Establishment of a Sandwich ELISA for Detection of Pan-Merbecoviruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Viruses
2.3. Plasmids
2.4. Preparation of Recombinant N Proteins
2.5. Generation of Monoclonal Antibodies Against VsCoV-1 N Protein
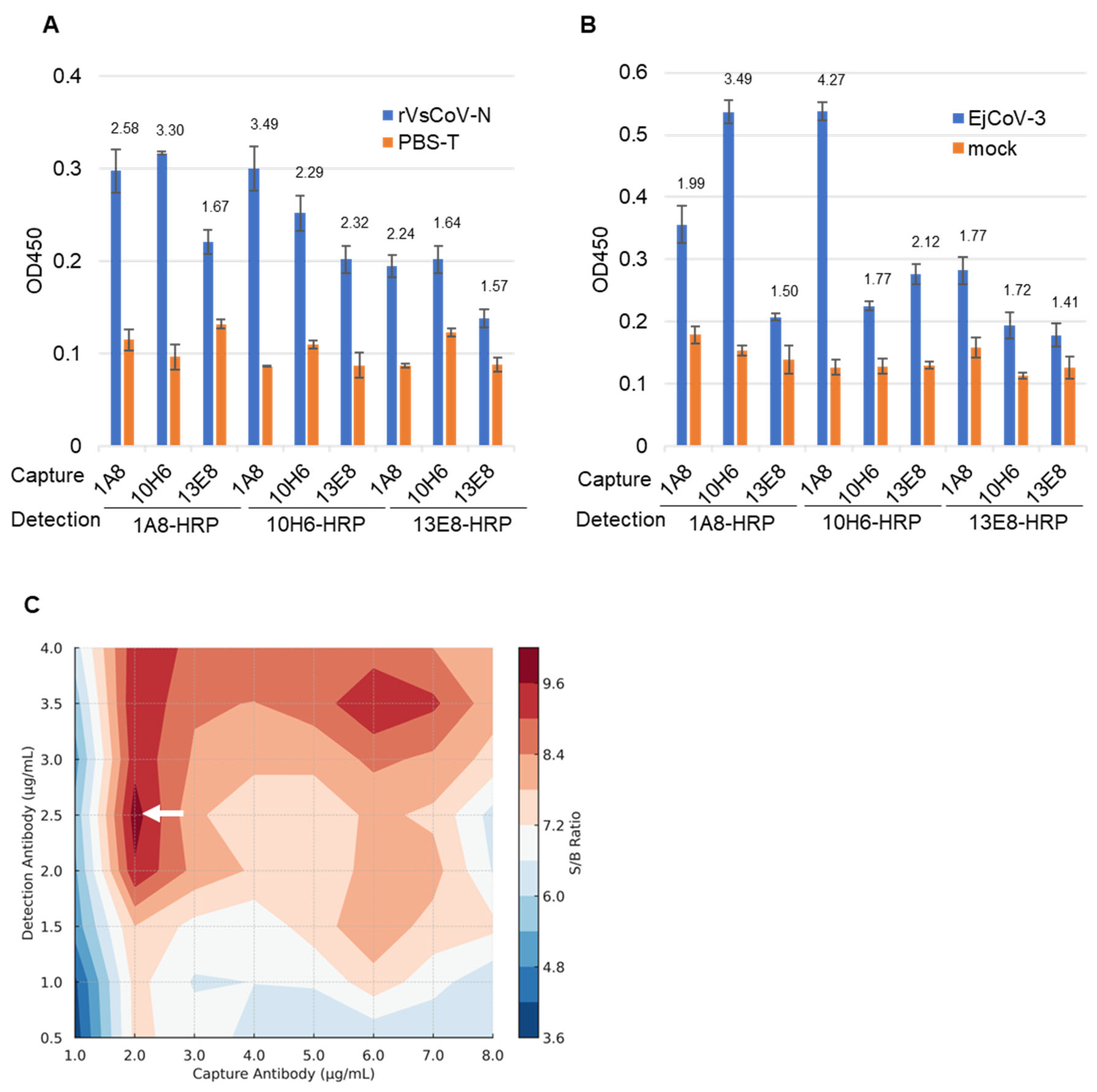
2.6. Development and Optimization of a Sandwich ELISA for Detection of Merbecoviruses
2.7. Evaluation of Sandwich ELISA Performance
2.8. Detection Limit of Sandwich ELISA
2.9. Ethics Statement
3. Results
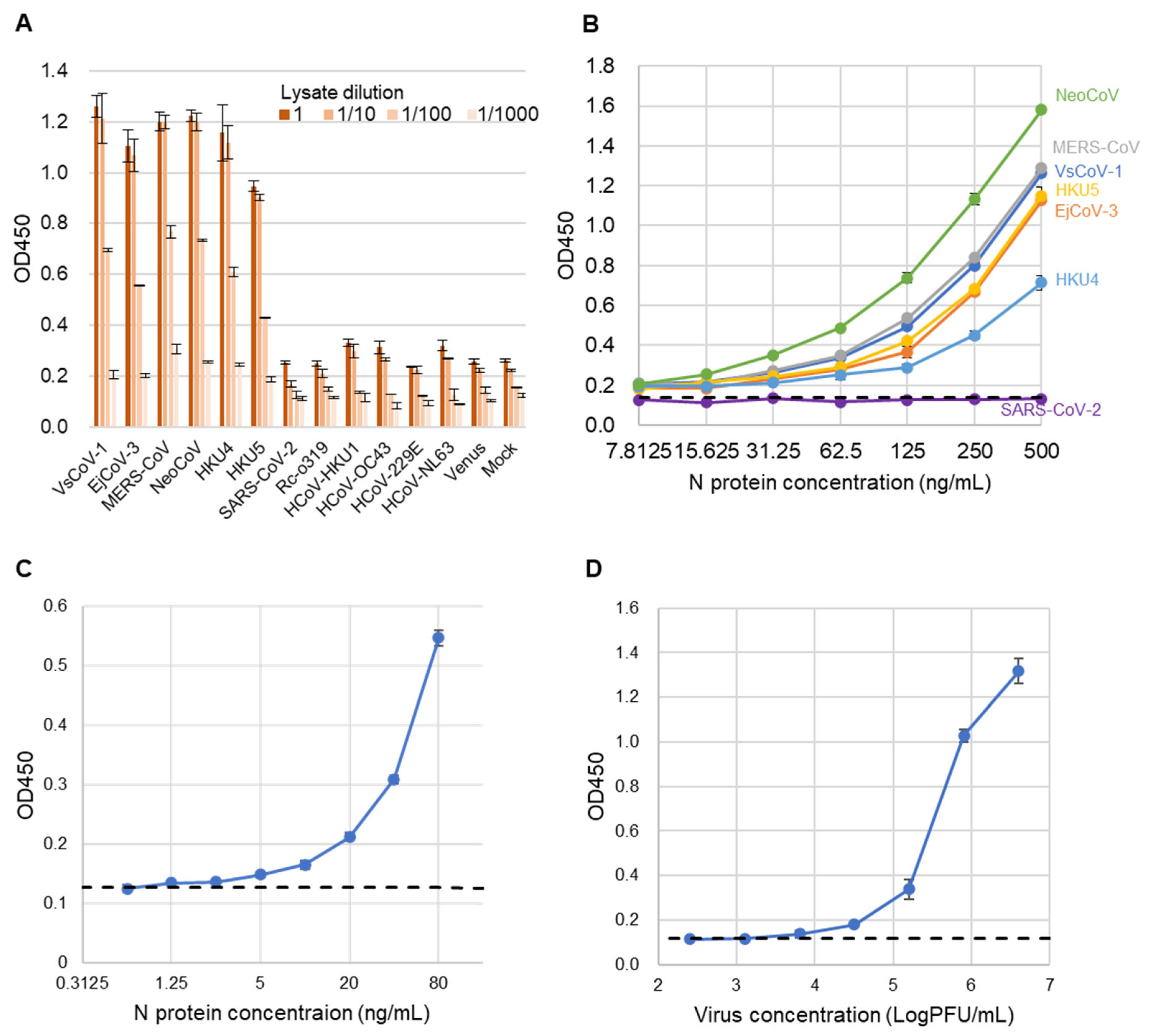
3.1. Generation and Characterization of Monoclonal Antibodies
3.2. Selection of Antibody Pairs for Sandwich ELISA
3.3. Performance Evaluation of the Sandwich ELISA
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ELISA | Enzyme-linked immunosorbent assay |
| CoV | Coronavirus |
| mAb | Monoclonal antibody |
| N | Nucleocapsid |
| LOD | Limit of detection |
| SARS | Severe acute respiratory syndrome |
| MERS | Middle East respiratory syndrome |
| COVID-19 | Coronavirus disease 2019 |
| RT-PCR | Real-time polymerase chain reaction |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | Fetal bovine serum |
| MDCK | Madin–Darby canine kidney cell |
| MEM | Eagle’s minimum essential medium |
| NBS | Newborn bovine serum |
| BAC | Bacterial artificial chromosome |
| PFU | Plaque-forming unit |
| HRP | Horseradish peroxidase |
| PEI | Polyethyleneimine |
| SD | Standard deviation |
| IFA | Indirect immunofluorescence assay |
| S/B | Signal-to-background ratio |
| CCHFV | Crimean–Congo hemorrhagic fever virus |
References
- Memish, Z.A.; Mishra, N.; Olival, K.J.; Fagbo, S.F.; Kapoor, V.; Epstein, J.H.; Alhakeem, R.; Durosinloun, A.; Al Asmari, M.; Islam, A.; et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg. Infect. Dis. 2013, 19, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.P.; Woo, P.C.Y.; Li, K.S.M.; Huang, Y.; Tsoi, H.-W.; Wong, B.H.L.; Wong, S.S.Y.; Leung, S.-Y.; Chan, K.-H.; Yuen, K.-Y. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. USA 2005, 102, 14040–14045. