Bat Influenza M2 Shows Functions Similar to Those of Classical Influenza A Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids and Virus Rescue
2.2. Antiviral Compounds
2.3. Virus Growth Kinetics
2.4. Plaque Assay
2.5. Statistical Analysis
2.6. Data Availability
3. Results
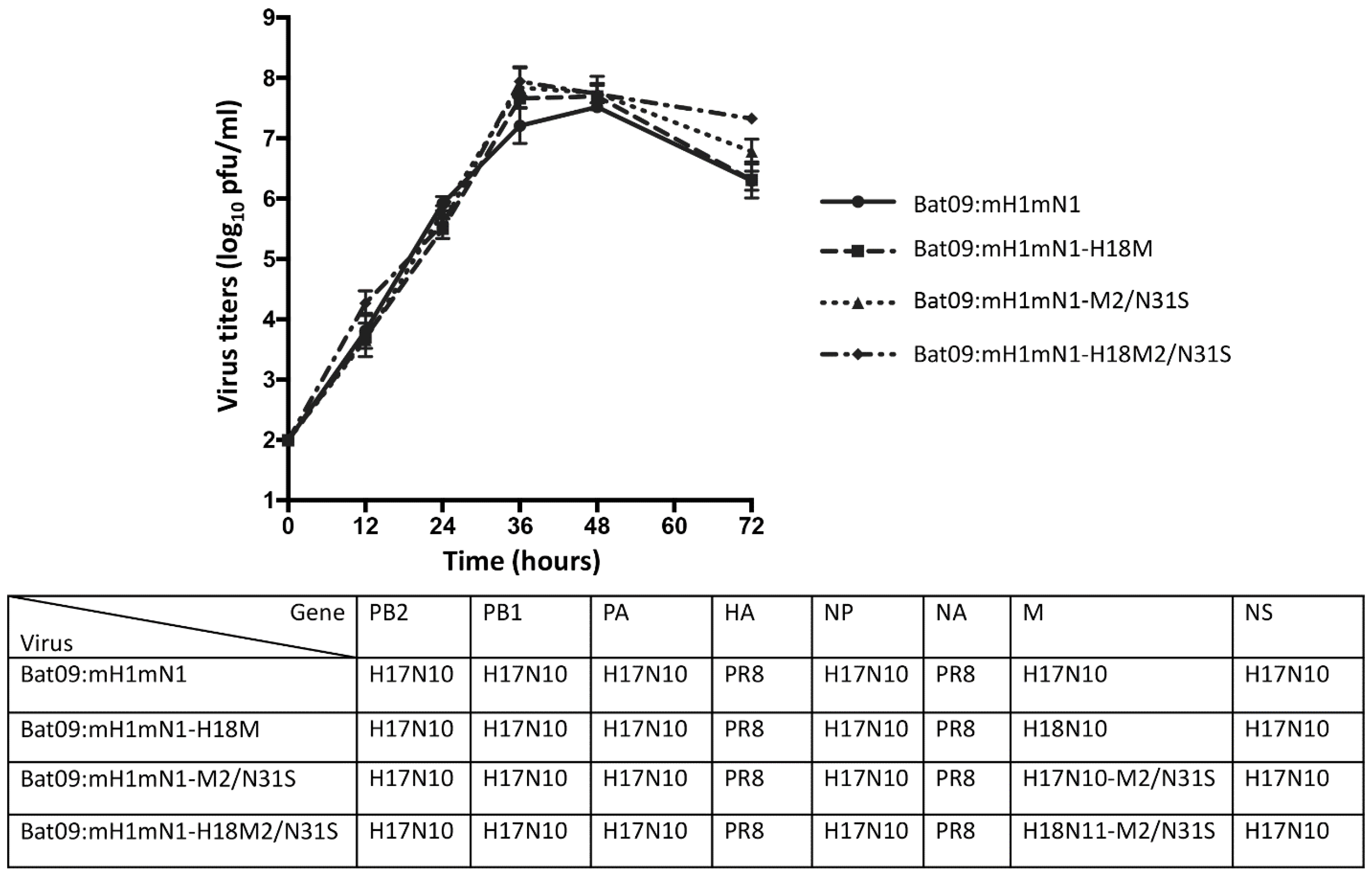
3.1. Generation and Characterization of Chimeric Bat Influenza Virus and Its Recombinant and Single-M2-N31S-Mutant Viruses
3.2. Chimeric and Recombinant Bat Influenza Viruses as Well as Their Single-Mutant Viruses Show Different Sensitivities to Antivirals Targeting the M2 Ion Channel
3.3. M2-H37G and M2-W41A Substitutions Critical for the Chimeric H17 but Not for the Recombinant H18 Virus
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef] [PubMed]
- Fouchier, R.A.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005, 79, 2814–2822. [Google Scholar] [CrossRef] [PubMed]
- Karakus, U.; Mena, I.; Kottur, J.; El Zahed, S.S.; Seoane, R.; Yildiz, S.; Chen, L.; Plancarte, M.; Lindsay, L.; Halpin, R.; et al. H19 influenza A virus exhibits species-specific MHC class II receptor usage. Cell Host Microbe 2024, 32, 1089–1102.e10. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Zhu, X.; Li, Y.; Shi, M.; Zhang, J.; Bourgeois, M.; Yang, H.; Chen, X.; Recuenco, S.; Gomez, J.; et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013, 9, e1003657. [Google Scholar] [CrossRef]
- Tong, S.; Li, Y.; Rivailler, P.; Conrardy, C.; Castillo, D.A.; Chen, L.M.; Recuenco, S.; Ellison, J.A.; Davis, C.T.; York, I.A.; et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. USA 2012, 109, 4269–4274. [Google Scholar] [CrossRef]
- Yang, W.; Schountz, T.; Ma, W. Bat Influenza Viruses: Current Status and Perspective. Viruses 2021, 13, 547. [Google Scholar] [CrossRef]
- Campos, A.C.A.; Goes, L.G.B.; Moreira-Soto, A.; de Carvalho, C.; Ambar, G.; Sander, A.L.; Fischer, C.; Ruckert da Rosa, A.; Cardoso de Oliveira, D.; Kataoka, A.P.G.; et al. Bat Influenza A(HL18NL11) Virus in Fruit Bats, Brazil. Emerg. Infect. Dis. 2019, 25, 333–337. [Google Scholar] [CrossRef]
- Moreira, E.A.; Locher, S.; Kolesnikova, L.; Bolte, H.; Aydillo, T.; Garcia-Sastre, A.; Schwemmle, M.; Zimmer, G. Synthetically derived bat influenza A-like viruses reveal a cell type- but not species-specific tropism. Proc. Natl. Acad. Sci. USA 2016, 113, 12797–12802. [Google Scholar] [CrossRef]
- Karakus, U.; Thamamongood, T.; Ciminski, K.; Ran, W.; Gunther, S.C.; Pohl, M.O.; Eletto, D.; Jeney, C.; Hoffmann, D.; Reiche, S.; et al. MHC class II proteins mediate cross-species entry of bat influenza viruses. Nature 2019, 567, 109–112. [Google Scholar] [CrossRef]
