Abstract
Novel bat influenza viruses show different features in contrast to classical influenza A viruses (IAVs). The M2 of IAVs functions as an ion channel that plays an important role in virus entry, viral assembly, and release and also serves as the antiviral target. To date, whether bat influenza M2 functions as the ion channel like classical IAV M2 remains unknown. Here, we show that the bat influenza M2 amino acid at position 31 (N/S) is critical for sensitivity to antivirals targeting the ion channel such as amantadine and other tested antivirals and that the amino acids at position 37 (H/G) and 41 (W/A) are crucial for virus replication and survival. The results indicate that bat influenza M2 functions similarly to conventional IAVs despite the low identity between the two.
1. Introduction
The influenza A virus (IAV) is an important zoonotic pathogen that can infect birds and mammalian species including humans. IAV infections cause significant morbidity and mortality in humans annually and are responsible for seasonal influenza epidemics and pandemics. According to the antigenicity of the viral surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), IAVs are divided into H1-H19 and N1-N11 subtypes. The H1-16, H19, and N1-9 subtypes of IAVs have been isolated from waterfowl and shorebirds that are considered the natural hosts of IAVs [1,2,3], while the sequences of the H17-18 and N10-11 subtypes of IAVs were only detected in bats [4,5,6]. Since the genome sequences of H17N10 and H18N11 viruses have been identified from bat samples, the host species of IAVs have been expanded to include bats [4,5,7].
Both H17N10 and H18N11 viruses have similar genomes and are closely related to known IAVs, but they are distinct from conventional IAVs [4,5]. In particular, both surface HA and NA proteins of bat influenza viruses show low identities and different characteristics in contrast to the counterparts of conventional IAVs, resulting in unique features of virus cultivation and cellular receptor usage [8,9,10]. The HA proteins of bat influenza H17N10 and H18N11 viruses do not recognize sialic acid receptors used by conventional IAVs; instead, they employ the MHC class II molecules to mediate cell entry [9,10]. The NA proteins of both H17N10 and H18N11 viruses do not have neuraminidase activity [11], and their exact functions remain unclear [12,13]. The M2 of IAVs is the third membrane protein that plays an important role in virus entry, viral assembly, and release [14,15,16] and is also an antiviral target [17]. The transmembrane domain (amino acids 22-46) of M2 protein is a homotetramer that functions as the proton-selective channel, which causes acidification of the interior of the virus particle to initiate genome transcription and replication [18]. In the M2 pore, a histidine (H) residue at position 37 acts as a proton selectivity filter, and a valine (V) residue at position 27 and a tryptophan (W) residue at position 41 serve as channel gates [19,20,21]. The amantadine family of drug inhibitors targets the ion channel of the M2 protein by binding to 27V and 37H with a high affinity, resulting in physical occlusion and antiviral effects [17,22,23]. Although the amantadine family of antivirals has been shown to be effective and has been widely used for many years, the emergence and persistence of drug-resistant influenza strains have compromised its effectiveness. Mutations in the M2 transmembrane domain including L26F, V27A, A30T/V, S31N, and G34E are known to confer resistance to amantadine [24,25]. In previous studies, the M2-S31N mutation has been shown to be key for amantadine-resistant IAVs, and the M2-S31N mutant persists in 95% of the currently circulating IAVs [26,27]. Furthermore, potentially broadly effective antiviral compounds targeting the M2 ion channel have been developed and were shown to be effective in inhibiting amantadine-resistant IAVs [26,28]. In contrast to other bat influenza internal genes, bat influenza M2 shows high homology with the counterparts of classical IAVs at the nucleotide level but only displays an average 45% identity with those of classical IAVs at the amino acid level. Notably, the M2 of both H17N10 and H18N11 bat influenza viruses has N at position 31, which is associated with adamantane resistance [29].
Wild-type bat H17N10 and H18N11 viruses have been successfully generated using reverse genetics and can replicate in limited cell lines effectively [8]. However, it is still challenging to study these novel viruses since they do not induce cytopathic effects (CPEs) in susceptible cells and require specific conditions to amplify these viruses [8,12]. In our and other former studies, chimeric bat influenza viruses, which contain six internal genes (PB1, PB2, PA, NP, M, and NS) from the bat influenza virus and two surface HA and NA coding regions from the classical IAVs with respective bat influenza virus gene packaging sequences, replicate efficiently and induce CPEs in normal substrates for classical IAVs [30,31], making them useful tools to study the internal gene functions of bat influenza viruses. In this study, we focus on the characteristics of the bat M2 protein by using the chimeric bat influenza virus to test antiviral compounds including amantadine and compounds that are effective against amandine-resistant influenza strains and by mutating both H37 and W41 amino acids located in the proton channel to determine their impacts on the replication and lifecycle of bat influenza viruses.
