape -Presence of Ag oxidation states -Negative solvation layer | [49] |
| Thymol NP | Antimicrobial activity | In vitro | -Affects membrane permeability and structure -Are nanoencapsulated to increase bioavailability and improve thymol stability -Gradual release into target cells | [50,51,52,53,54] |
| Fosetyl–Al NP | Systemic fungicide with antibacterial activity | In vitro | Optimization of diffusion at the target site with nanoformulation by sonification to break chemical bonds and reduce particle size | [55,56,57,58] |
| Zinkicide® | Antimicrobial activity | In vitro and in vivo | -Particle size (4 nm) -Production of reactive oxygen species (ROS) -Accumulation of Zn ions -Lipid peroxidation -Cell membrane disruption | [59,60,61] |
| Calcium carbonate nanocarriers | Antibacterial activity | In vitro and in vivo | -Different ways of integrating molecules -They act as nanocarriers of micro and macromolecules | [62,63,64] |
| Molecule | Treated Plant | In Vitro | In Vivo | Ref. | |||
|---|---|---|---|---|---|---|---|
| Xf Subsp. | Bacterial Titer Reduction | Biofilm Reduction | Symptom Reduction | Bacterial Titer Reduction | |||
| N-acetylcysteine | Sweet orange | pauca | Yes | Yes | Yes | Yes | [16] |
| Olive | pauca | No | - | Partial | Partial | [23] | |
| fastidiosa | Yes | Yes | - | - | [27,30,31] | ||
| Phenolic compounds | multiplex | Yes | - | - | - | [27] | |
| Olive | pauca | Yes | Yes | Partial | - | [31] | |
| Oxylipins | pauca | - | Yes (dioxygenase) No (lipoxygenase) | - | - | [34,36] | |
| fastidiosa and multiplex | - | Yes | - | - | [37] | ||
| NuovOlivo® | Olive | pauca | - | - | Yes | Yes | [38,39] |
| Menadione and benzethonium chloride | Grapevine | fastidiosa | Yes | - | Yes | - | [44] |
| Ag nanoparticles | pauca | Yes | Yes | - | - | [65] | |
| Thymol nanoparticles | Olive | pauca | Yes | - | Yes | Yes | [66] |
| Fosetyl–Al nanocrystals+ chitosan | fastidiosa | Yes | Yes | - | - | [68] | |
| pauca | Yes | No | - | - | [68] | ||
| Zinkicide® | N. tabacum | fastidiosa | Yes | Yes | Yes | Yes | [71] |
| V. corymbosum | multiplex | Yes | Yes | Yes | Yes | [71] | |
| Calcium carbonate nanocarriers | Olive | pauca | Yes | - | No | No | [73] |
| Antimicrobial peptides | N. tabacum | fastidiosa, multiplex and pauca | Yes | Yes | Partial | - | [81,82,83] |
| N. benthamiana | fastidiosa | Yes | Yes | Yes | Yes | [86] | |
| Gomesina | N. clevelandii | pauca | Yes | No | Yes | No | [85] |
| Kalex Zn® Kalex Cu® | Olive | pauca, sandyi and multiplex | Yes | - | Yes | Yes | [87] |
| Siliforce® | pauca, sandyi and multiplex | Yes | - | - | - | [87] | |
| Ammonium chloride | Olive | pauca | Yes | Yes | Yes | No | [90] |
| Dentamet® | Olive | pauca, fastidiosa and multiplex | Yes | Yes | Yes | Partial | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portaccio, L.; Vergine, M.; Bene, A.; De Pascali, M.; Sabella, E.; De Bellis, L.; Luvisi, A. Chemical Treatments Tested Against Xylella fastidiosa: Strategies, Successes and Limitations. Pathogens 2025, 14, 840. https://doi.org/10.3390/pathogens14090840
Portaccio L, Vergine M, Bene A, De Pascali M, Sabella E, De Bellis L, Luvisi A. Chemical Treatments Tested Against Xylella fastidiosa: Strategies, Successes and Limitations. Pathogens. 2025; 14(9):840. https://doi.org/10.3390/pathogens14090840
Chicago/Turabian StylePortaccio, Letizia, Marzia Vergine, Alessandro Bene, Mariarosaria De Pascali, Erika Sabella, Luigi De Bellis, and Andrea Luvisi. 2025. "Chemical Treatments Tested Against Xylella fastidiosa: Strategies, Successes and Limitations" Pathogens 14, no. 9: 840. https://doi.org/10.3390/pathogens14090840
APA StylePortaccio, L., Vergine, M., Bene, A., De Pascali, M., Sabella, E., De Bellis, L., & Luvisi, A. (2025). Chemical Treatments Tested Against Xylella fastidiosa: Strategies, Successes and Limitations. Pathogens, 14(9), 840. https://doi.org/10.3390/pathogens14090840