- Giotis, E.S.; Carnell, G.; Young, E.F.; Ghanny, S.; Soteropoulos, P.; Wang, L.F.; Barclay, W.S.; Skinner, M.A.; Temperton, N. Entry of the bat influenza H17N10 virus into mammalian cells is enabled by the MHC class II HLA-DR receptor. Nat. Microbiol. 2019, 4, 2035–2038. [Google Scholar] [CrossRef]
- Garcia-Sastre, A. The neuraminidase of bat influenza viruses is not a neuraminidase. Proc. Natl. Acad. Sci. USA 2012, 109, 18635–18636. [Google Scholar] [CrossRef] [PubMed]
- Ciminski, K.; Ran, W.; Gorka, M.; Lee, J.; Malmlov, A.; Schinkothe, J.; Eckley, M.; Murrieta, R.A.; Aboellail, T.A.; Campbell, C.L.; et al. Bat influenza viruses transmit among bats but are poorly adapted to non-bat species. Nat. Microbiol. 2019, 4, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Garcia-Sastre, A.; Schwemmle, M. Expected and Unexpected Features of the Newly Discovered Bat Influenza A-like Viruses. PLoS Pathog. 2015, 11, e1004819. [Google Scholar] [CrossRef] [PubMed]
- Rossman, J.S.; Lamb, R.A. Influenza virus assembly and budding. Virology 2011, 411, 229–236. [Google Scholar] [CrossRef]
- Chen, B.J.; Leser, G.P.; Jackson, D.; Lamb, R.A. The influenza virus M2 protein cytoplasmic tail interacts with the M1 protein and influences virus assembly at the site of virus budding. J. Virol. 2008, 82, 10059–10070. [Google Scholar] [CrossRef]
- Rossman, J.S.; Jing, X.; Leser, G.P.; Lamb, R.A. Influenza virus M2 protein mediates ESCRT-independent membrane scission. Cell 2010, 142, 902–913. [Google Scholar] [CrossRef]
- Rosenberg, M.R.; Casarotto, M.G. Coexistence of two adamantane binding sites in the influenza A M2 ion channel. Proc. Natl. Acad. Sci. USA 2010, 107, 13866–13871. [Google Scholar] [CrossRef]
- Ma, C.; Polishchuk, A.L.; Ohigashi, Y.; Stouffer, A.L.; Schon, A.; Magavern, E.; Jing, X.; Lear, J.D.; Freire, E.; Lamb, R.A.; et al. Identification of the functional core of the influenza A virus A/M2 proton-selective ion channel. Proc. Natl. Acad. Sci. USA 2009, 106, 12283–12288. [Google Scholar] [CrossRef]
- Venkataraman, P.; Lamb, R.A.; Pinto, L.H. Chemical rescue of histidine selectivity filter mutants of the M2 ion channel of influenza A virus. J. Biol. Chem. 2005, 280, 21463–21472. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zaitseva, F.; Lamb, R.A.; Pinto, L.H. The gate of the influenza virus M-2 proton channel is formed by a single tryptophan residue. J. Biol. Chem. 2002, 277, 39880–39886. [Google Scholar] [CrossRef]
- Cross, T.A.; Dong, H.; Sharma, M.; Busath, D.D.; Zhou, H.X. M2 protein from Influenza A: From multiple structures to biophysical and functional insights. Curr. Opin. Virol. 2012, 2, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Takeuchi, K.; Holsinger, L.J.; Lamb, R.A.; Pinto, L.H. Ion Channel Activity of the M2 Protein of Influenza A-Virus. Biophys. J. 1993, 64, A94. [Google Scholar]