2. Materials and Methods
2.1. Plasmids and Virus Rescue
Six synthesized internal genes (PB1, PB2, PA, NP, M, and NS) from the A/little yellow-shouldered bat/Guatemala/164/2009 (H17N10) virus and two surface gene coding regions (mHA and mNA) from the PR8 A/Puerto Rico/8/1934 (H1N1) with the respective bat H17N10 gene packaging sequences were cloned into the plasmid PBZ, respectively as described previously [30]. These eight plasmids (pBZ-PB1, -PB2, -PA, -NP, -M, -NS, -mHA, and -mNA) were used to generate the chimeric Bat09:mH1mN1 virus. The M gene from the A/flat-faced bat/Peru/033/2010 (H18N11) was cloned into a pHW2000 vector, resulting in the plasmid pHW-H18-M as described previously [30]. To generate a recombinant Bat09:mH1mN1-H18M virus, the plasmid pBZ-M was replaced with the pHW-H18-M in the chimeric Bat09:mH1mN1 genetic background. Single substitutions in H17N10-M2/N31S, H17N10-M2/H37G, H17N10-M2/W41A, H17N10-M2/L36I, H18N11-M2/N31S, H18N11-M2/H37G, or H18N11-M2/W41A and double substitutions in H17N10-M2/L36I W41A were introduced using site-directed mutagenesis PCR based on plasmid pBZ-M or pHW-H18-M, resulting in plasmids with the expected single or double substitutions. To rescue the single- or double-mutant viruses, the plasmid pBZ-M was replaced by the plasmid containing each single or double substitutions in the chimeric Bat09:mH1mN1 genetic background.
To rescue chimeric and recombinant viruses as well as their single- or double-mutant viruses, eight gene plasmids (0.5 μg of each plasmid) were mixed and incubated with 8 μL LipofectamineTM 3000 (ThermoFisher, Waltham, MA, USA) in 250 μL Opti-MEM medium (ThermoFisher, Waltham, MA, USA) at room temperature for 15 min. The transfection mixture was transferred to 90% confluent 293T cells in 6-well plates and incubated at 37 °C with 5% CO2 for 48 h. The 0.5 mL supernatants were then collected and passaged on Madin-Darby canine kidney (MDCK) cells three times in 1.5 mL Minimum Essential Medium (MEM, Gibco, Waltham, MA, USA) with 0.3% bovine serum albumin (BSA, Sigma, St. Louis, MO, USA), 1% antibiotic–antimycotic solution (Gibco, Waltham, MA, USA) and 1 μg/mL TPCK-trypsin (Worthington-Biochem, Lakewood, NJ, USA) in 6-well plates until CPEs were present. The virus titers of each rescued virus were determined by plaque assay on MDCK cells.
2.2. Antiviral Compounds
Amantadine was purchased from Sigma (St. Louis, MA, USA), and Jun7-12-2 and Jun7-9 were synthesized and prepared by Dr. Jun Wang’s lab [32,33]. Jun7-12-2 is an M2-S31N inhibitor, and Jun7-9 is a dual inhibitor of M2-WT and M2-S31N.
2.3. Virus Growth Kinetics
MDCK cells in 12-well plates were infected with each virus at a multiplicity of infection (MOI) of 0.001. After 1 h of incubation at 37 °C, the supernatants were removed from the infected cells that were then washed twice with phosphate buffered saline (PBS). The MDCK cells were cultured in MEM medium with 0.3% BSA and 1 μg/mL TPCK-trypsin. The supernatants were collected at 12 h, 24 h, 36 h, 48 h, 60 h, and 72 h postinfection, and the virus titer in the supernatants was determined on MDCK cells by plaque assay.
2.4. Plaque Assay
To determine virus titers, the supernatant collected from infected cells or virus stock was serially diluted from 10−1 to 10−8 using MEM infection medium, which contains 0.3% BSA, 1% antibiotic–antimycotic solution, and 1 µg/mL of TPCK-trypsin. Confluent MDCK cells in 6-well plates were infected with the diluted virus and incubated for 1 h at 37 °C, after which the inoculum was removed. Next, 2 mL of MEM agarose overlay, which contains a 1:1 ratio of 2% low-melting agarose (Lonza, Walkersville, MD, USA) and 2 × MEM supplemented with 2 µg/mL of TPCK-treated trypsin, was added to each well. After the agarose solidified at room temperature, the plates were incubated upside down at 37 °C for 2–3 days until translucent plaques appeared. Cold methanol was added into the plate to fix the cells, followed by the crystal violet staining. The number of plaques was counted to determine virus titers using the Reed–Muench method.
To determine the efficacy of antivirals in inhibiting each tested virus, plaque reduction assays were performed. Confluent monolayers of MDCK cells in 6-well plates were infected with approximately 100 PFU/well of each tested virus in MEM medium with 0.3% BSA, 1% antibiotic–antimycotic solution, and 1 μg/mL TPCK-trypsin at 37 °C for 1 h. The inoculum was removed from the infected MDCK cells, which were then washed twice with PBS. The infected cells were treated with different concentrations (30 μM, 10 μM, 3 μM, 1 μM, 0.3 μM, and 0.1 μM) of each antiviral drug (amantadine, Jun7-9, or Jun7-12-2) in 2 mL of MEM agarose overlay per well. After the agarose in each well solidified at room temperature, the plates were incubated upside down at 37 °C for 2–3 days until translucent plaques appeared. The plaque size was measured using ImageJ v1.54g software [34]. The area of each well of the 6-well plate was individually measured and determined to be 9.5 cm2. For each indicated virus, the areas of all the plaques with clear boundaries were measured and normalized to 9.5 cm2 (shown in mm2 format).
