2. Materials and Methods
Preparation of alkoxyamines and reference samples. All reagents including anhydrous solvents used for the chemical synthesis were purchased from commercially available sources such as Merck, TCI, and Fluorochem, and were used without further purification. All air and/or water-sensitive reactions were carried out under an argon atmosphere with dry solvents using standard syringe-cannula/septa techniques. Routine monitoring of reactions was performed using Merck Silica gel 60 F254, aluminum-supported TLC plates; spots were visualized using UV light and ethanolic acidic para-anisaldehyde solution or ethanolic phosphomolybdic solution, followed by heating. Purifications by means of column chromatography were performed with Silica gel 60 (230–400 mesh). 1H, 13C, and 19F NMR spectra were obtained in CDCl3, MeOD (with residual H2O), D2O, DMSO-d6, or C6D6 solutions on by using Bruker AC400 (400 MHz) and Bruker AC300 (300 MHz) spectrometers. High-resolution mass spectra (HRMS) have been performed using a SYNAPT G2 HDMS (Waters) mass spectrometer equipped with a pneumatically assisted atmospheric pressure ionization (API) source. The sample was ionized in positive electrospray mode under the following conditions: electrospray voltage: 2.8 kV; port voltage: 20 V; Nebulizing gas flow rate (nitrogen): 100 L/h or in negative electrospray mode under the following conditions: electrospray voltage: −2.27 kV; port voltage: −20 V; Nebulizing gas flow rate (nitrogen): 100 L/h. The mass spectra (MS) were obtained with a time-of-flight (TOF) analyzer. The exact mass measurements were carried out in triplicate with external calibration. Homolysis constants were measured by EPR on an EMX Bruker spectrometer from 10−4 M samples in tertbutyl benzene or in a mixture of H2O-nPrOH (1:1; v:v).
General ethyl chloroformate/triethylamine procedure GP1. A solution of N-Boc-phenylalanine (1.3 eq) in THF (150 mL) is cooled to −15 °C under an argon atmosphere. Triethylamine (1.3 eq) is added, and then the ethyl chloroformate (1.3 eq). The reaction mixture is stirred at −15 °C for 1 h. Then, alkylaniline (1.0 eq) dissolved in THF (20 mL) is added and the reaction is stirred for 1 h at −15 °C, then at room temperature overnight. The solvent is removed in vacuo, and the residue is taken up in DCM. The organic layer is washed sequentially with 1 M HCl twice, saturated NaHCO3 solution twice and brine twice, dried over MgSO4, and evaporated under reduced pressure. The purification is performed by flash column chromatography (EtOAc/Petroleum ether).
General Salen/MnCl2 alkoxyamine synthesis procedure GP2. To a stirred solution of N,N’-bis(salicylidene)ethylene diamine (salen ligand, 0.25 eq.) in THF (10 mL) is added MnCl2 (0.25 eq) in an open flask. After 10 min of stirring at room temperature, a solution of TEMPO (1.0 eq) and 4-vinylanilido phenylalanine-Boc (1.2 eq) in THF (50 mL) is added, and then solid NaBH4 is added (4.0 eq) in four portions, every 10 min. The mixture is stirred between 24 h and 48 h at room temperature. At the end, the solution is dissolved in EtOAc (100 mL) in an Erlenmeyer and carefully quenched at 0 °C by the addition of 1 M HCl until the solution becomes colorless or slightly orange. Then, NaHCO3 is added up to neutral pH and the organic layer is washed with water (80 mL) and brine (80 mL). The organic layer is dried on MgSO4, filtered, and evaporated to dryness. The purification is performed by flash column chromatography (EtOAc/Petroleum ether).
General Boc deprotection procedure GP3. The Boc-residue (1.0 eq) is dissolved in DCM (45 mL, VDCM = 4VTFA) and TFA (10.0 eq) is added under air. The reaction mixture is stirred at room temperature. Once the substrate is fully consumed, toluene (25 mL) is added, and the solvents are removed in vacuo. The co-evaporation is repeated twice. The purification by flash column chromatography (EtOAc/Petroleum ether) is performed.
General DCC/HOBt procedure GP4. Diisopropylethylamine (1.0 eq) is added to a stirred solution of TFA salt (1.0 eq) under argon. The mixture is stirred for 10 min at room temperature, then Boc-dipeptide (1.1 eq) and HOBt (1.1 eq) are added and stirred until dissolved. The mixture is cooled to 0 °C, and DCC or EDCI (1.1 eq) is added. The mixture is stirred overnight at room temperature. The mixture is filtered with cold DCM, and washed with 1 M HCl, NaHCO3 (saturated solution), and brine. The purification is performed by flash column chromatography (EtOAc/Petroleum ether).
General TFA removing procedure GP5. 1 M NaOH solution (6 mL) is added to a stirred solution of TFA salt (1.0 eq) under air. The mixture is stirred for 10 min at room temperature, the mixture becomes cloudy. Then, a few drops of MeOH are added and the aqueous phase is extracted three times with DCM (5 mL). The organic layer is dried with MgSO4, filtered, and the solvent is evaporated to dryness.
General hydrogenation procedure GP6. To a solution of the benzylated compound (1.0 eq) in MeOH (20 mL) is added Pd/(C) (10% weight). Then, the atmosphere in the flask is replaced by the H2 atmosphere and stirred. Once the substrate is fully consumed, the mixture is filtrated through a pad of celite, rinsed with MeOH, and the solvent is evaporated to dryness.