- Cady, S.D.; Schmidt-Rohr, K.; Wang, J.; Soto, C.S.; DeGrado, W.F.; Hong, M. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature 2010, 463, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Durrant, M.G.; Eggett, D.L.; Busath, D.D. Investigation of a recent rise of dual amantadine-resistance mutations in the influenza A M2 sequence. BMC Genet. 2015, 16, S3. [Google Scholar] [CrossRef]
- Kode, S.S.; Pawar, S.D.; Tare, D.S.; Keng, S.S.; Mullick, J. Amantadine resistance markers among low pathogenic avian influenza H9N2 viruses isolated from poultry in India, during 2009–2017. Microb. Pathog. 2019, 137, 103779. [Google Scholar] [CrossRef]
- Li, F.; Ma, C.; DeGrado, W.F.; Wang, J. Discovery of Highly Potent Inhibitors Targeting the Predominant Drug-Resistant S31N Mutant of the Influenza A Virus M2 Proton Channel. J. Med. Chem. 2016, 59, 1207–1216. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, J.; Wang, J. Pharmacological Characterization of the Spectrum of Antiviral Activity and Genetic Barrier to Drug Resistance of M2-S31N Channel Blockers. Mol. Pharmacol. 2016, 90, 188–198. [Google Scholar] [CrossRef]
- Rey-Carrizo, M.; Gazzarrini, S.; Llabres, S.; Frigole-Vivas, M.; Juarez-Jimenez, J.; Font-Bardia, M.; Naesens, L.; Moroni, A.; Luque, F.J.; Vazquez, S. New polycyclic dual inhibitors of the wild type and the V27A mutant M2 channel of the influenza A virus with unexpected binding mode. Eur. J. Med. Chem. 2015, 96, 318–329. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Ma, C.; Fiorin, G.; Wang, J.; Pinto, L.H.; Lamb, R.A.; Klein, M.L.; Degrado, W.F. Structure and inhibition of the drug-resistant S31N mutant of the M2 ion channel of influenza A virus. Proc. Natl. Acad. Sci. USA 2013, 110, 1315–1320. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, J.; Liu, Q.; Bawa, B.; Wang, W.; Shabman, R.S.; Duff, M.; Lee, J.; Lang, Y.; Cao, N.; et al. Characterization of uncultivable bat influenza virus using a replicative synthetic virus. PLoS Pathog. 2014, 10, e1004420. [Google Scholar] [CrossRef]
- Juozapaitis, M.; Aguiar Moreira, E.; Mena, I.; Giese, S.; Riegger, D.; Pohlmann, A.; Hoper, D.; Zimmer, G.; Beer, M.; Garcia-Sastre, A.; et al. An infectious bat-derived chimeric influenza virus harbouring the entry machinery of an influenza A virus. Nat. Commun. 2014, 5, 4448. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Canturk, B.; Jo, H.; Ma, C.; Gianti, E.; Klein, M.L.; Pinto, L.H.; Lamb, R.A.; Fiorin, G.; Wang, J.; et al. Flipping in the pore: Discovery of dual inhibitors that bind in different orientations to the wild-type versus the amantadine-resistant S31N mutant of the influenza A virus M2 proton channel. J. Am. Chem. Soc. 2014, 136, 17987–17995. