Alterations in N-glycosylation of HCV E2 Protein in Children Patients with IFN-RBV Therapy Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Samples
2.2. Antibodies
2.3. Cell Lines
2.4. Construction of Chimeric E1E2 Expression Plasmids
2.5. In Situ Immunostaining of Cells
2.6. E1E2 Immunoprecipitation
2.7. GNA-Capture ELISA
2.8. SDS-PAGE and Western Blotting Analysis
2.9. Glycosylation Status Analysis
2.10. CD81-LEL-GST Fusion Protein Production
2.11. CD81-Binding Assay
2.12. ELISA with Conformation-Sensitive Antibodies
2.13. Statistics
3. Results
3.1. Collection and Analysis of Patient Samples
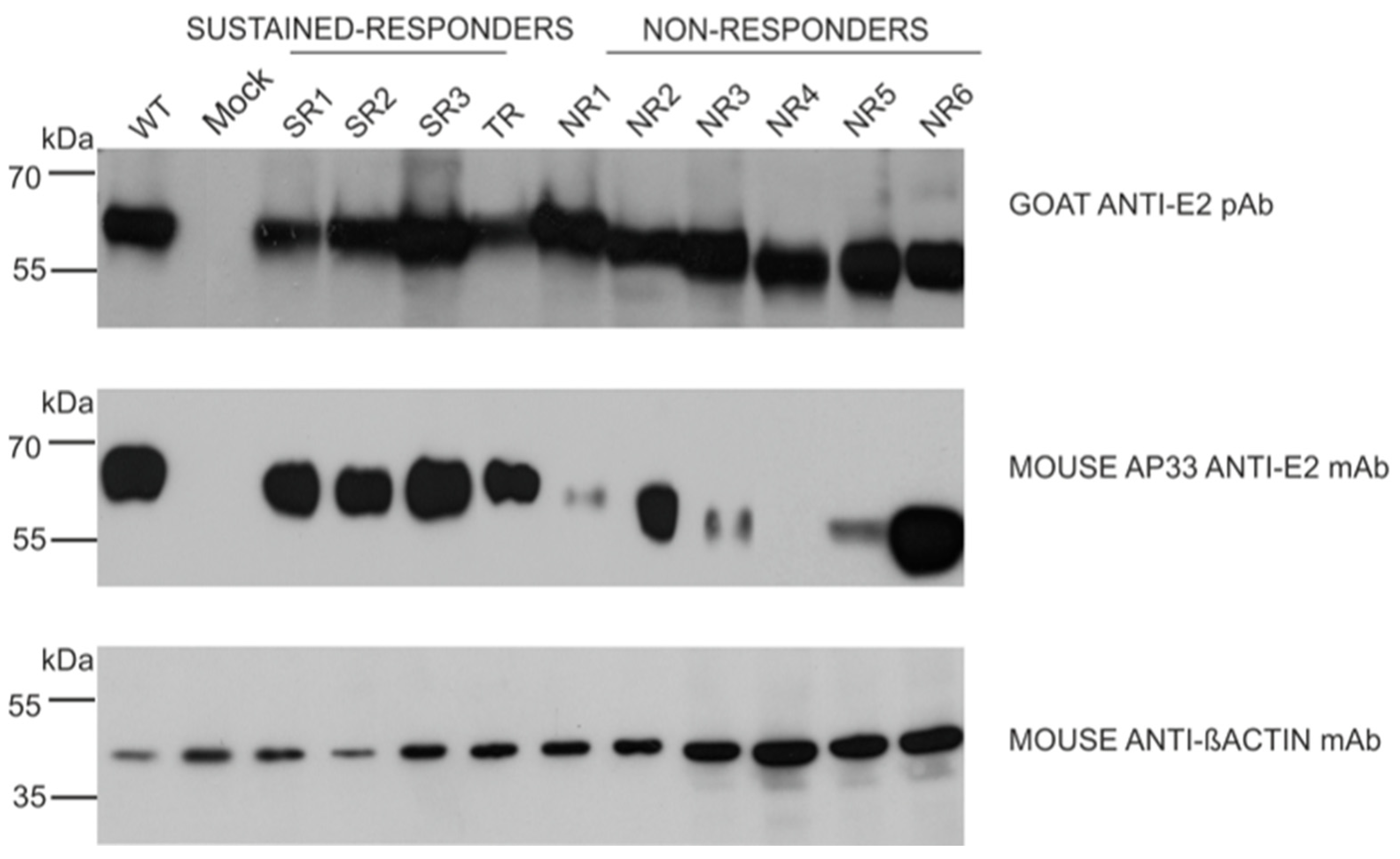
3.2. E1E2 Heterodimer Reconstruction
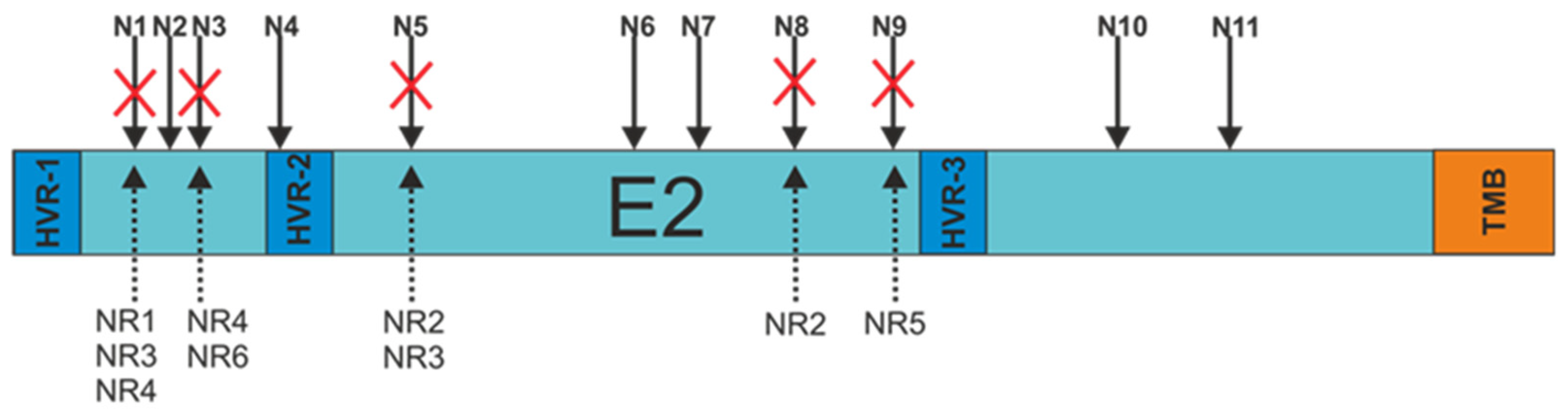
3.3. E2 Glycoprotein Variants N-glycosylation Status Analysis
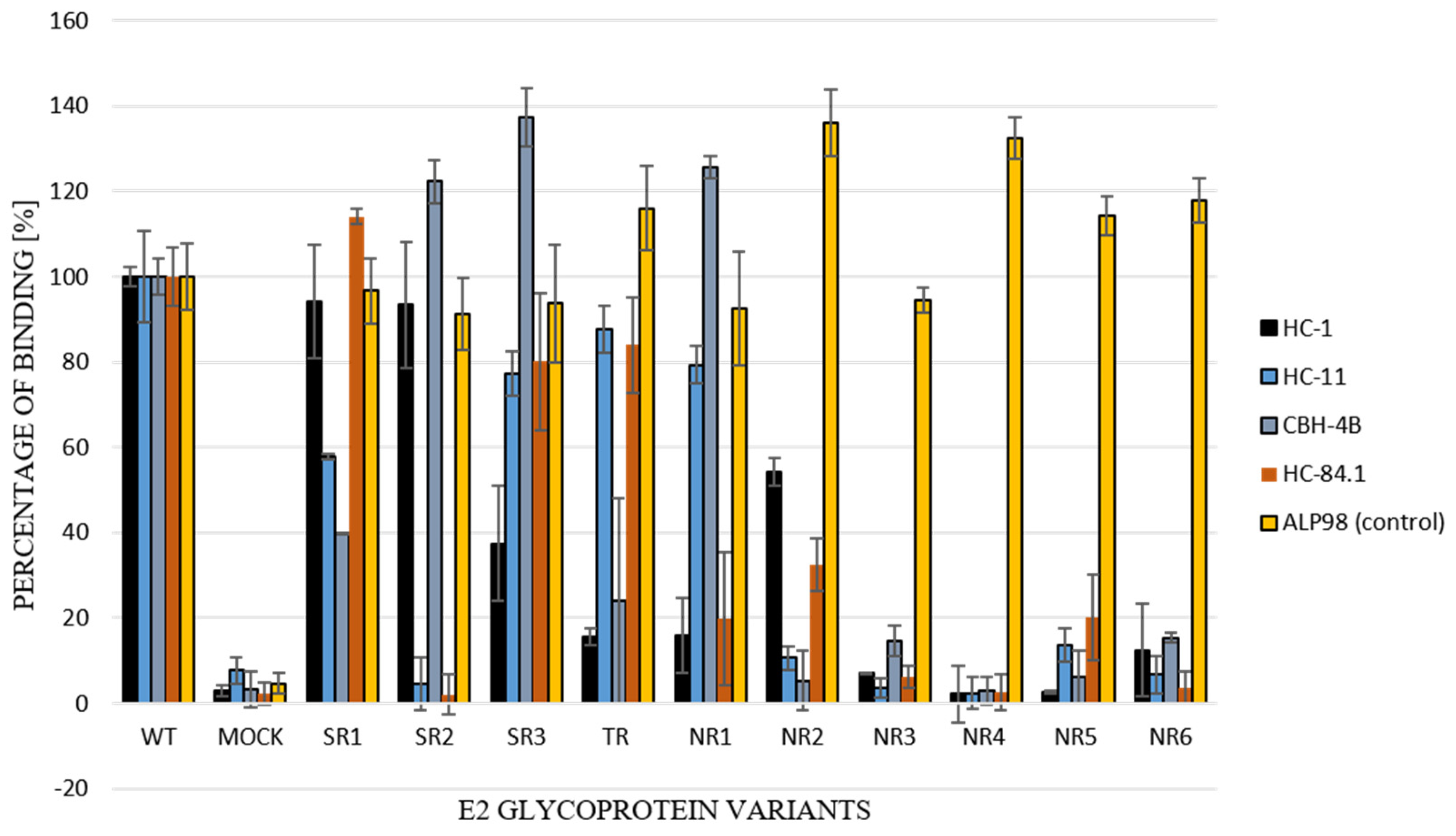
3.4. Functional Analysis of E1E2 Heterodimers
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hepatitis C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 1 February 2024).
- Kenfack-Momo, R.; Ngounoue, M.D.; Kenmoe, S.; Takuissu, G.R.; Ebogo-Belobo, J.T.; Kengne-Ndé, C.; Mbaga, D.S.; Zeuko’o Menkem, E.; Lontuo Fogang, R.; Tchatchouang, S.; et al. Global epidemiology of hepatitis C virus in dialysis patients: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0284169. [Google Scholar] [CrossRef]
- Marshall, A.D.; Willing, A.R.; Kairouz, A.; Cunningham, E.B.; Wheeler, A.; O’Brien, N.; Perera, V.; Ward, J.W.; Hiebert, L.; Degenhardt, L.; et al. Global HCV and HIV Treatment Restrictions Group. Direct-acting antiviral therapies for hepatitis C infection: Global registration, reimbursement, and restrictions. Lancet Gastroenterol. Hepatol. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Moustafa, R.; Dubuisson, J.; Lavie, M. Function of the HCV E1 envelope glycoprotein in viral entry and assembly. Future Virol. 2019, 14, 171–184. [Google Scholar] [CrossRef]