2.5. Statistical Analysis
The significance of differences between groups and virus titers was analyzed by using one-way ANOVA followed by Tukey’s multiple comparison, two-way ANOVA in GraphPad Prism 10 software (GraphPad Software Inc., San Diego, CA, USA), or Student’s t test. All the data are representative of at least three independent experiments, and the values are indicated as mean ± SD. The value p < 0.05 was determined to be statistically significant (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001).
2.6. Data Availability
The data presented in this study are available upon request. Materials are available upon request through the filing of material transfer agreements.
3. Results
3.1. Generation and Characterization of Chimeric Bat Influenza Virus and Its Recombinant and Single-M2-N31S-Mutant Viruses
As wild-type bat influenza viruses do not cause CPEs in susceptible cells such as MDCK II and RIE1495 [8], the chimeric Bat09:mH1mN1 virus, which has six internal genes from the bat H17N10 virus and two surface HA and NA coding regions from the PR8 H1N1 virus with respective bat H17N10 gene packing signals, was used in this study [30]. To investigate whether M genes from both H17N10 and H18N11 have similar functions and impacts on virus replication, the recombinant Bat09:mH1mN1-H18M was generated using reverse genetics by replacing the M gene of H17N10 with the H18N11 M gene. Previous studies have shown that conventional IAVs with serine (S) at position 31 in M2 are sensitive to amantadine [35,36], while the M2 from either H17N10 or H18N11 virus has an asparagine (N) at position 31, which is expected to confer resistance to amantadine. We generated single-M2-N31S-mutant viruses in the chimeric Bat09:mH1mN1 and recombinant Bat09:mH1mN1-H18M genetic backgrounds, resulting in single-mutant Bat09:mH1mN1-M2-N31S and Bat09:mH1mN1-H18M2-N31S viruses. Growth kinetics analysis showed that both Bat09:mH1mN1 and recombinant Bat09:mH1mN1-H18M replicated to a similar level on MDCK cells (Figure 1). Single N31S substitutions in H17 and H18 M2 in each virus did not impact virus replication on MDCK cells based on growth dynamics when compared with their respective parental viruses (Figure 1).
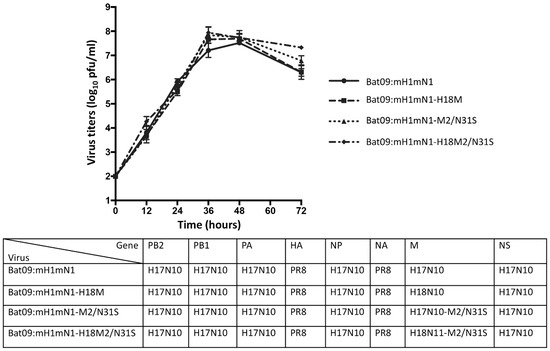
Figure 1.
Growth curves of bat influenza recombinant viruses and their M2/N31S mutant viruses. Generated recombinant viruses (Bat09:mH1mN1 and Bat09:mH1mN1-18M) and their M2/N31S mutant viruses (Bat09:mH1mN1-M2/N31S and Bat09:mH1mN1-18M2/N31S), which contain surface gene ORFs from PR8 A/Puerto Rico/8/1934 (H1N1), 5 internal genes from H17N10, and the M gene from either H17N10 or H18N11, were rescued. MDCK cells were infected with each indicated virus at an MOI of 0.001 in 12-well plates. The supernatant was collected at 12, 24, 36, 48, and 72 h postinfection and titrated on MDCK cells by plaque assay. Virus titers are presented as mean ± SD. Their growth curves were determined and compared in three independent experiments and analyzed by two-way ANOVA in GraphPad PRISM 10 software.
3.2. Chimeric and Recombinant Bat Influenza Viruses as Well as Their Single-Mutant Viruses Show Different Sensitivities to Antivirals Targeting the M2 Ion Channel
We determined whether the chimeric and recombinant bat influenza viruses as well as their single-mutant viruses were sensitive to antivirals targeting the M2 ion channel including amantadine, Jun7-12-2, and Jun7-9. Amantadine is an M2-WT (S31) inhibitor and does not inhibit M2-S31N [37]. Jun7-12-2 is an M2-S31N inhibitor and does not inhibit M2-WT (S31) [33]. Jun7-9 is a dual inhibitor of M2-S31N and M2-WT (S31) [32]. Their structures are shown in Figure 2A. The results showed that both chimeric Bat09:mH1mN1 and recombinant Bat09:mH1mN1-H18M were resistant to amantadine as expected, since both M2 from H17N10 and H18N11 have 31N. The antiviral EC50 values of amantadine in inhibiting chimeric Bat09:mH1mN1 and recombinant Bat09:mH1mN1-H18M were >30 μM (Figure 2B). In contrast, both Jun7-12-2 and Jun7-9 were more effective in inhibiting both chimeric Bat09:mH1mN1 and recombinant Bat09:mH1mN1-H18M. The antiviral EC50 values of Jun7-12-2 in inhibiting chimeric Bat09:mH1mN1 and recombinant Bat09:mH1mN1-H18M were 5.12 ± 0.12 μM and 7.00 ± 0.07 μM, respectively. Jun7-9 showed more-potent antiviral activity against both viruses with EC50 values of 2.14 ± 0.11 μM and 1.88 ± 0.06 μM, respectively (Figure 2B).