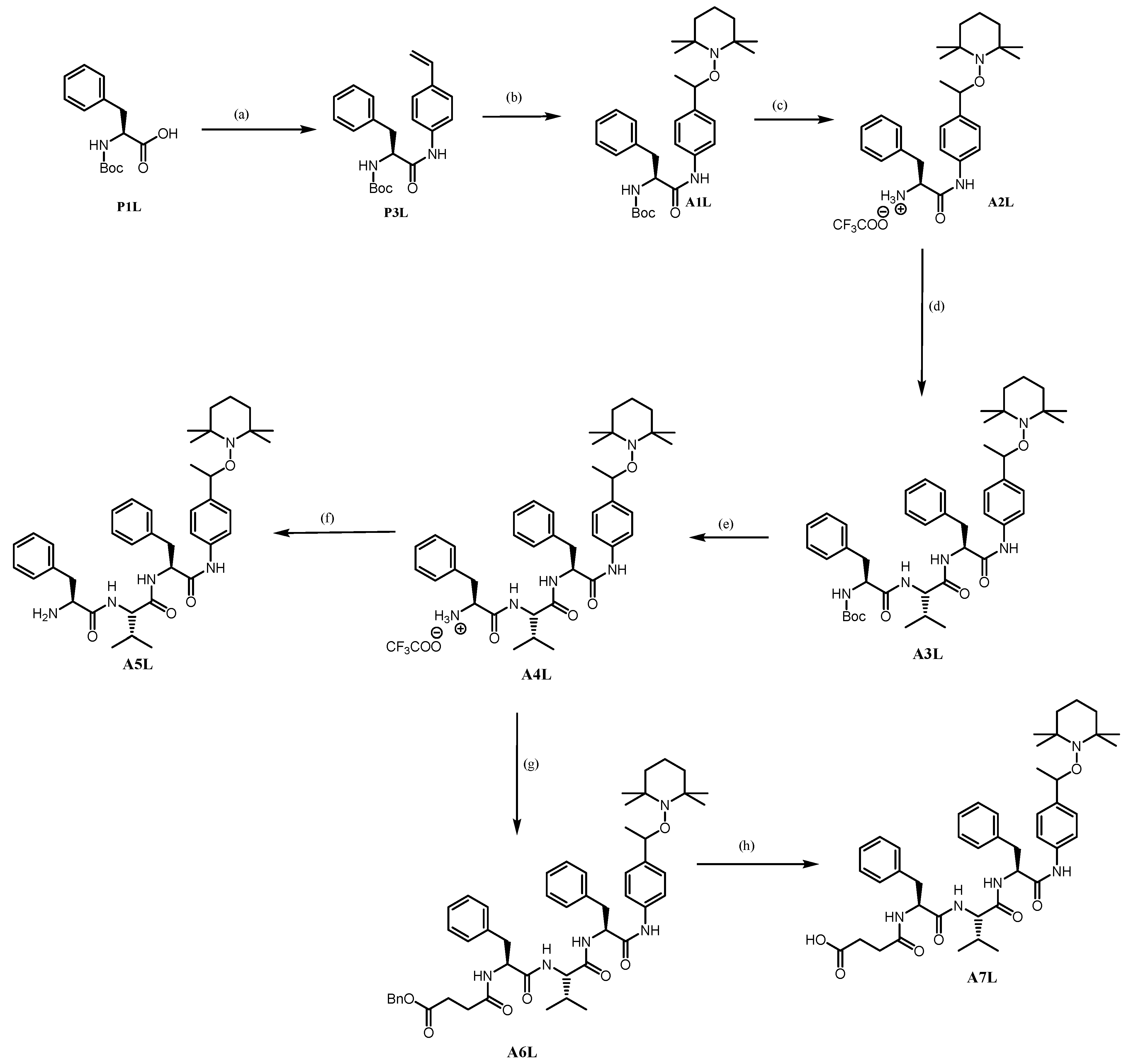
tert-butyl (S)-(1-oxo-3-phenyl-1-((4-vinylphenyl)amino)propan-2-yl)carbamate P3L. By using the general ethyl chloroformate/triethylamine procedure on N-Boc-L-phenylalanine P1L (12.44 g, 46.910 mmol, 1.3 eq) and 4-vinylaniline (4.30 g, 36.085 mmol, 1.0 eq) afforded P3L as a white solid (11.89 g, yield: 90%).
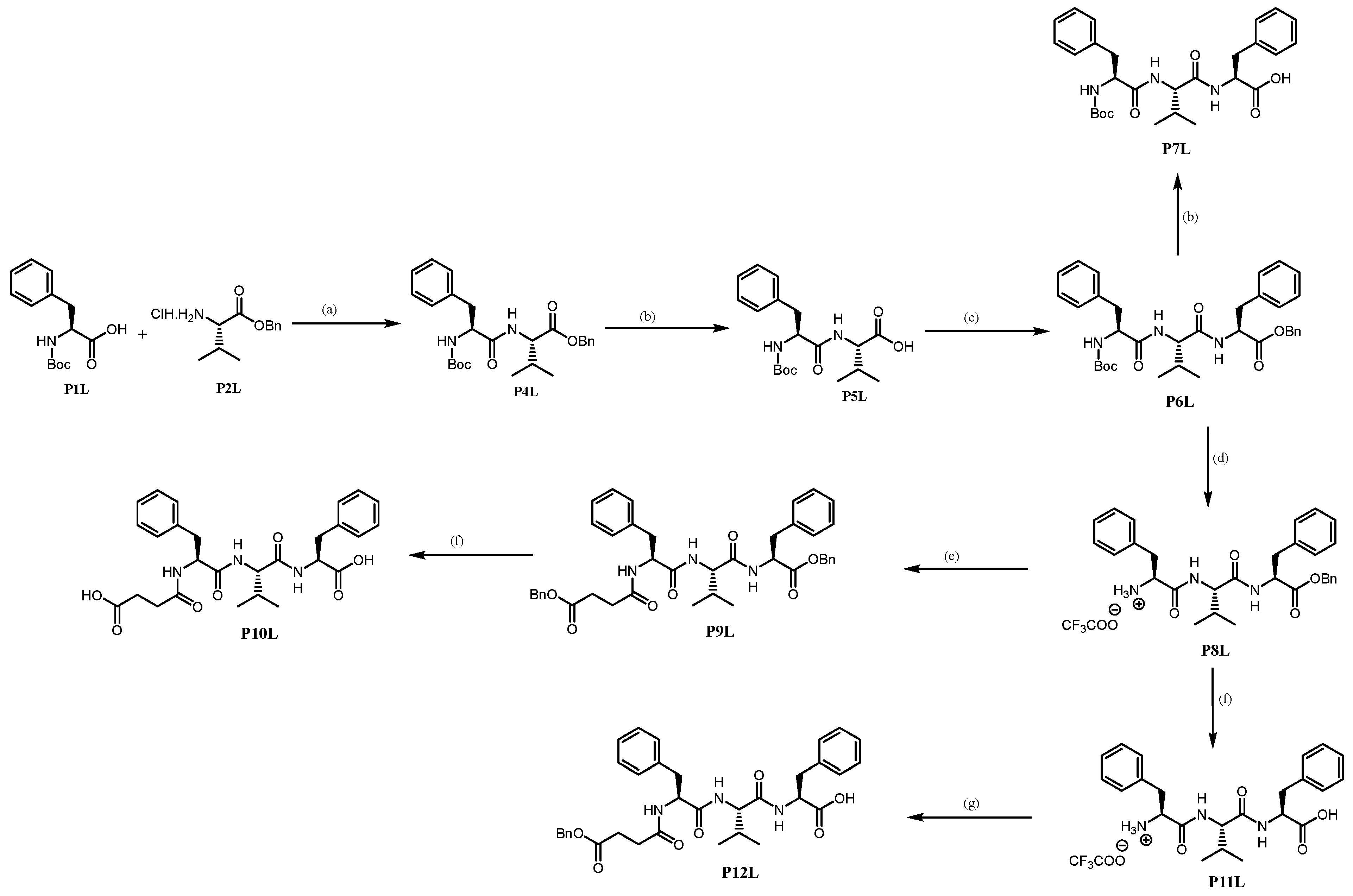
Benzyl (tert-butoxycarbonyl)-L-phenylalanyl-L-valinate P4L. Using GP4 with HCl.H
2N-L-Val-Bn
P2L (10.00 g, 41.03 mmol, 1.0 eq) and BocHN-L-Phe-OH
P1L (11.97 g, 45.13 mmol, 1.1 eq). The purification by column chromatography (EtOAc/Petroleum ether) of crude
P4L affords a white solid (18.07 g, 97%) as reported in the literature [
13].