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.J.; Johnson, C.K.; Greig, D.J.; Kramer, S.; Che, X.; Wells, H.; Hicks, A.L.; Joly, D.O.; Wolfe, N.D.; Daszak, P.; et al. Global patterns in coronavirus diversity. Virus Evol. 2017, 3, vex012. [Google Scholar] [CrossRef]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats are natural reservoirs of SARS-like coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef]
- Ruiz-Aravena, M.; McKee, C.; Gamble, A.; Lunn, T.; Morris, A.; Snedden, C.E.; Yinda, C.K.; Port, J.R.; Buchholz, D.W.; Yeo, Y.Y.; et al. Ecology, evolution and spillover of coronaviruses from bats. Nat. Rev. Microbiol. 2022, 20, 299–314. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Z.; Ren, X.; Yang, F.; Zhang, J.; He, G.; Dong, J.; Sun, L.; Zhu, Y.; Zhang, S.; et al. MERS–Related Betacoronavirus in Vespertilio superans Bats, China. Emerg. Infect. Dis. 2014, 20, 1260–1262. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses Virus Taxonomy: 2020 Release. Available online: https://ictv.global/taxonomy (accessed on 30 April 2025).
- Wang, L.; Fu, S.; Cao, Y.; Zhang, H.; Feng, Y.; Yang, W.; Nie, K.; Ma, X.; Liang, G. Discovery and genetic analysis of novel coronaviruses in least horseshoe bats in southwestern China. Emerg. Microbes Infect. 2017, 6, e14. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Chu, C.; Chan, K.; Tsoi, H.; Huang, Y.; Wong, B.H.L.; Poon, R.W.S.; Cai, J.J.; Luk, W.; et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005, 79, 884–895. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Zhang, L.; Luk, H.K.H.; Xiong, L.; Peng, X.; Li, K.S.M.; He, X.; Zhao, P.S.-H.; Fan, R.Y.Y.; Wong, A.C.P.; et al. Receptor Usage of a Novel Bat Lineage C Betacoronavirus Reveals Evolution of Middle East Respiratory Syndrome-Related Coronavirus Spike Proteins for Human Dipeptidyl Peptidase 4 Binding. J. Infect. Dis. 2018, 218, 197–207. [Google Scholar] [CrossRef]
- Speranskaya, A.S.; Artiushin, I.V.; Samoilov, A.E.; Korneenko, E.V.; Khabudaev, K.V.; Ilina, E.N.; Yusefovich, A.P.; Safonova, M.V.; Dolgova, A.S.; Gladkikh, A.S.; et al. Identification and Genetic Characterization of MERS-Related Coronavirus Isolated from Nathusius’ Pipistrelle (Pipistrellus nathusii) near Zvenigorod (Moscow Region, Russia). Int. J. Environ. Res. Public Health 2023, 20, 3702. [Google Scholar] [CrossRef]