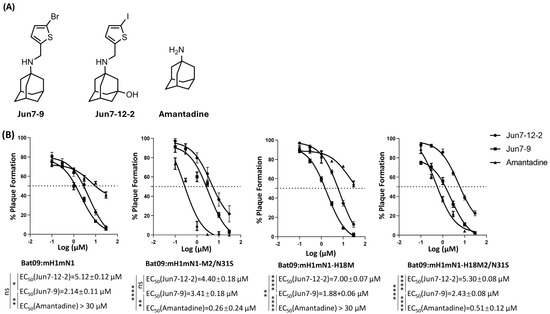
Figure 2.
Sensitivity of bat influenza recombinant viruses and their M2/N31S mutant viruses to antivirals targeting the M2 ion channel. Amantadine and compounds Jun7-12-2 and Jun7-9 were used to test the antiviral sensitivity of bat influenza recombinant viruses and their M2/N31S mutant viruses. (A) Structures of Jun7-12-2, Jun7-9, and amantadine. (B) Sensitivity of bat influenza recombinant viruses and their M2/N31S mutant viruses to the tested antivirals. Each virus was assayed for plaque formation on MDCK cells at different concentrations (30 μM, 10 μM, 3 μM, 1 μM, 0.3 μM, and 0.1 μM) of each tested antiviral. The EC50 of each antiviral against each virus was determined by a plaque reduction assay through three independent replicates. The dashed lines in these panels indicate 50% plaque formation. The inhibition efficacy of each compound was analyzed by nonlinear regression (curve fit) in GraphPad PRISM 10 software (ns, not significant; * p < 0.05; ** p < 0.01; **** p < 0.0001).
Both chimeric Bat09:mH1mN1-M2/N31S and recombinant Bat09:mH1mN1-H18M2/N31S with a single N31S mutation in M2 showed high sensitivity to amantadine with EC50 values of 0.26 ± 0.24 μM and 0.51 ± 0.12 μM, respectively. Similar to amantadine, Jun7-9 showed potent inhibition against Bat09:mH1mN1-M2/N31S and Bat09:mH1mN1-H18M2/N31S with EC50 values of 3.41 ± 0.18 µM and 2.43 ± 0.08 µM, respectively. In contrast, Jun7-12-2 was less effective against both viruses with a single N31S mutation in M2, and no complete inhibition was observed at the highest drug concentration tested (30 µM) (Figure 2B).
In conclusion, chimeric bat influenza viruses with H17N10 or H18N11 M2 carrying N31 are resistant to amantadine but sensitive to both Jun7-12-2 and Jun7-9. Jun7-9 shows inhibition against both amantadine-sensitive and -resistant influenza viruses, while Jun7-12-2 is only active against amantadine-resistant S31N-containing strains.
3.3. M2-H37G and M2-W41A Substitutions Critical for the Chimeric H17 but Not for the Recombinant H18 Virus
Both histidine (H) at position 37 and tryptophan (W) at position 41 are the two most conserved residues in the M2 protein of IAVs and are essential for ion channel function [38]. A previous study using a mutagenesis approach indicates that W41 functions as the gate in cooperation with H37 [20]. In contrast to H37, the two mutants (W41A and W41F) that abolish gating [20] actually have enhanced tetramer stability [39], indicating that this residue has evolved for function rather than stability.
To determine the roles of both H37 and W41 in bat influenza M2, we introduced the individual substitutions H37G or W41A in both H17 and H18 M2 and tried to rescue the single-mutant viruses. The results showed that both H18-M2 single-mutant viruses (Bat09:mH1mN1-H18M2/H37G and Bat09:mH1mN1-H18M2/W41A) were successfully rescued, but only the H17-M2/H37G (Bat09:mH1mN1-M2/H37G) single-mutant virus, not the single-mutant virus with W41A in H17-M2, was rescued (Figure 3). Compared with the parental Bat09:mH1mN1 virus, Bat09:mH1mN1-M2/H37G formed significantly smaller plaques on MDCK cells, while the plaque size of the H18-M2 single-mutant viruses was similar to that of those formed by the parental Bat09:mH1mN1-H18M2 virus (Figure 3). The results of growth dynamics revealed that the single H37G mutation in H17-M2 resulted in virus attenuation compared with the parental Bat09:mH1mN1 virus, and significantly lower titers were observed at 48 h postinfection but not at other time points. In contrast, lower titers were found in both Bat09:mH1mN1-H18M2/H37G and Bat09:mH1mN1-H18M2/W41A viruses after 36 h postinfection compared with the parental Bat09:mH1mN1-H18M2 virus, but no significant differences in virus titers were observed among these viruses (Figure 3). All these results indicate that both H37G and W41A substitutions in M2 significantly affect the survival or replication of chimeric viruses in the H17N10 background but do not have a significant impact on chimeric viruses with H18N11-M2.
Figure 3.
