Epidemiology, Clinical Signs, and Risk Factors Associated with Theileriosis in Australian Cattle (2006–2022)
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Clinical Analysis
2.3. Mapping and Statistical Analysis
3. Results
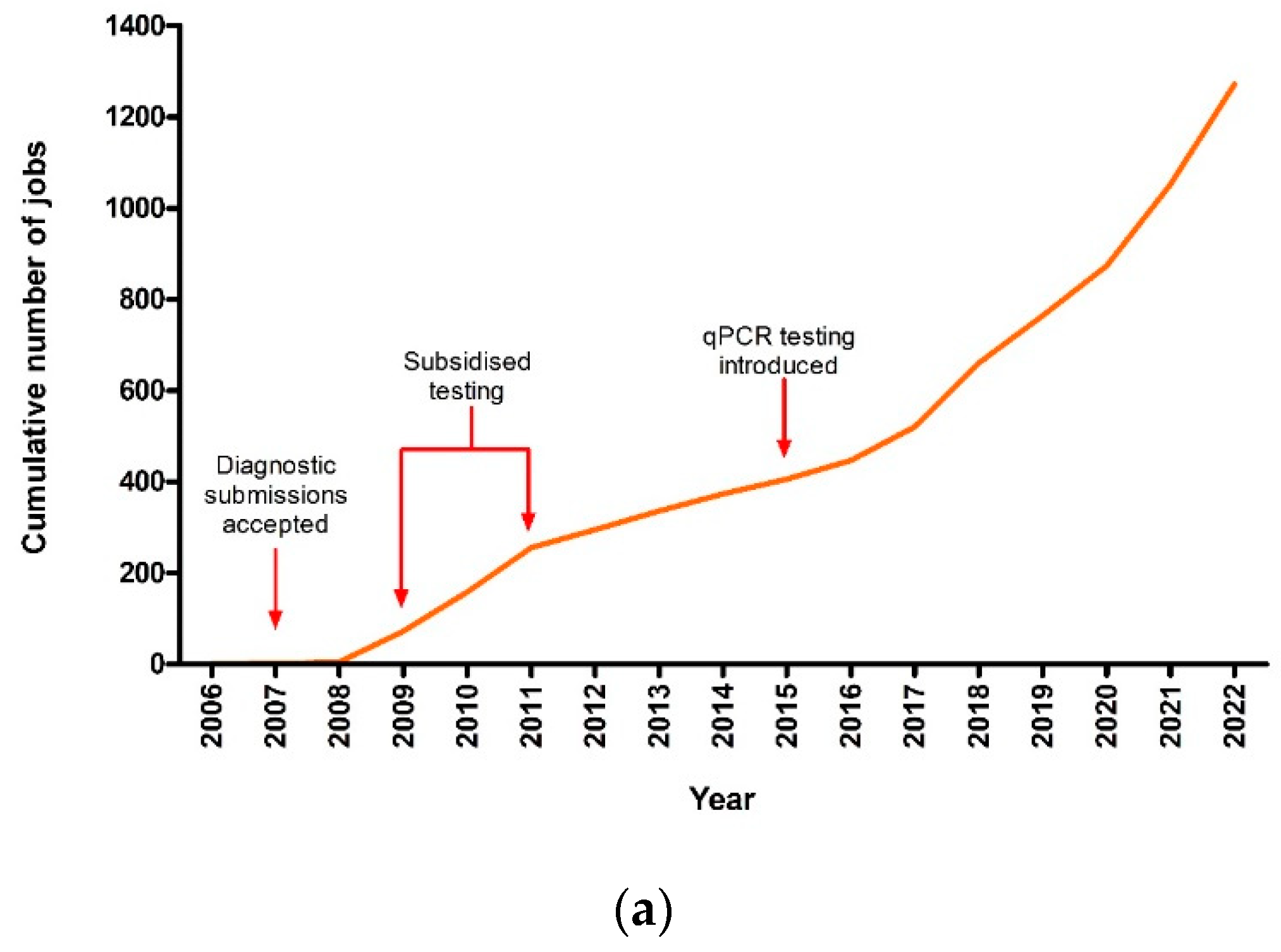
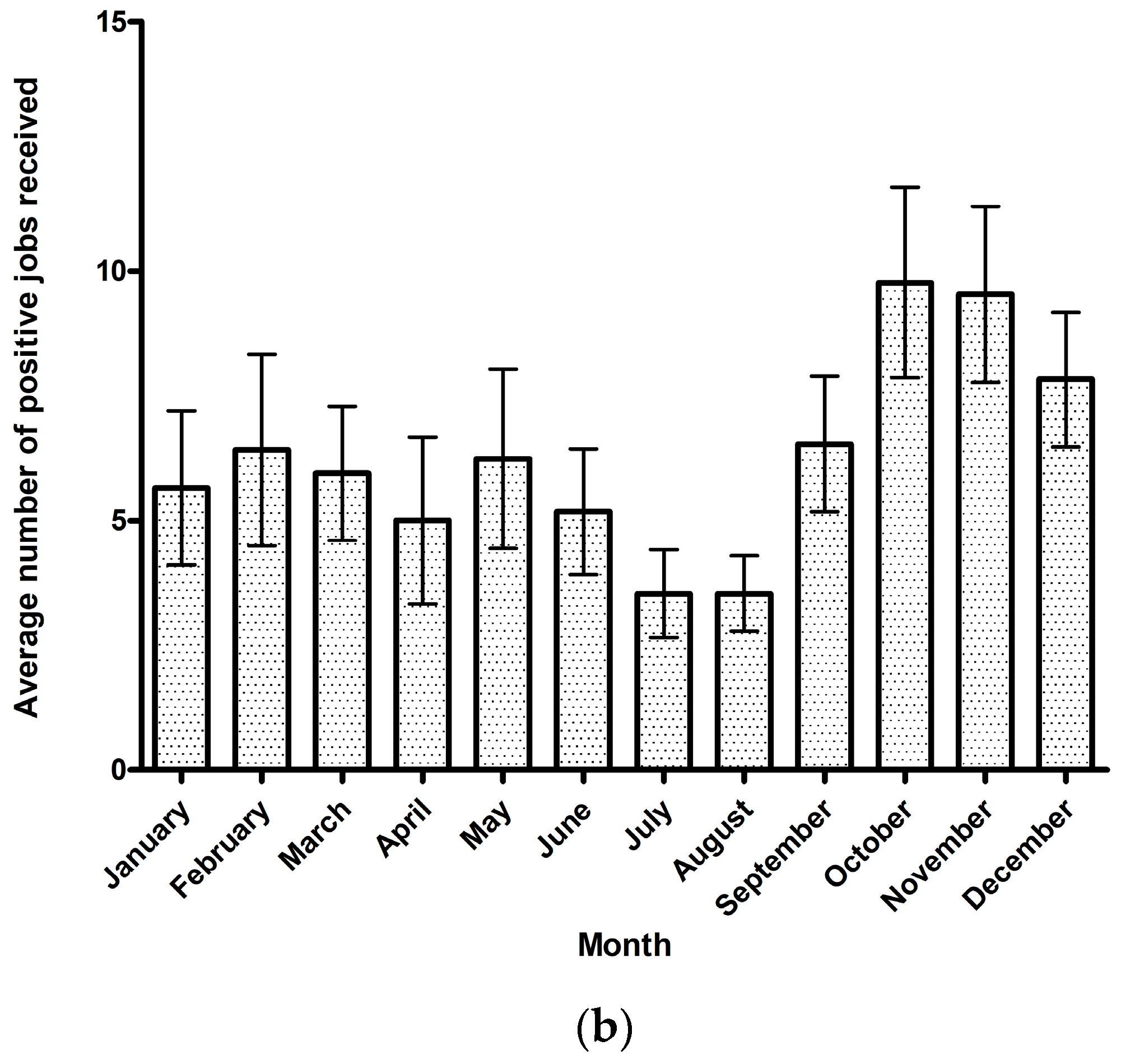
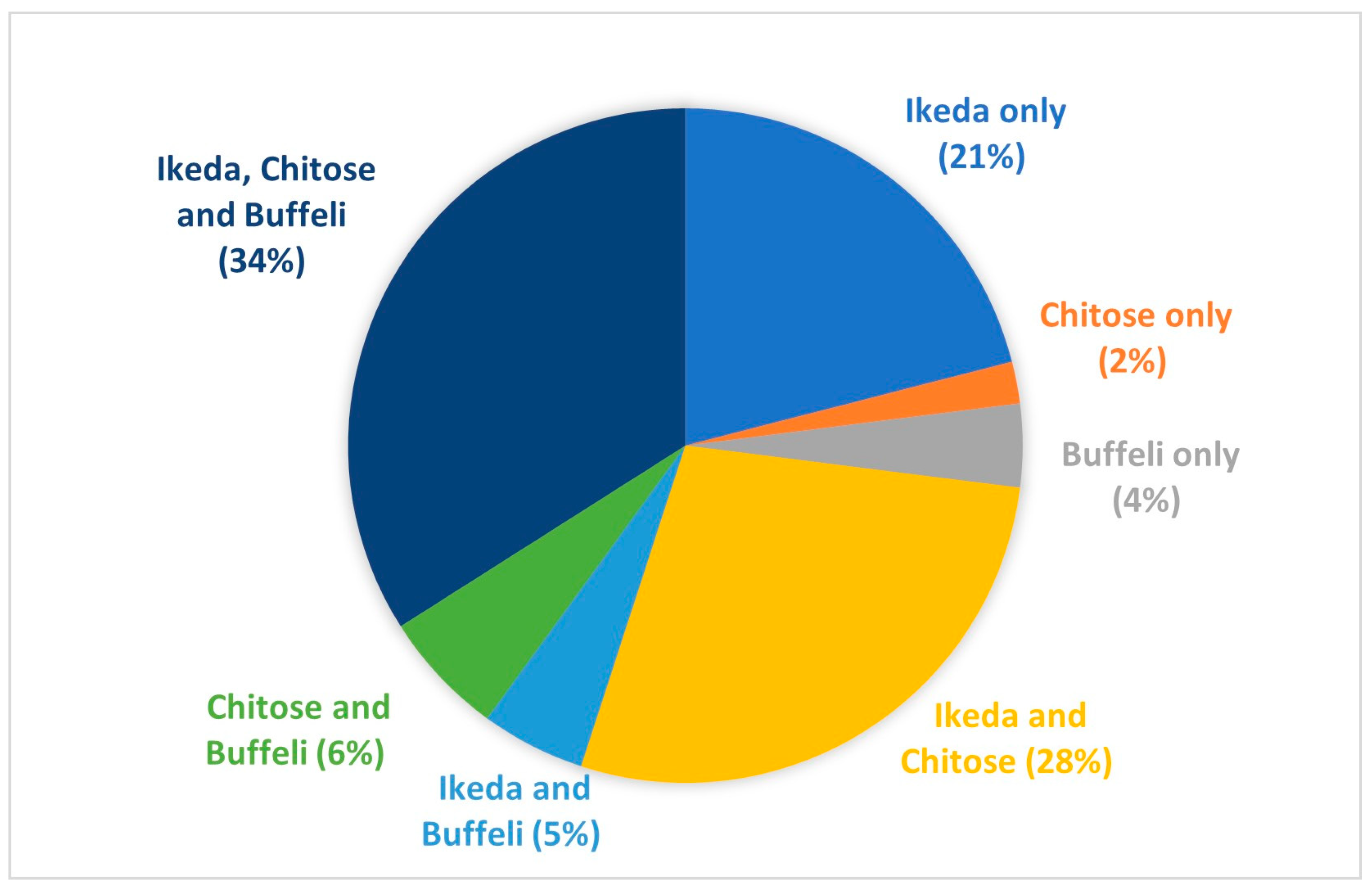
3.1. Theileriosis Sample Submission Summary and Overall Trends
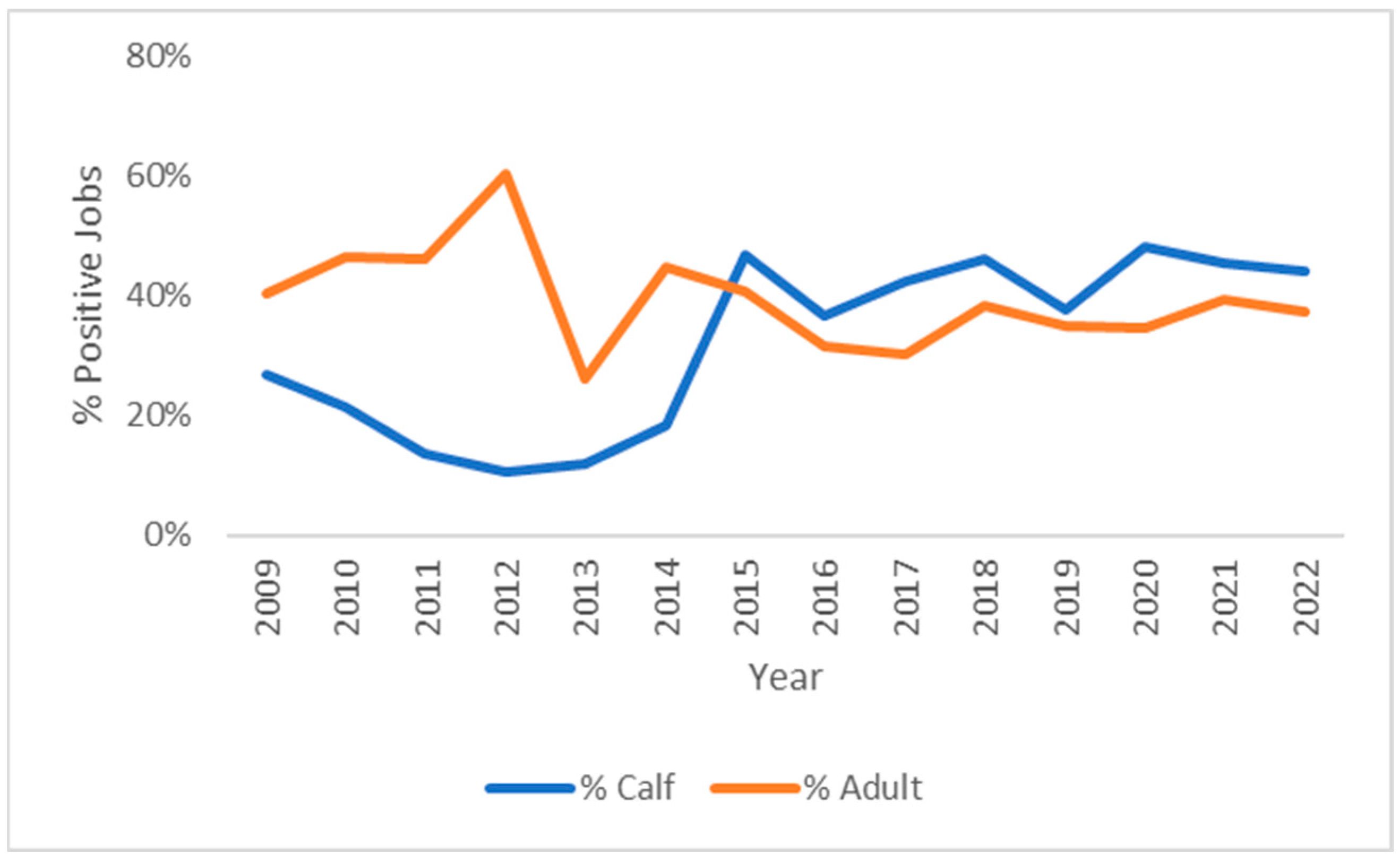
3.2. Breed and Age
3.3. Distribution of Clinical Theileriosis in Australia
3.4. Clinical Signs and Parasitaemia
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agina, O.A.; Shaari, M.R.; Isa, N.M.M.; Ajat, M.; Zamri-Saad, M.; Hamzah, H. Clinical Pathology, Immunopathology and Advanced Vaccine Technology in Bovine Theileriosis: A Review. Pathogens 2020, 9, 697. [Google Scholar] [CrossRef]
- Yokoyama, N.; Sivakumar, T.; Ota, N.; Igarashi, I.; Nakamura, Y.; Yamashina, H.; Matsui, S.; Fukumoto, N.; Hata, H.; Kondo, S.; et al. Genetic diversity of Theileria orientalis in tick vectors detected in Hokkaido and Okinawa, Japan. Infect. Genet. Evol. 2012, 12, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Gebrekidan, H.; Gasser, R.B.; Baneth, G.; Yasur-Landau, D.; Nachum-Biala, Y.; Hailu, A.; Jabbar, A. Molecular characterization of Theileria orientalis from cattle in Ethiopia. Ticks Tick-Borne Dis. 2016, 7, 742–747. [Google Scholar] [CrossRef]
- Savini, G.; Onuma, M.; Scaramozzino, P.; Kakuda, T.; Semproni, G.; Langella, V. First report of Theileria sergenti and T. buffeli/orientalis in cattle in Italy. Ann. N. Y. Acad. Sci. 1998, 849, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Soares, R.; Santos, M.; Santos-Gomes, G.; Botelho, A.; Amaro, A.; Inácio, J. Detection of Theileria and Babesia infections amongst asymptomatic cattle in Portugal. Ticks Tick-Borne Dis. 2013, 4, 148–151. [Google Scholar] [CrossRef]
- Gebrekidan, H.; Nelson, L.; Smith, G.; Gasser, R.B.; Jabbar, A. An outbreak of oriental theileriosis in dairy cattle imported to Vietnam from Australia. Parasitology 2016, 144, 738–746. [Google Scholar] [CrossRef]
- Kim, S.; Yu, D.-H.; Chae, J.-B.; Choi, K.-S.; Kim, H.-C.; Park, B.-K.; Chae, J.-S.; Park, J. Pathogenic genotype of major piroplasm surface protein associated with anemia in Theileria orientalis infection in cattle. Acta Vet. Scand. 2017, 59, 51. [Google Scholar] [CrossRef]