- Corman, V.M.; Kallies, R.; Philipps, H.; Göpner, G.; Müller, M.A.; Eckerle, I.; Brünink, S.; Drosten, C.; Drexler, J.F. Characterization of a novel betacoronavirus related to middle East respiratory syndrome coronavirus in European hedgehogs. J. Virol. 2014, 88, 717–724. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Li, Y.; Liu, C.; Dong, T.; Chen, H.; Wu, C.; Su, J.; Li, B.; Zhang, W.; et al. Bat-infecting merbecovirus HKU5-CoV lineage 2 can use human ACE2 as a cell entry receptor. Cell 2025, 188, 1729–1742.e16. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Mahajan, S.; Agrawal, R.; Kumar, M.; Mohan, A.; Pande, N. Comparative evaluation of RT-PCR with sandwich-ELISA for detection of Peste des petits ruminant in sheep and goats. Vet. World 2013, 6, 288. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Park, S.; Kim, M.; Baek, K.; Kang, M.; Choi, J.-K.; Maharjan, S.; Akauliya, M.; Lee, Y.; et al. Production of SARS-CoV-2 N Protein-Specific Monoclonal Antibody and Its Application in an ELISA-Based Detection System and Targeting the Interaction Between the Spike C-Terminal Domain and N Protein. Front. Microbiol. 2021, 12, 726231. [Google Scholar] [CrossRef]
- Murakami, S.; Kitamura, T.; Matsugo, H.; Yamamoto, T.; Mineshita, K.; Sakuyama, M.; Sasaki, R.; Takenaka-Uema, A.; Horimoto, T. Detection and genetic characterization of bat MERS-related coronaviruses in Japan. Transbound. Emerg. Dis. 2022, 69, 3388–3396. [Google Scholar] [CrossRef]
- Matsugo, H.; Kitamura, T.; Takahashi, N.; Chambers, J.; Ichikawa, A.; Katayama, M.; Li, K.; Sekine, W.; Ohira, K.; Ishida, H.; et al. In vitro and in vivo characterization of a bat merbecovirus with ACE2- and DPP4-independent cell entry. J. Virol. 2025, in press. [Google Scholar] [CrossRef]
- Komabayashi, K.; Matoba, Y.; Seto, J.; Ikeda, Y.; Tanaka, W.; Aoki, Y.; Ikeda, T.; Matsuzaki, Y.; Itagaki, T.; Shirato, K.; et al. Isolation of Human Coronaviruses OC43, HKU1, NL63, and 229E in Yamagata, Japan, Using Primary Human Airway Epithelium Cells Cultured by Employing an Air-Liquid Interface Culture. Jpn. J. Infect. Dis. 2021, 74, 285–292. [Google Scholar] [CrossRef]