Plaque size and growth curves of bat influenza recombinant viruses and their mutant viruses. (A) Plaques formed by bat influenza recombinant viruses and their M2/N31S, M2/H37G, and M2/W41A mutant viruses. The red arrows indicate tiny plaques. The plaque picture represents one of two biological replicates. (B) The plaque size formed by each virus was analyzed using ImageJ software. The plaque sizes of each indicated virus were analyzed by one-way ANOVA with follow-up Tukey’s multiple comparison in GraphPad PRISM 10 software (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001). (C) Growth curves of bat influenza recombinant viruses and their M2/H37G and M2/W41A mutant viruses. MDCK cells were infected with each indicated virus at an MOI of 0.001 in 12-well plates. The supernatants of infected cells were collected at 12, 24, 36, 48, and 72 h postinfection and titrated by plaque assay. Their growth curves were determined and compared across three independent experiments. Error bars show the standard deviation. The growth curves of viruses were analyzed by two-way ANOVA in GraphPad PRISM 10 software (* p < 0.05).
4. Discussion
The identification of novel bat influenza viruses has challenged the dogma that all known IAVs originate from aquatic waterfowl, which are considered the natural reservoir of IAVs. Although significant progress on novel bat influenza viruses has been made [6,40], many questions about these mysterious viruses remain unresolved, such as those regarding NA and M2 functions. In the present study, we determined the M2 characteristics of bat influenza viruses and showed that chimeric bat influenza viruses are resistant to amantadine but sensitive to Jun7-12-2 and Jun7-9, which is consistent with the bat influenza M2 having N31 as expected. When N31 is substituted with S in bat M2, this results in chimeric viruses that are sensitive to amantadine, similar to the findings in conventional IAVs [41,42,43,44,45]. This indicates that the M2 proton channel of bat influenza viruses is a target of the amantadine family of antiviral drugs, similar to that of conventional IAVs.
We further revealed that both His 37 as a proton-selective filter and Trp 41 as a channel gate in the M2 proton channel from H17N10 and H18N11 viruses show different impacts on virus survival and replication. Both H37G and W41A substitutions in H18 M2 do not impact virus survival since single-mutant viruses with either substitution can be rescued. However, they still affect virus replication (lower virus titers were detected at 48 h and 72 h) in contrast to the parental Bat09:mH1mN1-H18M2 virus, despite similar plaque sizes being observed among single-mutant and parental Bat09:mH1mN1-H18M2 viruses. Interestingly, both single-mutant viruses (Bat09:mH1mN1-H18M2/H37G and Bat09:mH1mN1-H18M2/W41A) show similar growth dynamics on MDCK cells. In contrast, the single W41A substitution in H17 M2 results in failure to rescue the virus, while the single H37G substitution does not impact virus survival but causes the formation of small plaques in the single-mutant Bat09:mH1mN1-M/H37G virus when compared with the parental virus. The M2 transmembrane domain between amino acids 22 and 46 in H17N10 and H18N11 viruses is very conserved; there is only one amino acid difference at position 36 between the two M2 proteins (L36 in H17-M2; I36 in H18-M2). Our results showed that the single-mutant virus with the L36I substitution (H17N10-M2/L36I) or the double-mutant virus with L36I and W41A (H17N10-M2/L36I W41A) in H17-M2 cannot be rescued. In contrast, these two amino acids in the M2 ion channel do not impact H18N11 M2 functions in the chimeric viruses. These results suggest that the loss of the M2 ion channel gate function is fatal for the H17N10 M2, and impaired functioning of the M2 proton selectivity filter might affect the replication of the H17N10 M2-based viruses. The difference in rescuing the single-W41A-mutant virus in both H17 and H18 M2 could also be due to the genetic background used, which may impact protein–protein interactions, as our previous studies have shown that uncoating of the recombinant PR8 H1N1 viruses driven by the H17N10 M2 ion channel is much less efficient than that driven by the H18N11 M2 [46]. The underlying mechanisms by which both bat M2s show different features remain unknown and need to be investigated in future studies.
Although M2 proton channel activity is necessary for virus replication and the viral lifecycle, some IAVs are able to survive and replicate without proton channel activity. In a previous study, Watanabe et al. found that the A/Udorn/307/72 (H3N2) virus can undergo multiple cycles of replication without M2 proton channel activity due to the deletion of M2 amino acids 29-31 [47]. It should be noted that the heart of acid activation and proton conductance is H37xxxW41 in the M2 transmembrane region [20]. The H37G substitution in the M2 proton channel has been confirmed as a poorly selective cation channel [19], and the W41A substitution in the M2 results in abolished gating [20]. It remains unclear whether and how the mutation of H37xxxW41 actually affects the virus replication or lifecycle of IAVs since conflicting results have been obtained in previous studies. Gannage et al. showed that a recombinant PR8 H1N1 that contains only the extracellular domain of M2 (amino acids 1-25) is able to replicate for a single round in vitro [48]. Wang et al. failed to rescue the recombinant A/duck/Hubei/Hangmei01/2006 (H5N1) virus that had the single H37G substitution in M2 [49]. Jing et al. successfully rescued the A/Udorn/72 (H3N2) virus with the M2-W41A substitution that replicates very efficiently on MDCK cells [50]. In the present study, we were able to rescue the recombinant virus with either H37G or W41A substitution in H18 M2 but only with H37G and not W41A substitution in H17 M2. According to previous studies and our results, this phenomenon is most likely strain-dependent, and the underlying mechanisms need to be investigated in future studies. Taken together, our results demonstrate that the M2 of novel bat influenza viruses has similar proton channel functions to those of classical IAVs, which impacts virus replication and antiviral resistance.