- Izzo, M.; Poe, I.; Horadagoda, N.; De Vos, A.; House, J. Haemolytic anaemia in cattle in NSW associated with Theileria infections. Aust. Vet. J. 2010, 88, 45–51. [Google Scholar] [CrossRef] [PubMed]
- McFadden, A.M.J.; Rawdon, T.G.; Meyer, J.; Makin, J.; Morley, C.M.; Clough, R.R.; Tham, K.; Müllner, P.; Geysen, D. An outbreak of haemolytic anaemia associated with infection of Theileria orientalis in naïve cattle. N. Z. Vet. J. 2011, 59, 79–85. [Google Scholar] [CrossRef]
- Oakes, V.J.; Yabsley, M.J.; Schwartz, D.; LeRoith, T.; Bissett, C.; Broaddus, C.; Schlater, J.L.; Todd, S.M.; Boes, K.M.; Brookhart, M.; et al. Theileria orientalis Ikeda Genotype in Cattle, Virginia, USA. Emerg. Infect. Dis. 2019, 25, 1653–1659. [Google Scholar] [CrossRef]
- Minami, T.; Fujinaga, T.; Furuya, K.; Ishihara, T. Clinico-hematologic and serological comparison of Japanese and Russian strains of Theileria sergenti. Natl. Inst. Anim. Health Q. 1980, 20, 44–52. [Google Scholar]