- Pantua, H.; Diao, J.; Ultsch, M.; Hazen, M.; Mathieu, M.; McCutcheon, K.; Takeda, K.; Date, S.; Cheung, T.K.; Phung, Q.; et al. Glycan shifting on hepatitis C virus (HCV) E2 glycoprotein is a mechanism for escape from broadly neutralizing antibodies. J. Mol. Biol. 2013, 425, 1899–1914. [Google Scholar] [CrossRef]
- Anjum, S.; Wahid, A.; Afzal, M.S.; Albecka, A.; Alsaleh, K.; Ahmad, T.; Baumert, T.F.; Wychowski, C.; Qadri, I.; Penin, F.; et al. Additional glycosylation within a specific hypervariable region of subtype 3a of hepatitis C virus protects against virus neutralization. J. Infect. Dis. 2013, 208, 1888–1897. [Google Scholar] [CrossRef]
- LeBlanc, E.V.; Kim, Y.; Capicciotti, C.J.; Colpitts, C.C. Hepatitis C Virus Glycan-Dependent Interactions and the Potential for Novel Preventative Strategies. Pathogens 2021, 10, 685. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; von Schaewen, M.; Wang, X.; Tao, W.; Zhang, Y.; Li, L.; Heller, B.; Hrebikova, G.; Deng, Q.; Ploss, A.; et al. Altered Glycosylation Patterns Increase Immunogenicity of a Subunit Hepatitis C Virus Vaccine, Inducing Neutralizing Antibodies Which Confer Protection in Mice. J. Virol. 2016, 90, 10486–10498. [Google Scholar] [CrossRef] [PubMed]
- Czarnota, A.; Offersgaard, A.; Owsianka, A.; Alzua, G.P.; Bukh, J.; Gottwein, J.M.; Patel, A.H.; Bieńkowska-Szewczyk, K.; Grzyb, K. Effect of Glycan Shift on Antibodies against Hepatitis C Virus E2 412–425 Epitope Elicited by Chimeric sHBsAg-Based Virus-Like Particles. Microbiol. Spectr. 2023, 11, e0254622. [Google Scholar] [CrossRef]
- Figlerowicz, M.; Sluzewski, W.; Kowala-Piaskowska, A.; Mozer-Lisewska, I. Interferon alpha and ribavirin in the treatment of children with chronic hepatitis C. Eur. J. Pediatr. 2004, 163, 265–267. [Google Scholar] [CrossRef]
- Figlerowicz, M.; Jackowiak, P.; Formanowicz, P.; Kędziora, P.; Alejska, M.; Malinowska, N.; Błażewicz, J.; Figlerowicz, M. Hepatitis C virus quasispecies in chronically infected children subjected to interferon-ribavirin therapy. Arch. Virol. 2010, 155, 1977–1987. [Google Scholar] [CrossRef]