(Tert-butoxycarbonyl)-L-phenylalanyl-L-valine P5L. Using GP6 with
P4L (12.00 g, 26.417 mmol, 1.0 eq) affords
P5L as a white solid (9.62 g, quantitative yield) as described in the literature [
14].
Benzyl (tert-butoxycarbonyl)-L-phenylalanyl-L-valyl-L-phenylalaninate P6L. Using GP4 with L-Phenylalanine benzyl ester hydrochloride (2.000 g, 6.854 mmol, 1.0 eq) and P5L. The purification by flash column chromatography (DCM/MeOH, 98: 2, v/v) of the crude P6L affords a white solid (3.00 g, yield 73%).
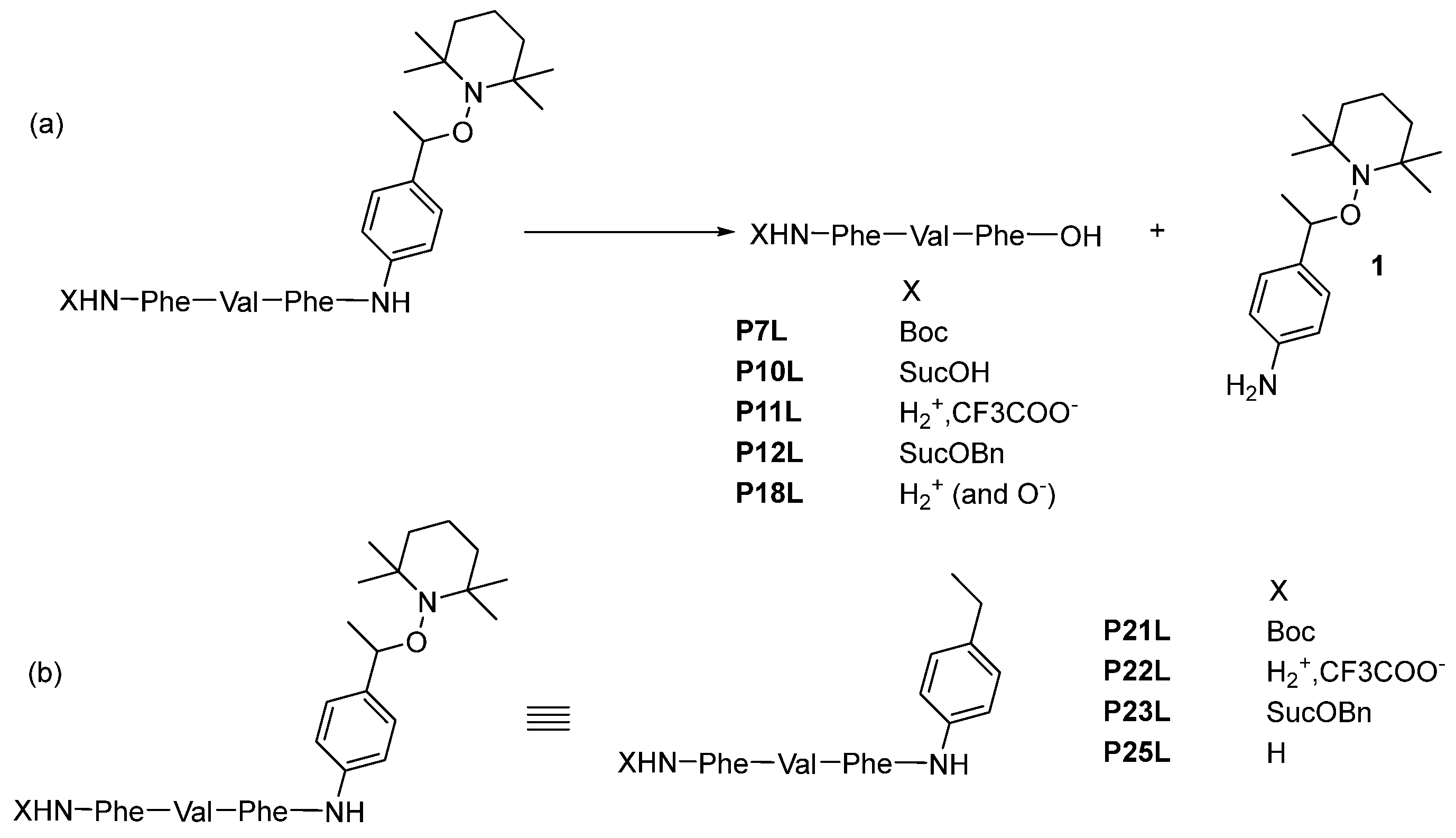
(Tert-butoxycarbonyl)-L-phenylalanyl-L-valyl-L-phenylalanine P7L. Using GP6 with
P6L (0.53 g, 0.881 mmol, 1.0 eq) and Pd/(C) (0.053 g, 10% weight) and the solvent is evaporated to dryness to afford the pure
P7L as white solid (0.44 g, 90%) as reported in the literature [
15].
(S)-1-(((S)-1-(((S)-1-(benzyloxy)-1-oxo-3-phenylpropan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)amino)-1-oxo-3-phenylpropan-2-aminium trifluoroacetate P8L. By usingGP3 with P6L (0.60 g, 0.997 mmol, 1.0 eq) afforded P8L as a white solid (0.49 g, yield 79%).