- Murakami, S.; Kitamura, T.; Matsugo, H.; Kamiki, H.; Oyabu, K.; Sekine, W.; Takenaka-Uema, A.; Sakai-Tagawa, Y.; Kawaoka, Y.; Horimoto, T. Isolation of Bat Sarbecoviruses, Japan. Emerg. Infect. Dis. 2022, 28, 2500–2503. [Google Scholar] [CrossRef]
- Corman, V.M.; Eckerle, I.; Bleicker, T.; Zaki, A.; Landt, O.; Eschbach-Bludau, M.; van Boheemen, S.; Gopal, R.; Ballhause, M.; Bestebroer, T.M.; et al. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2012, 17, 20285. [Google Scholar] [CrossRef] [PubMed]
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; de Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect. Dis. 2020, 26, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Liew, O.W.; Ling, S.S.M.; Lilyanna, S.; Zhou, Y.; Wang, P.; Chong, J.P.C.; Ng, Y.X.; Lim, A.E.S.; Leong, E.R.Y.; Lin, Q.; et al. Epitope-directed monoclonal antibody production using a mixed antigen cocktail facilitates antibody characterization and validation. Commun. Biol. 2021, 4, 441. [Google Scholar] [CrossRef]
- Starcevic Manning, M.; Hassanein, M.; Partridge, M.A.; Jawa, V.; Mora, J.; Ryman, J.; Barker, B.; Braithwaite, C.; Carleton, K.; Hay, L.; et al. Comparison of Titer and Signal to Noise (S/N) for Determination of Anti-drug Antibody Magnitude Using Clinical Data from an Industry Consortium. AAPS J. 2022, 24, 81. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, N.; Kumar, J.S.; Yadav, P.; Shete, A.M.; Jain, R.; Shrivastava, A.; Dash, P.K. Development of double antibody sandwich ELISA as potential diagnostic tool for rapid detection of Crimean-Congo hemorrhagic fever virus. Sci. Rep. 2021, 11, 14699. [Google Scholar] [CrossRef]
- Mladenovic Stokanic, M.; Simovic, A.; Jovanovic, V.; Radomirovic, M.; Udovicki, B.; Krstic Ristivojevic, M.; Djukic, T.; Vasovic, T.; Acimovic, J.; Sabljic, L.; et al. Sandwich ELISA for the Quantification of Nucleocapsid Protein of SARS-CoV-2 Based on Polyclonal Antibodies from Two Different Species. Int. J. Mol. Sci. 2023, 25, 333. [Google Scholar] [CrossRef]