- Jackowiak, P.; Kowala-Piaskowska, A.; Figlerowicz, M.; Alejska, M.; Malinowska, N.; Figlerowicz, M. Evolution of hepatitis C virus hypervariable region 1 in chronically infected children. Virus. Res. 2012, 167, 380–384. [Google Scholar] [CrossRef]
- Owsianka, A.; Tarr, A.W.; Juttla, V.S.; Lavillette, D.; Bartosch, B.; Cosset, F.L.; Ball, J.K.; Patel, A.H. Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J. Virol. 2005, 79, 11095–11104. [Google Scholar] [CrossRef]
- Clayton, R.F.; Owsianka, A.; Aitken, J.; Graham, S.; Bhella, D.; Patel, A.H. Analysis of antigenicity and topology of E2 glycoprotein present on recombinant hepatitis C virus-like particles. J. Virol. 2002, 76, 7672–7682. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, K.G.; Lanford, R.E.; Perkins, S.; Rowe, J.; Yang, Q.; Levy, S.; Pileri, P.; Abrignani, S.; Foung, S.K. Human monoclonal antibodies that inhibit binding of hepatitis C virus E2 protein to CD81 and recognize conserved conformational epitopes. J. Virol. 2000, 74, 10407–10416. [Google Scholar] [CrossRef] [PubMed]
- Keck, Z.Y.; Op De Beeck, A.; Hadlock, K.G.; Xia, J.; Li, T.K.; Dubuisson, J.; Foung, S.K. Hepatitis C virus E2 has three immunogenic domains containing conformational epitopes with distinct properties and biological functions. J. Virol. 2004, 78, 9224–9232. [Google Scholar] [CrossRef] [PubMed]
- Keck, Z.Y.; Xia, J.; Wang, Y.; Wang, W.; Krey, T.; Prentoe, J.; Carlsen, T.; Li, A.Y.; Patel, A.H.; Lemon, S.M.; et al. Human monoclonal antibodies to a novel cluster of conformational epitopes on HCV E2 with resistance to neutralization escape in a genotype 2a isolate. PLoS Pathog. 2012, 8, e1002653. [Google Scholar] [CrossRef]
- Yanagi, M.; Purcell, R.H.; Emerson, S.U.; Bukh, J. Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc. Natl. Acad. Sci. USA 1997, 94, 8738–8743. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Thuru, X. Targeting of Tetraspanin CD81 with Monoclonal Antibodies and Small Molecules to Combat Cancers and Viral Diseases. Cancers 2023, 15, 2186. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Humphreys, I.; Flaxman, A.; Brown, A.; Cooke, G.S.; Pybus, O.G.; Barnes, E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015, 61, 77–87. [Google Scholar] [CrossRef]