- Ota, N.; Mizuno, D.; Kuboki, N.; Igarashi, I.; Nakamura, Y.; Yamashina, H.; Hanzaike, T.; Fujii, K.; Onoe, S.; Hata, H.; et al. Epidemiological Survey of Theileria orientalis Infection in Grazing Cattle in the Eastern Part of Hokkaido, Japan. J. Vet. Med. Sci. 2009, 71, 937–944. [Google Scholar] [CrossRef]
- Kamau, J.; de Vos, A.J.; Playford, M.; Salim, B.; Kinyanjui, P.; Sugimoto, C. Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks. Parasites Vectors 2011, 4, 22. [Google Scholar] [CrossRef]
- Sivakumar, T.; Hayashida, K.; Sugimoto, C.; Yokoyama, N. Evolution and genetic diversity of Theileria. Infect. Genet. Evol. 2014, 27, 250–263. [Google Scholar] [CrossRef]
- Pulford, D.; McFadden, A.; Hamilton, J.; Donald, J. Investigation of the index case herd and identification of the genotypes of Theileria orientalis associated with outbreaks of bovine anaemia in New Zealand in 2012. N. Z. Vet. J. 2016, 64, 21–28. [Google Scholar] [CrossRef]
- Thompson, A.T.; White, S.; Shaw, D.; Egizi, A.; Lahmers, K.; Ruder, M.G.; Yabsley, M.J. Theileria orientalis Ikeda in host-seeking Haemaphysalis longicornis in Virginia, U.S.A. Ticks Tick-Borne Dis. 2020, 11, 101450. [Google Scholar] [CrossRef]