Benzyl 4-(((S)-1-(((S)-1-(((S)-1-(benzyloxy)-1-oxo-3-phenylpropan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-4-oxobutanoate P9L. Using GP4 (DCC is replaced by EDCI.HCl) with P8L (0.20 g, 0.391 mmol, 1.0 eq) affords crude P9L. The purification by column chromatography (DCM/MeOH) affords P9L as a white solid (0.17 g, yield 62%).
4-(((S)-1-(((S)-1-(((S)-1-carboxy-2-phenylethyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-4-oxobutanoic acid P10L. Using GP6 with P9L (0.12 g, 0.173 mmol, 1.0 eq) affords P10L as a white solid (0.08 g, yield 94%).
(S)-1-(((S)-1-(((S)-1-carboxy-2-phenylethyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-1-oxo-3-phenylpropan-2-aminium trifluoroacetate P11L. Using GP6 with P8L (2.50 g, 4.063 mmol, 1.0 eq) affords P11L as a white solid (2.00 g, yield 94%).
(4-(benzyloxy)-4-oxobutanoyl)-L-phenylalanyl-L-valyl-L-phenylalanine P12L. 4-(benzyloxy)-4-oxobutanoic acid (0.087 g, 0.418 mmol, 1.1 eq) and HOBt (0.056 g, 0.418 mmol, 1.1 eq) are dissolved in DCM, under argon, and stirred until clearance. The mixture is cooled to 0 °C and EDCI.HCl (0.080 g, 0.418 mmol, 1.1 eq) was added. The mixture is stirred for 1 h at 0 °C. Then, a solution of diisopropylethylamine (0.06 mL, 0.380 mmol, 1.0 eq) and peptide TFA salt P11L (0.20 g, 0.380 mmol, 1.0 eq) in DCM is added dropwise. The mixture is stirred for 30 min at 0 °C and overnight at room temperature. The mixture is washed with 1 M HCl and brine. The organic layer was dried with MgSO4, filtered, and the solvent was evaporated to dryness. The purification by column chromatography (DCM/MeOH) affords P12L as a white solid (0.108 g, 47%).
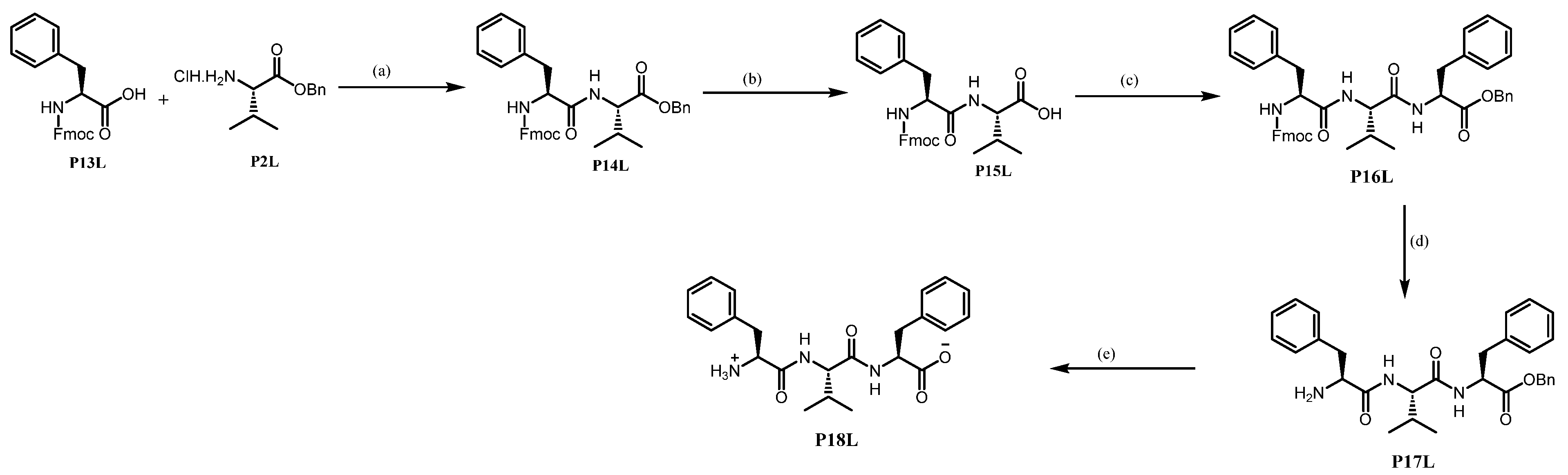
Benzyl (((9H-fluoren-9-yl)methoxy)carbonyl)-L-phenylalanyl-L-valinate P14L. Using GP4 with HCl.H2N-L-Val-Bn P2L (2.00 g, 8.205 mmol, 1.0 eq), Fmoc-L-Phe-OH P13L (3.49 g, 9.025 mmol, 1.1 eq) and HOBt (1.22 g, 9.025 mmol, 1.1 eq) affords P14L. The purification by column chromatography (EtOAc/Petroleum ether) affords P14L as a white solid (4.06 g, 86%).
(((9H-fluoren-9-yl)methoxy)carbonyl)-L-phenylalanyl-L-valine P15L. Using GP6 with
P14L (3.80 g, 6.589 mmol, 1.0 eq) affords
P15L as a white solid (3.15 g, yield 98%). Already described in the literature [
16].
Benzyl (((9H-fluoren-9-yl)methoxy)carbonyl)-L-phenylalanyl-L-valyl-L-phenylalaninate P16L. Using GP4 with Fmoc-L-Phe-L-Val-OH P15L (0.16 mg, 0.328 mmol, 1.0 eq) and HCl.H2N-L-Phe-Bn (0.11 mg, 0.360 mmol, 1.1 eq) affords crude P16L. The purification by column chromatography (DCM/MeOH) yields P16L as a white solid (0.17 g, 71%).
L-phenylalanyl-L-valyl-L-phenylalanine P18L. To a solution of the amino acid Fmoc-L-Phe-L-Val-L-Phe-OBn P16L (1.300 g; 1.795 mmol; 1.0 eq.) in DCM (15 mL) at 0 °C under Argon was added in one fraction DBU (0.29 mL; 1.975 mmol; 1.1 eq.). After 3 h, the mixture was directly loaded on a silica gel column chromatography (DCM/MeOH) to obtain the primary amine. Then, GP6 is applied to this product (0.25 g, 0.498 mmol, 1.0 eq) affording P18L as a white solid (0.11 g, yield 52%).