- Goffard, A.; Callens, N.; Bartosch, B.; Wychowski, C.; Cosset, F.L.; Montpellier, C.; Dubuisson, J. Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J. Virol. 2005, 79, 8400–8409. [Google Scholar] [CrossRef]
- Deleersnyder, V.; Pillez, A.; Wychowski, C.; Blight, K.; Xu, J.; Hahn, Y.S.; Rice, C.M.; Dubuisson, J. Formation of native hepatitis C virus glycoprotein complexes. J. Virol. 1997, 71, 697–704. [Google Scholar] [CrossRef]
- Dubuisson, J.; Hsu, H.H.; Cheung, R.C.; Greenberg, H.B.; Russell, D.G.; Rice, C.M. Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J. Virol. 1994, 68, 6147–6160. [Google Scholar] [CrossRef] [PubMed]
- Lavie, M.; Hanoulle, X.; Dubuisson, J. Glycan Shielding and Modulation of Hepatitis C Virus Neutralizing Antibodies. Front. Immunol. 2018, 9, 910. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S.; Witteveldt, J.; Gatherer, D.; Owsianka, A.M.; Zeisel, M.B.; Zahid, M.N.; Rychłowska, M.; Foung, S.K.; Baumert, T.F.; Angus, A.G.; et al. Mutations within a conserved region of the hepatitis C virus E2 glycoprotein that influence virus-receptor interactions and sensitivity to neutralizing antibodies. J. Virol. 2010, 84, 5494–5507. [Google Scholar] [CrossRef] [PubMed]
- Pileri, P.; Uematsu, Y.; Campagnoli, S.; Galli, G.; Falugi, F.; Petracca, R.; Weiner, A.J.; Houghton, M.; Rosa, D.; Grandi, G.; et al. Binding of hepatitis C virus to CD81. Science 1998, 282, 938–941. [Google Scholar] [CrossRef]
- Fénéant, L.; Levy, S.; Cocquerel, L. CD81 and hepatitis C virus (HCV) infection. Viruses 2014, 6, 535–572. [Google Scholar] [CrossRef]
- Drummer, H.E.; Boo, I.; Maerz, A.L.; Poumbourios, P. A conserved Gly436-Trp-Leu-Ala-Gly-Leu-Phe-Tyr motif in hepatitis C virus glycoprotein E2 is a determinant of CD81 binding and viral entry. J. Virol. 2006, 80, 7844–7853. [Google Scholar] [CrossRef]