Author Contributions
W.M. developed the concept of the study; W.M., W.Y. and J.W. designed the study; W.Y., L.W., L.S., J.Z. and H.L. performed the experiments; W.Y., L.S. and W.M. analyzed the data. W.Y. and W.M. wrote the first manuscript; J.W. and W.M. edited and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by NIH/NIAID 1R01AI134768, the Centers of Excellence in Influenza Research and Response (CEIRR) funded by NIAID, contract number 75N93021C00016 and University of Missouri Startup funds.
Institutional Review Board Statement
The study was approved by the Institutional Biosafety Committee of the University of Missouri (protocol #12100 and approval on 3 November 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef] [PubMed]
- Fouchier, R.A.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005, 79, 2814–2822. [Google Scholar] [CrossRef] [PubMed]
- Karakus, U.; Mena, I.; Kottur, J.; El Zahed, S.S.; Seoane, R.; Yildiz, S.; Chen, L.; Plancarte, M.; Lindsay, L.; Halpin, R.; et al. H19 influenza A virus exhibits species-specific MHC class II receptor usage. Cell Host Microbe 2024, 32, 1089–1102.e10. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Zhu, X.; Li, Y.; Shi, M.; Zhang, J.; Bourgeois, M.; Yang, H.; Chen, X.; Recuenco, S.; Gomez, J.; et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013, 9, e1003657. [Google Scholar] [CrossRef]
- Tong, S.; Li, Y.; Rivailler, P.; Conrardy, C.; Castillo, D.A.; Chen, L.M.; Recuenco, S.; Ellison, J.A.; Davis, C.T.; York, I.A.; et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. USA 2012, 109, 4269–4274. [Google Scholar] [CrossRef]
- Yang, W.; Schountz, T.; Ma, W. Bat Influenza Viruses: Current Status and Perspective. Viruses 2021, 13, 547. [Google Scholar] [CrossRef]
- Campos, A.C.A.; Goes, L.G.B.; Moreira-Soto, A.; de Carvalho, C.; Ambar, G.; Sander, A.L.; Fischer, C.; Ruckert da Rosa, A.; Cardoso de Oliveira, D.; Kataoka, A.P.G.; et al. Bat Influenza A(HL18NL11) Virus in Fruit Bats, Brazil. Emerg. Infect. Dis. 2019, 25, 333–337. [Google Scholar] [CrossRef]
- Moreira, E.A.; Locher, S.; Kolesnikova, L.; Bolte, H.; Aydillo, T.; Garcia-Sastre, A.; Schwemmle, M.; Zimmer, G. Synthetically derived bat influenza A-like viruses reveal a cell type- but not species-specific tropism. Proc. Natl. Acad. Sci. USA 2016, 113, 12797–12802. [Google Scholar] [CrossRef]
- Karakus, U.; Thamamongood, T.; Ciminski, K.; Ran, W.; Gunther, S.C.; Pohl, M.O.; Eletto, D.; Jeney, C.; Hoffmann, D.; Reiche, S.; et al. MHC class II proteins mediate cross-species entry of bat influenza viruses. Nature 2019, 567, 109–112. [Google Scholar] [CrossRef]
- Giotis, E.S.; Carnell, G.; Young, E.F.; Ghanny, S.; Soteropoulos, P.; Wang, L.F.; Barclay, W.S.; Skinner, M.A.; Temperton, N. Entry of the bat influenza H17N10 virus into mammalian cells is enabled by the MHC class II HLA-DR receptor. Nat. Microbiol. 2019, 4, 2035–2038. [Google Scholar] [CrossRef]
- Garcia-Sastre, A. The neuraminidase of bat influenza viruses is not a neuraminidase. Proc. Natl. Acad. Sci. USA 2012, 109, 18635–18636. [Google Scholar] [CrossRef] [PubMed]
- Ciminski, K.; Ran, W.; Gorka, M.; Lee, J.; Malmlov, A.; Schinkothe, J.; Eckley, M.; Murrieta, R.A.; Aboellail, T.A.; Campbell, C.L.; et al. Bat influenza viruses transmit among bats but are poorly adapted to non-bat species. Nat. Microbiol. 2019, 4, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Garcia-Sastre, A.; Schwemmle, M. Expected and Unexpected Features of the Newly Discovered Bat Influenza A-like Viruses. PLoS Pathog. 2015, 11, e1004819. [Google Scholar] [CrossRef] [PubMed]
- Rossman, J.S.; Lamb, R.A. Influenza virus assembly and budding. Virology 2011, 411, 229–236. [Google Scholar] [CrossRef]
- Chen, B.J.; Leser, G.P.; Jackson, D.; Lamb, R.A. The influenza virus M2 protein cytoplasmic tail interacts with the M1 protein and influences virus assembly at the site of virus budding. J. Virol. 2008, 82, 10059–10070. [Google Scholar] [CrossRef]
- Rossman, J.S.; Jing, X.; Leser, G.P.; Lamb, R.A. Influenza virus M2 protein mediates ESCRT-independent membrane scission. Cell 2010, 142, 902–913. [Google Scholar] [CrossRef]