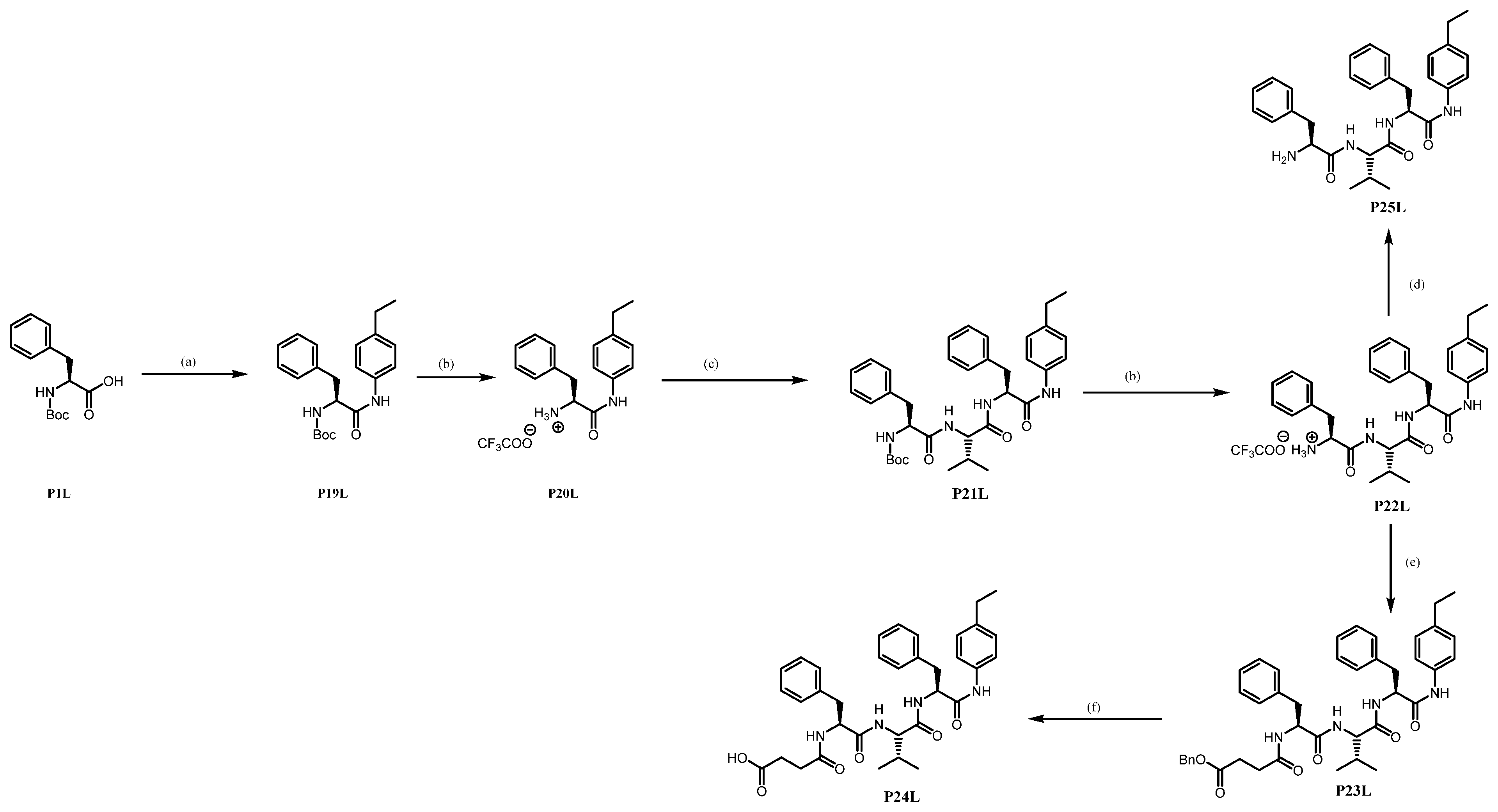
tert-butyl (S)-(1-oxo-3-phenyl-1-((4-vinylphenyl)amino)propan-2-yl)carbamate P19L. Using GP1, N-Boc-phenylalanine P1L (11.38 g, 42.911 mmol, 1.3 eq), triethylamine (5.79 mL, 42.911 mmol, 1.3 eq) and ethyl chloroformate (3.81 mL, 42.911 mmol, 1.3 eq) provides P19L as a white solid (10.72 g, 88%).
(S)-1-((4-ethylphenyl)amino)-1-oxo-3-phenylpropan-2-aminium trifluoroacetate P20L: Using GP3 with the Boc-residue P19L (5.00 g, 13.58 mmol, 1.0 eq) and TFA (11.65 mL, 152.23 mmol, 10.0 eq) afforded P20L as a white solid (5.20 g, quant).
tert-butyl ((S)-1-(((S)-1-(((S)-1-((4-ethylphenyl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)carbamate P21L. By using GP4 with P20L (2.00 g, 5.233 mmol, 1.0 eq), the Purification by flash column chromatography (EtOAc/Petroleum ether) of the crude product afforded a white solid (3.00 g, yield 93%). Product containing less than 8% residual DCU.
(S)-1-(((S)-1-(((S)-1-((4-ethylphenyl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)amino)-1-oxo-3-phenylpropan-2-aminium trifluoroacetate P22L. By GP3 with P21L (3.20 g, 5.208 mmol, 1.0 eq) afforded P22L as a white solid (1.96 g, yield 60%).
Benzyl 4-(((S)-1-(((S)-1-(((S)-1-((4-ethylphenyl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-4-oxobutanoate P23L. Using GP4 (DCC is replaced by EDCI.HCl) with P22L (1.83 g, 2.917 mmol, 1.0 eq) affords P23L as a white solid (1.05 g, yield 50%).
4-(((S)-1-(((S)-1-(((S)-1-carboxy-2-phenylethyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-4-oxobutanoic acid P24L. Using GP6 with P23L (0.12 g, 0.173 mmol, 1.0 eq) affords P24L as a white solid (0.08 g, yield 94%).