- Kong, L.; Giang, E.; Nieusma, T.; Kadam, R.U.; Cogburn, K.E.; Hua, Y.; Dai, X.; Stanfield, R.L.; Burton, D.R.; Ward, A.B.; et al. Hepatitis C virus E2 envelope glycoprotein core structure. Science 2013, 342, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Ströh, L.J.; Krey, T. Structural insights into hepatitis C virus neutralization. Curr. Opin. Virol. 2023, 60, 101316. [Google Scholar] [CrossRef]
- Khera, T.; Behrendt, P.; Bankwitz, D.; Brown, R.J.P.; Todt, D.; Doepke, M.; Khan, A.G.; Schulze, K.; Law, J.; Logan, M.; et al. Functional and immunogenic characterization of diverse HCV glycoprotein E2 variants. J. Hepatol. 2019, 70, 593–602. [Google Scholar] [CrossRef]
- Zeisel, M.B.; Barth, H.; Schuster, C.; Baumert, T.F. Hepatitis C virus entry: Molecular mechanisms and targets for antiviral therapy. Front. Biosci. 2009, 14, 3274–3285. [Google Scholar] [CrossRef]
- Prentoe, J.; Velázquez-Moctezuma, R.; Augestad, E.H.; Galli, A.; Wang, R.; Law, M.; Alter, H.; Bukh, J. Hypervariable region 1 and N-linked glycans of hepatitis C regulate virion neutralization by modulating envelope conformations. Proc. Natl. Acad. Sci. USA 2019, 116, 10039–10047. [Google Scholar] [CrossRef]
- Pierce, B.G.; Keck, Z.Y.; Lau, P.; Fauvelle, C.; Gowthaman, R.; Baumert, T.F.; Fuerst, T.R.; Mariuzza, R.A.; Foung, S.K.H. Global mapping of antibody recognition of the hepatitis C virus E2 glycoprotein: Implications for vaccine design. Proc. Natl. Acad. Sci. USA 2016, 113, E6946–E6954. [Google Scholar] [CrossRef]
- Muñoz de Rueda, P.; Casado, J.; Patón, R.; Quintero, D.; Palacios, A.; Gila, A.; Quiles, R.; León, J.; Ruiz-Extremera, A.; Salmerón, J. Mutations in E2-PePHD, NS5A-PKRBD, NS5A-ISDR, and NS5A-V3 of hepatitis C virus genotype 1 and their relationships to pegylated interferon-ribavirin treatment responses. J. Virol. 2008, 82, 6644–6653. [Google Scholar] [CrossRef]
- Helle, F.; Vieyres, G.; Elkrief, L.; Popescu, C.I.; Wychowski, C.; Descamps, V.; Castelain, S.; Roingeard, P.; Duverlie, G.; Dubuisson, J. Role of N-linked glycans in the functions of hepatitis C virus envelope proteins incorporated into infectious virions. J. Virol. 2010, 84, 11905–11915. [Google Scholar] [CrossRef] [PubMed]