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hau, R.K.; Wang, Y.; Tuohy, P.; Zhang, Y.; Xu, S.; Ma, C.; Wang, J. Structure-Property Relationship Studies of Influenza A Virus AM2-S31N Proton Channel Blockers. ACS Med. Chem. Lett. 2018, 9, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Furuse, Y.; Suzuki, A.; Oshitani, H. Large-scale sequence analysis of M gene of influenza A viruses from different species: Mechanisms for emergence and spread of amantadine resistance. Antimicrob. Agents Chemother. 2009, 53, 4457–4463. [Google Scholar] [CrossRef]
- Hay, A.J.; Wolstenholme, A.J.; Skehel, J.J.; Smith, M.H. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985, 4, 3021–3024. [Google Scholar] [CrossRef]
- Jalily, P.H.; Duncan, M.C.; Fedida, D.; Wang, J.; Tietjen, I. Put a cork in it: Plugging the M2 viral ion channel to sink influenza. Antivir. Res. 2020, 178, 104780. [Google Scholar] [CrossRef]
- Cady, S.D.; Luo, W.; Hu, F.; Hong, M. Structure and function of the influenza A M2 proton channel. Biochemistry 2009, 48, 7356–7364. [Google Scholar] [CrossRef]
- Howard, K.P.; Lear, J.D.; DeGrado, W.F. Sequence determinants of the energetics of folding of a transmembrane four-helix-bundle protein. Proc. Natl. Acad. Sci. USA 2002, 99, 8568–8572. [Google Scholar] [CrossRef]
- Ciminski, K.; Schwemmle, M. Bat-Borne Influenza A Viruses: An Awakening. Cold Spring Harb. Perspect. Med. 2021, 11, a038612. [Google Scholar] [CrossRef]
- Krumbholz, A.; Schmidtke, M.; Bergmann, S.; Motzke, S.; Bauer, K.; Stech, J.; Durrwald, R.; Wutzler, P.; Zell, R. High prevalence of amantadine resistance among circulating European porcine influenza A viruses. J. Gen. Virol. 2009, 90, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Li, D.; Suzuki, Y.; Sato, I.; Masaki, H.; Nishimura, H.; Kawashima, T.; Shirahige, Y.; Shimomura, C.; Asoh, N.; et al. High prevalence of amantadine-resistance influenza a (H3N2) in six prefectures, Japan, in the 2005–2006 season. J. Med. Virol. 2007, 79, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Deyde, V.; Garten, R.; Sheu, T.; Smith, C.; Myrick, A.; Barnes, J.; Xu, X.; Shaw, M.; Klimov, A.; Gubareva, L. Genomic events underlying the changes in adamantane resistance among influenza A(H3N2) viruses during 2006–2008. Influenza Other Respir. Viruses 2009, 3, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Deyde, V.M.; Xu, X.; Bright, R.A.; Shaw, M.; Smith, C.B.; Zhang, Y.; Shu, Y.; Gubareva, L.V.; Cox, N.J.; Klimov, A.I. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 2007, 196, 249–257. [Google Scholar] [CrossRef]