(S)-2-((S)-2-amino-3-phenylpropanamido)-N-((S)-1-((4-ethylphenyl)amino)-1-oxo-3-phenylpropan-2-yl)-3-methylbutanamide P25L. Using GP5 with P22L (0.33 g, 0.525 mmol, 1.0 eq) affords P25L as a white solid (0.25 g, yield 92%).
tert-butyl (R)-(1-oxo-3-phenyl-1-((4-vinylphenyl)amino)propan-2-yl)carbamate P3D. By using GP1 with N-Boc-D-phenylalanine P1D (12.44 g, 46.910 mmol, 1.3 eq) and 4-vinylaniline (4.30 g, 36.085 mmol, 1.0 eq) afforded P3D as a white solid (11.23 g, yield 85%).
Benzyl (tert-butoxycarbonyl)-D-phenylalanyl-D-valinate P4D. Using GP4 with Boc-D-Phenylalanine P1D (7.69 g, 28.99 mmol, 1.1 eq) and D-valine benzyl ester p-toluenesulfonate P2D (10.00 g, 26.35 mmol, 1.0 eq). The purification by column chromatography (EtOAc/Petroleum ether) of crude P4D affords a white solid (10.30 g, 86%).
(Tert-butoxycarbonyl)-D-phenylalanyl-D-valine P5D. Using GP6 with
P4D (9.00 g, 19.808 mmol, 1.0 eq) affords
P4D as a white solid (6.49 g, yield 90%) as described in the literature [
17].
Benzyl (tert-butoxycarbonyl)-D-phenylalanyl-D-valyl-D-phenylalaninate P6D. Using GP4 with L-Phenylalanine benzyl ester hydrochloride P5D (3.00 g, 8.237 mmol, 1.0 eq) affords crude P6D. The purification by flash column chromatography (DCM/MeOH, 98:2, v/v) yields P6D as a white solid (4.22 g, yield 84%).
(Tert-butoxycarbonyl)-D-phenylalanyl-D-valyl-D-phenylalanine P7D. Using GP6 with P6D (2.50 g, 4.157 mmol, 1.0 eq) affords P7D as a white solid (2.12 g, quantitative yield).
(R)-1-(((R)-1-(((R)-1-(benzyloxy)-1-oxo-3-phenylpropan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)amino)-1-oxo-3-phenylpropan-2-aminium trifluoroacetate P8D. By using GP3 with P6D (0.60 g, 0.997 mmol, 1.0 eq) afforded P8D as a crude white solid (0.31 g, yield 50%).
(R)-1-(((R)-1-(((R)-1-carboxy-2-phenylethyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-1-oxo-3-phenylpropan-2-aminium trifluoroacetate P11D. Using GP6 with P8D (0.24 g, 0.377 mmol, 1.0 eq) affords P11D as a white solid (0.17 g, yield 85%).
Tert-butyl ((R)-1-(((R)-1-(((R)-1-((4-ethylphenyl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)carbamate P21D. A solution of the N-Boc-D-Phe-D-Val-D-Phe-OH P7D (2.14 g, 4.185 mmol, 1.2 eq) in THF (50 mL) is cooled to −15 °C under argon atmosphere. Triethylamine (0.56 mL, 4.185 mmol, 1.2 eq) is added, and then ethyl chloroformate (0.37 mL, 4.185 mmol, 1.2 eq). The reaction mixture is stirred at −15 °C. After 1 h, 4-ethylaniline (0.43 g, 3.487 mmol, 1.0 eq) dissolved in THF (15 mL) was added and the reaction was allowed to stir for 1 h at −15 °C, then at room temperature overnight. The solvent is removed in vacuo, and the residue is taken up in DCM. The organic layer is washed sequentially with 1 M HCl twice, saturated NaHCO3 solution twice and brine twice, dried over MgSO4, and evaporated under reduced pressure. The purification by flash column chromatography (EtOAc/Petroleum ether) affords P21D as a white solid (0.47 mg, 22%).
(R)-1-(((R)-1-(((R)-1-((4-ethylphenyl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)amino)-1-oxo-3-phenylpropan-2-aminium trifluoroacetate P22D. By using GP3 with P21D (0.27 g, 0.439 mmol, 1.0 eq) afforded P22D as a white solid; (0.25 g, yield 92%).
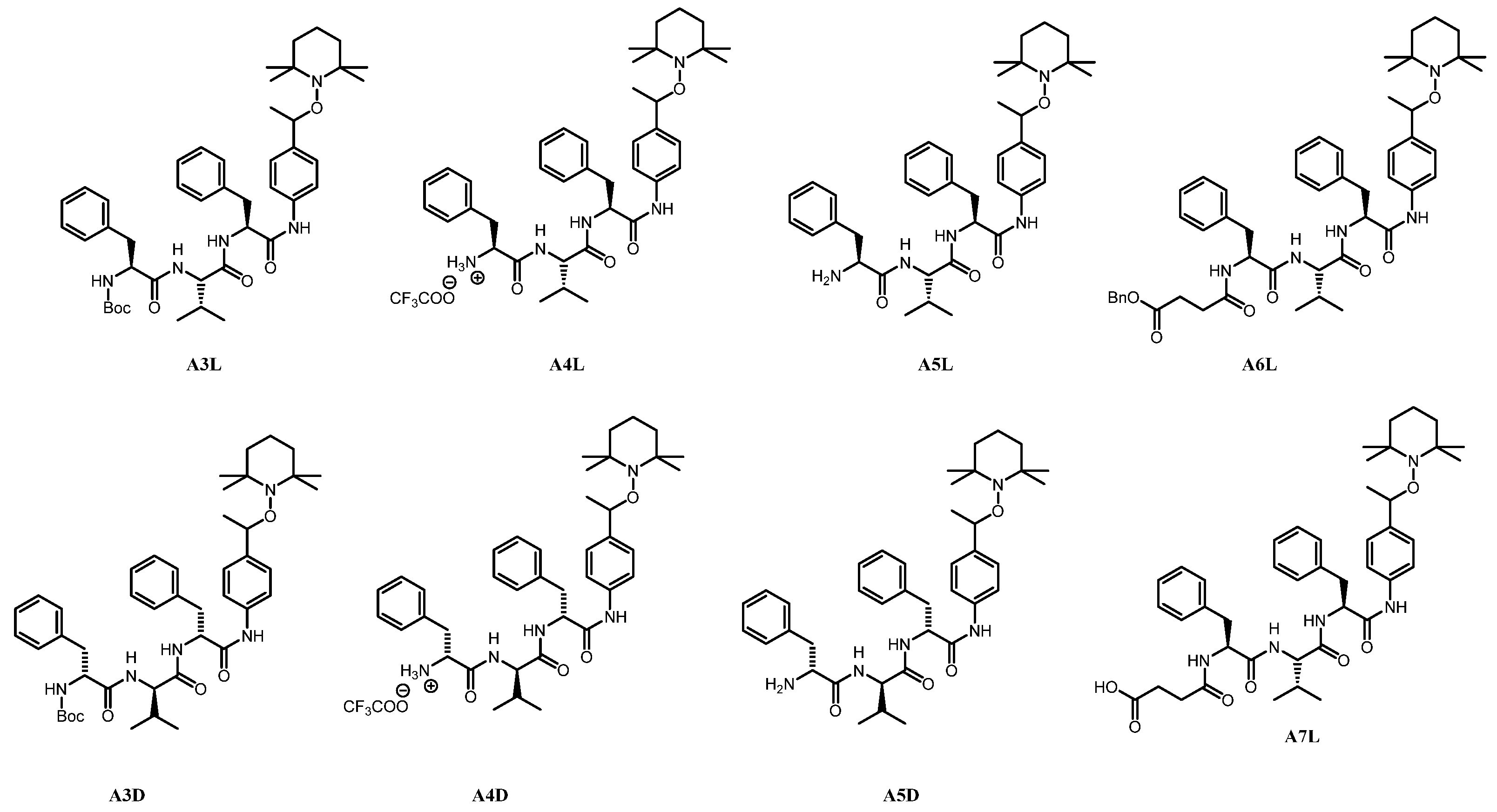