- Orlova, O.V.; Drutsa, V.L.; Spirin, P.V.; Prasolov, V.S.; Rubtsov, P.M.; Kochetkov, S.N.; Beljelarskaya, S.N. The role of HCV e2 protein glycosylation in functioning of virus envelope proteins in insect and Mammalian cells. Acta Naturae 2015, 7, 87–97. [Google Scholar] [CrossRef]
- Altman, M.O.; Angel, M.; Košík, I.; Trovão, N.S.; Zost, S.J.; Gibbs, J.S.; Casalino, L.; Amaro, R.E.; Hensley, S.E.; Nelson, M.I.; et al. Human Influenza A Virus Hemagglutinin Glycan Evolution Follows a Temporal Pattern to a Glycan Limit. mBio 2019, 10, e00204-19. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Upadhyay, C.; Alcami Pertejo, J.; Hioe, C.E.; Arora, S.K. Heterogeneity in glycan composition on the surface of HIV-1 envelope determines virus sensitivity to lectins. PLoS ONE 2018, 13, e0194498. [Google Scholar] [CrossRef]
- Newby, M.L.; Allen, J.D.; Crispin, M. Influence of glycosylation on the immunogenicity and antigenicity of viral immunogens. Biotechnol. Adv. 2023, 70, 108283. [Google Scholar] [CrossRef]
- Baboo, S.; Diedrich, J.K.; Torres, J.L.; Copps, J.; Singh, B.; Garrett, P.T.; Ward, A.B.; Paulson, J.C.; Yates, J.R., 3rd. Evolving spike-protein N-glycosylation in SARS-CoV-2 variants. bioRxiv 2023. [Google Scholar] [CrossRef]
- Bagdonaite, I.; Wandall, H.H. Global aspects of viral glycosylation. Glycobiology 2018, 28, 443–467. [Google Scholar] [CrossRef] [PubMed]




| Sample No. | Patient No. | Therapy Outcome | Age (years) | Sex | Pretreatment Viremia (copies/mL) | Pretreatment ALT (IU/L) | Pretreatment Anti-HCV Antibodies |
|---|---|---|---|---|---|---|---|
| SR1 | P2-17 | Sustained Response | 8 | Male | 56,300,000 | 19 | + |
| SR2 | P2-18 | Sustained Response | 15 | Female | 696,000 | 54 | + |
| SR3 | P2-20 | Sustained Response | 15 | Female | 152,000 | 37 | + |
| TR | P2-24 | Transient Response | 16 | Female | 286,000 | 28 | + |
| NR1 | P2-10 | No Response | 11 | Male | 2,670,000 | 45 | - |