- Sheu, T.G.; Fry, A.M.; Garten, R.J.; Deyde, V.M.; Shwe, T.; Bullion, L.; Peebles, P.J.; Li, Y.; Klimov, A.I.; Gubareva, L.V. Dual resistance to adamantanes and oseltamivir among seasonal influenza A(H1N1) viruses: 2008–2010. J. Infect. Dis. 2011, 203, 13–17. [Google Scholar] [CrossRef]
- Wang, L.; Shi, L.; Liu, H.; Zhang, J.; Yang, W.; Schountz, T.; Ma, W. Incompatible packaging signals and impaired protein functions hinder reassortment of bat H17N10 or H18N11 segment 7 with human H1N1 influenza A viruses. J. Virol. 2024, 98, e0086424. [Google Scholar] [CrossRef]
- Watanabe, T.; Watanabe, S.; Ito, H.; Kida, H.; Kawaoka, Y. Influenza a virus can undergo multiple cycles of replication without M2 ion channel activity. J. Virol. 2001, 75, 5656–5662. [Google Scholar] [CrossRef]
- Gannage, M.; Dormann, D.; Albrecht, R.; Dengjel, J.; Torossi, T.; Ramer, P.C.; Lee, M.; Strowig, T.; Arrey, F.; Conenello, G.; et al. Matrix Protein 2 of Influenza A Virus Blocks Autophagosome Fusion with Lysosomes. Cell Host Microbe 2009, 6, 367–380. [Google Scholar] [CrossRef]
- Wang, R.F.; Zhu, Y.X.; Lin, X.; Ren, C.W.; Zhao, J.C.; Wang, F.F.; Gao, X.C.; Xiao, R.; Zhao, L.Z.; Chen, H.C.; et al. Influenza M2 protein regulates MAVS-mediated signaling pathway through interacting with MAVS and increasing ROS production. Autophagy 2019, 15, 1163–1181. [Google Scholar] [CrossRef]
- Jing, X.H.; Ma, C.L.; Ohigashi, Y.; Oliveira, F.A.; Jardetzky, T.S.; Pinto, L.H.; Lamb, R.A. Functional studies indicate amantadine binds to the pore of the influenza A virus M2 proton-selective ion channel. Proc. Natl. Acad. Sci. USA 2008, 105, 10967–10972. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Wang, L.; Shi, L.; Zhang, J.; Liu, H.; Wang, J.; Ma, W. Bat Influenza M2 Shows Functions Similar to Those of Classical Influenza A Viruses. Pathogens 2025, 14, 599. https://doi.org/10.3390/pathogens14060599
Yang W, Wang L, Shi L, Zhang J, Liu H, Wang J, Ma W. Bat Influenza M2 Shows Functions Similar to Those of Classical Influenza A Viruses. Pathogens. 2025; 14(6):599. https://doi.org/10.3390/pathogens14060599
Chicago/Turabian StyleYang, Wenyu, Liping Wang, Lei Shi, Jialin Zhang, Heidi Liu, Jun Wang, and Wenjun Ma. 2025. "Bat Influenza M2 Shows Functions Similar to Those of Classical Influenza A Viruses" Pathogens 14, no. 6: 599. https://doi.org/10.3390/pathogens14060599
APA StyleYang, W., Wang, L., Shi, L., Zhang, J., Liu, H., Wang, J., & Ma, W. (2025). Bat Influenza M2 Shows Functions Similar to Those of Classical Influenza A Viruses. Pathogens, 14(6), 599. https://doi.org/10.3390/pathogens14060599