tert-butyl ((2S)-1-oxo-3-phenyl-1-((4-(1-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)ethyl)phenyl)amino)propan-2-yl)carbamate A1L. Using GP2 with salen ligand (0.64 g, 2.389 mmol, 0.25 eq), MnCl2 (0.47 g, 2.389 mmol, 0.25 eq), TEMPO (1.49 g, 9.56 mmol, 1.0 eq) and 4-vinylanilido phenylalanine-Boc P3L (4.20 g, 11.469 mmol, 1.2 eq) afforded A1L as a white solid (2.70 mg, 54%, mixture of diastereomers, 1:1).
(2S)-1-oxo-3-phenyl-1-((4-(1-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)ethyl)phenyl)amino)propan-2-aminium trifluoroacetate A2L. By using GP3 with A1L (0.86 g, 1.643 mmol, 1.0 eq) afforded A2L as a white solid (0.71 g, 81%). The product was directly used in the coupling reaction without characterization.
tert-butyl ((2S)-1-(((2S)-3-methyl-1-oxo-1-(((2S)-1-oxo-3-phenyl-1-((4-(1-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)ethyl)phenyl)amino)propan-2-yl)amino)butan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)carbamate A3L. Using GP4 with A2L (0.72 g, 1.336 mmol, 1.0 eq) and DCC was replaced by EDCI.HCl to prevent the formation of DCU (a by-product that is difficult to remove entirely) affords crude A3L. The Purification by flash column chromatography (EtOAc/Petroleum ether) of the crude product afforded a white solid (0.76 g, yield 74%).
(2S)-1-(((2S)-3-methyl-1-oxo-1-(((2S)-1-oxo-3-phenyl-1-((4-(1-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)ethyl)phenyl)amino)propan-2-yl)amino)butan-2-yl)amino)-1-oxo-3-phenylpropan-2-aminium trifluoroacetate A4L. By using GP3 with A3L (0.15 g, 0.195 mmol, 1.0 eq) afforded A4L as a white solid (0.091 g, yield 60%).
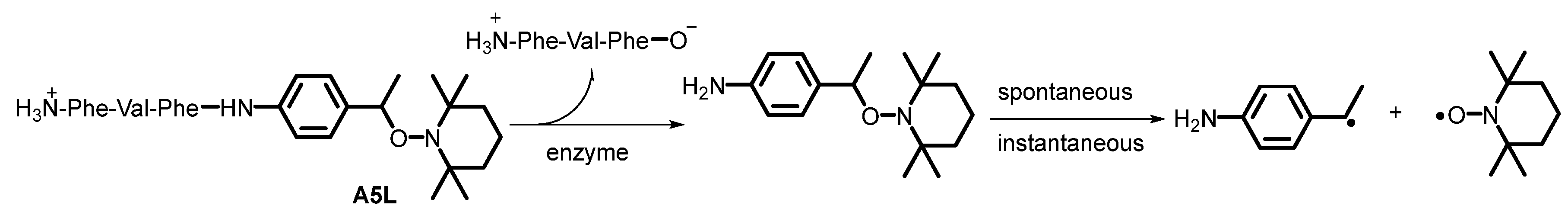
(2S)-2-((S)-2-amino-3-phenylpropanamido)-3-methyl-N-((2S)-1-oxo-3-phenyl-1-((4-(1-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)ethyl)phenyl)amino)propan-2-yl)butanamide A5L. Using GP5 with A4L (0.045 mg, 0.057 mmol, 1.0 eq) affords A5L as a white solid (0.033 g, 87%).
Benzyl 4-(((2S)-1-(((2S)-3-methyl-1-oxo-1-(((2S)-1-oxo-3-phenyl-1-((4-(1-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)ethyl)phenyl)amino)propan-2-yl)amino)butan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-4-oxobutanoate A6L. Using GP4 (DCC is replaced by EDCI.HCl) with A4L (1.48 g, 1.887 mmol, 1.0 eq) and 4-(benzyloxy)-4-oxobutanoic acid (0.43 g, 2.075 mmol, 1.1 eq) affords crude A6L. The purification by column chromatography (DCM/MeOH) affords A6L as a white solid (1.20 g, 73%).
4-(((2S)-1-(((2S)-3-methyl-1-oxo-1-(((2S)-1-oxo-3-phenyl-1-((4-(1-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)ethyl)phenyl)amino)propan-2-yl)amino)butan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-4-oxobutanoic acid A7L. Using GP6 with A6L (0.521 g, 0.605 mmol, 1.0 eq) affords A7L as a white solid (0.422 g, yield 90%).
tert-butyl ((2R)-1-oxo-3-phenyl-1-((4-(1-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)ethyl)phenyl)amino)propan-2-yl)carbamate A1D. By using GP2 with salen ligand (0.64 g, 2.39 mmol, 0.25 eq.), MnCl2 (0.47 g, 2.389 mmol, 0.25 eq), TEMPO (1.49 g, 9.56 mmol, 1.0 eq) and 4-vinylaniline D-phenylalanine-Boc P3D (3.50 g, 9.557 mmol, 1.2 eq) afforded A1D as a white solid (2.23 g, 58%, mixture of diastereomers, 1:1).