| mAb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1A8 | 2B4 | 2H5 | 3B4 | 5C11 | 5F3 | 7D5 | 10H6 | 12F6 | 13E8 | |
| Heavy chain | IgG1 | IgG2b | IgG2a | IgG1 | IgG2a | IgG2a | IgG1 | IgG1 | IgG1 | IgG1 |
| Light chain | κ | κ | κ | κ | Κ | Λ | κ | Κ | κ | κ |
| Virus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mAb | VsCoV | EjCoV | MERS | NeoCoV | HKU4 | HKU5 | SARS-CoV-2 | Rc-o319 | HKU1 | OC43 | 229E | NL63 |
| 1A8 | + | + | + | + | + | + | − | − | − | − | − | − |
| 2B4 | + | + | + | − | − | − | − | − | − | − | − | − |
| 2H5 | + | − | − | − | − | − | − | − | − | − | − | − |
| 3B4 | + | + | + | + | − | − | − | − | − | − | − | − |
| 5C11 | + | + | + | − | − | − | − | − | − | − | − | − |
| 5F3 | + | + | + | − | − | − | − | − | − | − | − | − |
| 7D5 | + | + | − | − | − | − | − | − | − | − | − | − |
| 10H6 | + | + | + | + | + | + | − | − | − | − | − | − |
| 12F6 | + | + | + | + | + | − | − | − | − | − | − | − |
| 13E8 | + | + | + | + | + | + | − | − | − | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Katayama, M.; Ichikawa, A.; Matsugo, H.; Wakabayashi, Y.; Takenaka-Uema, A.; Sekine, W.; Horimoto, T.; Murakami, S. Establishment of a Sandwich ELISA for Detection of Pan-Merbecoviruses. Pathogens 2025, 14, 605. https://doi.org/10.3390/pathogens14060605
Li K, Katayama M, Ichikawa A, Matsugo H, Wakabayashi Y, Takenaka-Uema A, Sekine W, Horimoto T, Murakami S. Establishment of a Sandwich ELISA for Detection of Pan-Merbecoviruses. Pathogens. 2025; 14(6):605. https://doi.org/10.3390/pathogens14060605
Chicago/Turabian StyleLi, Kaixin, Misa Katayama, Ayano Ichikawa, Hiromichi Matsugo, Yuta Wakabayashi, Akiko Takenaka-Uema, Wataru Sekine, Taisuke Horimoto, and Shin Murakami. 2025. "Establishment of a Sandwich ELISA for Detection of Pan-Merbecoviruses" Pathogens 14, no. 6: 605. https://doi.org/10.3390/pathogens14060605
APA StyleLi, K., Katayama, M., Ichikawa, A., Matsugo, H., Wakabayashi, Y., Takenaka-Uema, A., Sekine, W., Horimoto, T., & Murakami, S. (2025). Establishment of a Sandwich ELISA for Detection of Pan-Merbecoviruses. Pathogens, 14(6), 605. https://doi.org/10.3390/pathogens14060605