- Rosenberg, M.R.; Casarotto, M.G. Coexistence of two adamantane binding sites in the influenza A M2 ion channel. Proc. Natl. Acad. Sci. USA 2010, 107, 13866–13871. [Google Scholar] [CrossRef]
- Ma, C.; Polishchuk, A.L.; Ohigashi, Y.; Stouffer, A.L.; Schon, A.; Magavern, E.; Jing, X.; Lear, J.D.; Freire, E.; Lamb, R.A.; et al. Identification of the functional core of the influenza A virus A/M2 proton-selective ion channel. Proc. Natl. Acad. Sci. USA 2009, 106, 12283–12288. [Google Scholar] [CrossRef]
- Venkataraman, P.; Lamb, R.A.; Pinto, L.H. Chemical rescue of histidine selectivity filter mutants of the M2 ion channel of influenza A virus. J. Biol. Chem. 2005, 280, 21463–21472. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zaitseva, F.; Lamb, R.A.; Pinto, L.H. The gate of the influenza virus M-2 proton channel is formed by a single tryptophan residue. J. Biol. Chem. 2002, 277, 39880–39886. [Google Scholar] [CrossRef]
- Cross, T.A.; Dong, H.; Sharma, M.; Busath, D.D.; Zhou, H.X. M2 protein from Influenza A: From multiple structures to biophysical and functional insights. Curr. Opin. Virol. 2012, 2, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Takeuchi, K.; Holsinger, L.J.; Lamb, R.A.; Pinto, L.H. Ion Channel Activity of the M2 Protein of Influenza A-Virus. Biophys. J. 1993, 64, A94. [Google Scholar]
- Cady, S.D.; Schmidt-Rohr, K.; Wang, J.; Soto, C.S.; DeGrado, W.F.; Hong, M. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature 2010, 463, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Durrant, M.G.; Eggett, D.L.; Busath, D.D. Investigation of a recent rise of dual amantadine-resistance mutations in the influenza A M2 sequence. BMC Genet. 2015, 16, S3. [Google Scholar] [CrossRef]
- Kode, S.S.; Pawar, S.D.; Tare, D.S.; Keng, S.S.; Mullick, J. Amantadine resistance markers among low pathogenic avian influenza H9N2 viruses isolated from poultry in India, during 2009–2017. Microb. Pathog. 2019, 137, 103779. [Google Scholar] [CrossRef]
- Li, F.; Ma, C.; DeGrado, W.F.; Wang, J. Discovery of Highly Potent Inhibitors Targeting the Predominant Drug-Resistant S31N Mutant of the Influenza A Virus M2 Proton Channel. J. Med. Chem. 2016, 59, 1207–1216. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, J.; Wang, J. Pharmacological Characterization of the Spectrum of Antiviral Activity and Genetic Barrier to Drug Resistance of M2-S31N Channel Blockers. Mol. Pharmacol. 2016, 90, 188–198. [Google Scholar] [CrossRef]
- Rey-Carrizo, M.; Gazzarrini, S.; Llabres, S.; Frigole-Vivas, M.; Juarez-Jimenez, J.; Font-Bardia, M.; Naesens, L.; Moroni, A.; Luque, F.J.; Vazquez, S. New polycyclic dual inhibitors of the wild type and the V27A mutant M2 channel of the influenza A virus with unexpected binding mode. Eur. J. Med. Chem. 2015, 96, 318–329. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Ma, C.; Fiorin, G.; Wang, J.; Pinto, L.H.; Lamb, R.A.; Klein, M.L.; Degrado, W.F. Structure and inhibition of the drug-resistant S31N mutant of the M2 ion channel of influenza A virus. Proc. Natl. Acad. Sci. USA 2013, 110, 1315–1320. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, J.; Liu, Q.; Bawa, B.; Wang, W.; Shabman, R.S.; Duff, M.; Lee, J.; Lang, Y.; Cao, N.; et al. Characterization of uncultivable bat influenza virus using a replicative synthetic virus. PLoS Pathog. 2014, 10, e1004420. [Google Scholar] [CrossRef]
- Juozapaitis, M.; Aguiar Moreira, E.; Mena, I.; Giese, S.; Riegger, D.; Pohlmann, A.; Hoper, D.; Zimmer, G.; Beer, M.; Garcia-Sastre, A.; et al. An infectious bat-derived chimeric influenza virus harbouring the entry machinery of an influenza A virus. Nat. Commun. 2014, 5, 4448. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Canturk, B.; Jo, H.; Ma, C.; Gianti, E.; Klein, M.L.; Pinto, L.H.; Lamb, R.A.; Fiorin, G.; Wang, J.; et al. Flipping in the pore: Discovery of dual inhibitors that bind in different orientations to the wild-type versus the amantadine-resistant S31N mutant of the influenza A virus M2 proton channel. J. Am. Chem. Soc. 2014, 136, 17987–17995. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hau, R.K.; Wang, Y.; Tuohy, P.; Zhang, Y.; Xu, S.; Ma, C.; Wang, J. Structure-Property Relationship Studies of Influenza A Virus AM2-S31N Proton Channel Blockers. ACS Med. Chem. Lett. 2018, 9, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Furuse, Y.; Suzuki, A.; Oshitani, H. Large-scale sequence analysis of M gene of influenza A viruses from different species: Mechanisms for emergence and spread of amantadine resistance. Antimicrob. Agents Chemother. 2009, 53, 4457–4463. [Google Scholar] [CrossRef]