(2R)-1-oxo-3-phenyl-1-((4-(1-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)ethyl)phenyl)amino)propan-2-aminium trifluoroacetate A2D. Using GP3 with A1D (1.50 g, 2.866 mmol, 1.0 eq) is afforded A2D as a white solid (1.33 g, yield: 86%). The product was used in coupling reaction without characterization.
tert-butyl ((2R)-1-(((2R)-3-methyl-1-oxo-1-(((2R)-1-oxo-3-phenyl-1-((4-(1-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)ethyl)phenyl)amino)propan-2-yl)amino)butan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)carbamate A3D. Using GP4 with A2D (1.333 g, 2.481 mmol, 1.0 eq) affords crude A3D. The Purification by flash column chromatography (EtOAc/Petroleum ether) yields A3D as a white solid (1.300 g, yield 68%, mixture of diastereomers, 1:1).
(2R)-1-(((2R)-3-methyl-1-oxo-1-(((2R)-1-oxo-3-phenyl-1-((4-(1-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)ethyl)phenyl)amino)propan-2-yl)amino)butan-2-yl)amino)-1-oxo-3-phenylpropan-2-aminium trifluoroacetate A4D. Using GP3 with A3D (0.30 g, 0.389 mmol, 1.0 eq) is afforded A4D as a white solid (0.16 g, 52%).
(2R)-2-((R)-2-amino-3-phenylpropanamido)-3-methyl-N-((2R)-1-oxo-3-phenyl-1-((4-(1-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)ethyl)phenyl)amino)propan-2-yl)butanamide A5D. Using GP5 with A4D (0.035 g, 0.052 mmol, 1.0 eq) affords A5D as a white solid (0.024 g, yield 80%).
4-(Benzyloxy)-4-oxobutanoic acid. Succinic anhydride (8.00 g, 79.944 mmol, 1.0 eq) was dissolved in the mixture of anhydrous dichloromethane (10 mL) and Benzyl alcohol (12 mL). Triethylamine (3.2 mL, 23.983 mmol, 0.3 eq) and DMAP (9.76 g, 79.944 mmol, 1.0 eq) were added to this solution. The resulting clear solution was stirred at ambient temperature overnight. After which time, the mixture was dissolved in EtOAc (150 mL) and washed with a saturated solution of NaHCO3 (3 × 50 mL). The aqueous extracts were carefully acidified to pH 2 with concentrated HCl and then extracted with DCM (3 × 50 mL) and subsequently dried (Mg2SO4), filtered, and concentrated to afford the title compound (16.23 g, 97%) as a white solid with no further purification needed.
Kinetic experiments. Kinetics of
A1–
A7 (
Scheme 2) for series L and D are performed by monitoring the growth of nitroxide by Electron Paramagnetic Resonance (X-band EMX Bruker machine) in
tert-butylbenzene and a water/
n-PrOH (for a good solubility of the alkoxyamine) as solvents and using O
2 as alkyl radical scavenger to suppress the back reaction (
kc in
Scheme 2) as already reported [
18]. Homolysis rate constants
kd are given by Equation (1) ([nitroxide]
∞ = [alkoxyamine]
0 = 0.1 mM, see
Table S1) and the subsequent activation energies
Ea given by Equation (2).
Cytotoxicity experiments.
A non-cancer line of Vero cells was used to determine the cytotoxicity of the compound against mammalian cells. They were evaluated at a final DMSO concentration of 0.5%. The culture medium was MEM (Dutscher, Bernolsheim, France) supplemented with 10% fetal bovine serum (Fisher Scientific, Illkirch, France), 1X non-essential amino acids (Fisher Scientific, Illkirch, France), 100 U/mL, 100 µg/mL penicillin/streptomycin (Fisher Scientific, Illkirch, France), and 2 mM L-glutamine (Fisher Scientific, Illkirch, France) at 37 °C in a humidified 5% CO
2 atmosphere [
19]. Vero cells (100 µL of 10
5 cells/mL per well) were plated in 96-well plates for 24 h then treated with 100 µL of compound dilutions (in duplicate) during 48 h. All molecules were tested from 5 nM to 50 µM. Each well was then examined under the microscope to detect any precipitate formation before the supernatant was removed by flicking the plate. A volume of 100 µL of 0.5 mg/mL in MEM from a stock solution at 5 mg/mL of PBS-dissolved MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma Aldrich/Merck, Darmstadt, Germany) was then added to each well [
20].
After incubation for 1 h at 37 °C and 5% CO2 the supernatant was removed and 100 µL of DMSO were added. Plates were gently shaken to dissolve formazan crystals resulting from the MTT reduction by living cells and read at 570 nm with the VICTOR Nivo plate reader (Perkin Elmer, Waltham, MA, USA). CC50 values were determined using GraphPad Prism 7 software (San Diego, CA, USA).
Experiments on the viability of Schistosoma mansoni adult worms. In vitro tests were performed on
Schistosoma mansoni NMRI strains. This parasite is lab maintained on
Biomphalaria glabrata (BRE strain) as intermediate mollusc host and Golden Hamster (Janvier Labs, Le Genest-Saint-Isle, France) as definitive vertebrate host. Methods, for molluscs’ and hamsters’ infection and for parasite recovery, were previously described [
21]. Adult worms were recovered by hepatic perfusion technique between 42 and 47 days post hamster exposition to parasite larvae. Worms were carefully collected and disposed in 12-well Falcon
® plate containing 2 mL of RPMI 1640 (supplemented with L-glutamine and Hepes 25 mM). The plates containing a minimum of 15 worms by well were then placed in an incubator chamber at 37 °C and 5% CO
2. The worms sex ratio was almost balanced in each well. The drug was first dissolved in DMSO (Sigma-Aldrich) to give a 100 mg/mL mother solution. Then the stock solution was complemented with Tween 80 and RPMI to obtain the following final ratio dilution: RPMI 1640/Tween 80/DMSO and 1000/0.95/3.8,
v/
v/
v.
All molecules were tested at a final concentration of 100 µg/mL and 10 µg/mL. A negative control consisted in adding the same RPMI 1640/Tween 80/DMSO solution, but without any drug and a positive control consisted in adding praziquantel [
22]. All molecules and control were performed in duplicates of well. The worm viability was checked after 1, 2, 3, 4, 5, 6, 7, 8, 24, 48, and 72 h after adding the molecule. Parasites exhibiting no body contractions during a 30 s observation were considered as dead.