| NR2 | P2-23 | No Response | 10 | Male | 840,000 | 63 | + |
| NR3 | P2-28 | No Response | 14 | Female | 365,000 | 51 | - |
| NR4 | P2-05 | No Response | 11 | Female | 2,170,000 | 53 | - |
| NR5 | P2-19 | No Response | 10 | Female | 726,000 | 35 | + |
| NR6 | P2-04 | No Response | 13 | Male | 11,280,000 | 315 | + |
| Sample | Lacking N-glycosylation Site (Amino Acid No.) | Wild-type N-glycosylation Site Consensus Sequence | Mutation in N-glycosylation Site Sequence |
|---|---|---|---|
| NR1 | N1 (AA417) | Asn-Gly-Ser | Asn-Gly-Asn |
| NR2 | N5 (AA476) | Asn-Gly-Ser | Asn-Gly-Arg |
| N8 (AA556) | Ans-Ser-Thr | Ser-Ser-Thr | |
| NR3 | N1 (AA417) | Asn-Gly-Ser | Asn-Gly-Asn |
| N5 (AA476) | Asn-Gly-Ser | Asp-Gly-Ser | |
| NR4 | N1 (AA417) | Asn-Gly-Ser | Asn-Gly-Arg |
| N3 (AA430) | Asn-Asp-Ser | Asp-Asp-Ser | |
| NR5 | N9 (AA576) | Asn-Asn-Thr | Asn-Asn-Ala |
| NR6 | N3 (AA430) | Asn-Asp-Ser | Asp-Asp-Ser |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmer, K.; Chmielewska, A.M.; Jackowiak, P.; Figlerowicz, M.; Bienkowska-Szewczyk, K. Alterations in N-glycosylation of HCV E2 Protein in Children Patients with IFN-RBV Therapy Failure. Pathogens 2024, 13, 256. https://doi.org/10.3390/pathogens13030256
Zimmer K, Chmielewska AM, Jackowiak P, Figlerowicz M, Bienkowska-Szewczyk K. Alterations in N-glycosylation of HCV E2 Protein in Children Patients with IFN-RBV Therapy Failure. Pathogens. 2024; 13(3):256. https://doi.org/10.3390/pathogens13030256
Chicago/Turabian StyleZimmer, Karolina, Alicja M. Chmielewska, Paulina Jackowiak, Marek Figlerowicz, and Krystyna Bienkowska-Szewczyk. 2024. "Alterations in N-glycosylation of HCV E2 Protein in Children Patients with IFN-RBV Therapy Failure" Pathogens 13, no. 3: 256. https://doi.org/10.3390/pathogens13030256
APA StyleZimmer, K., Chmielewska, A. M., Jackowiak, P., Figlerowicz, M., & Bienkowska-Szewczyk, K. (2024). Alterations in N-glycosylation of HCV E2 Protein in Children Patients with IFN-RBV Therapy Failure. Pathogens, 13(3), 256. https://doi.org/10.3390/pathogens13030256