- Hay, A.J.; Wolstenholme, A.J.; Skehel, J.J.; Smith, M.H. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985, 4, 3021–3024. [Google Scholar] [CrossRef]
- Jalily, P.H.; Duncan, M.C.; Fedida, D.; Wang, J.; Tietjen, I. Put a cork in it: Plugging the M2 viral ion channel to sink influenza. Antivir. Res. 2020, 178, 104780. [Google Scholar] [CrossRef]
- Cady, S.D.; Luo, W.; Hu, F.; Hong, M. Structure and function of the influenza A M2 proton channel. Biochemistry 2009, 48, 7356–7364. [Google Scholar] [CrossRef]
- Howard, K.P.; Lear, J.D.; DeGrado, W.F. Sequence determinants of the energetics of folding of a transmembrane four-helix-bundle protein. Proc. Natl. Acad. Sci. USA 2002, 99, 8568–8572. [Google Scholar] [CrossRef]
- Ciminski, K.; Schwemmle, M. Bat-Borne Influenza A Viruses: An Awakening. Cold Spring Harb. Perspect. Med. 2021, 11, a038612. [Google Scholar] [CrossRef]
- Krumbholz, A.; Schmidtke, M.; Bergmann, S.; Motzke, S.; Bauer, K.; Stech, J.; Durrwald, R.; Wutzler, P.; Zell, R. High prevalence of amantadine resistance among circulating European porcine influenza A viruses. J. Gen. Virol. 2009, 90, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Li, D.; Suzuki, Y.; Sato, I.; Masaki, H.; Nishimura, H.; Kawashima, T.; Shirahige, Y.; Shimomura, C.; Asoh, N.; et al. High prevalence of amantadine-resistance influenza a (H3N2) in six prefectures, Japan, in the 2005–2006 season. J. Med. Virol. 2007, 79, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Deyde, V.; Garten, R.; Sheu, T.; Smith, C.; Myrick, A.; Barnes, J.; Xu, X.; Shaw, M.; Klimov, A.; Gubareva, L. Genomic events underlying the changes in adamantane resistance among influenza A(H3N2) viruses during 2006–2008. Influenza Other Respir. Viruses 2009, 3, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Deyde, V.M.; Xu, X.; Bright, R.A.; Shaw, M.; Smith, C.B.; Zhang, Y.; Shu, Y.; Gubareva, L.V.; Cox, N.J.; Klimov, A.I. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 2007, 196, 249–257. [Google Scholar] [CrossRef]
- Sheu, T.G.; Fry, A.M.; Garten, R.J.; Deyde, V.M.; Shwe, T.; Bullion, L.; Peebles, P.J.; Li, Y.; Klimov, A.I.; Gubareva, L.V. Dual resistance to adamantanes and oseltamivir among seasonal influenza A(H1N1) viruses: 2008–2010. J. Infect. Dis. 2011, 203, 13–17. [Google Scholar] [CrossRef]
- Wang, L.; Shi, L.; Liu, H.; Zhang, J.; Yang, W.; Schountz, T.; Ma, W. Incompatible packaging signals and impaired protein functions hinder reassortment of bat H17N10 or H18N11 segment 7 with human H1N1 influenza A viruses. J. Virol. 2024, 98, e0086424. [Google Scholar] [CrossRef]
- Watanabe, T.; Watanabe, S.; Ito, H.; Kida, H.; Kawaoka, Y. Influenza a virus can undergo multiple cycles of replication without M2 ion channel activity. J. Virol. 2001, 75, 5656–5662. [Google Scholar] [CrossRef]
- Gannage, M.; Dormann, D.; Albrecht, R.; Dengjel, J.; Torossi, T.; Ramer, P.C.; Lee, M.; Strowig, T.; Arrey, F.; Conenello, G.; et al. Matrix Protein 2 of Influenza A Virus Blocks Autophagosome Fusion with Lysosomes. Cell Host Microbe 2009, 6, 367–380. [Google Scholar] [CrossRef]
- Wang, R.F.; Zhu, Y.X.; Lin, X.; Ren, C.W.; Zhao, J.C.; Wang, F.F.; Gao, X.C.; Xiao, R.; Zhao, L.Z.; Chen, H.C.; et al. Influenza M2 protein regulates MAVS-mediated signaling pathway through interacting with MAVS and increasing ROS production. Autophagy 2019, 15, 1163–1181. [Google Scholar] [CrossRef]
- Jing, X.H.; Ma, C.L.; Ohigashi, Y.; Oliveira, F.A.; Jardetzky, T.S.; Pinto, L.H.; Lamb, R.A. Functional studies indicate amantadine binds to the pore of the influenza A virus M2 proton-selective ion channel. Proc. Natl. Acad. Sci. USA 2008, 105, 10967–10972. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).