- Cufos, N.; Jabbar, A.; de Carvalho, L.M.; Gasser, R.B. Mutation scanning-based analysis of Theileria orientalis populations in cattle following an outbreak. Electro-Phoresis 2012, 33, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Micallef, M.; Alex, S.; Collins, D.; Djordjevic, S.; Bogema, D. Temporal dynamics and subpopulation analysis of Theileria orientalis genotypes in cattle. Infect. Genet. Evol. 2015, 32, 199–207. [Google Scholar] [CrossRef]
- Seddon, H.R. Diseases of Domestic Animals in Australia. Part 4, Protozoan and Virus Diseases/H.R. Seddon. Service Publications (Veterinary Hygiene), 8th ed.; Albiston, H.E., Australia, H., Eds.; Department of Health: Canberra, Australia, 1966. [Google Scholar]
- Callow, L.L. Animal Health in Australia: Protozoal and Rickettsial Diseases; AusInfo. 264: Canberra, Australia, 1984; Volume 5. [Google Scholar]
- Rogers, R.J.; Callow, L.L. Three fatal cases of Theileria mutans infection. Aust. Vet. J. 1966, 42, 42–46. [Google Scholar] [CrossRef]
- Eamens, G.J.; Gonsalves, J.R.; Jenkins, C.; Collins, D.; Bailey, G. Theileria orientalis MPSP types in Australian cattle herds associated with outbreaks of clinical disease and their association with clinical pathology findings. Vet. Parasitol. 2013, 191, 209–217. [Google Scholar] [CrossRef]
- Hammer, J.F.; Emery, D.; Bogema, D.R.; Jenkins, C. Detection of Theileria orientalis genotypes in Haemaphysalis longicornis ticks from southern Australia. Parasites Vectors 2015, 8, 229. [Google Scholar] [CrossRef]
- Heath, A.C.G. Biology, ecology and distribution of the tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae) in New Zealand. N. Z. Vet. J. 2016, 64, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, J.; Cui, X.; Jia, N.; Wei, J.; Xia, L.; Wang, H.; Zhou, Y.; Wang, Q.; Liu, X.; et al. Distribution of Haemaphysalis longicornis and associated pathogens: Analysis of pooled data from a China field survey and global published data. Lancet Planet. Health 2020, 4, e320–e329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, C.; Cheng, C.; Zhang, G.; Yu, T.; Lawrence, K.; Li, H.; Sun, J.; Yang, Z.; Ye, L.; et al. Rapid Spread of Severe Fever with Thrombocytopenia Syndrome Virus by Parthenogenetic Asian Longhorned Ticks. Emerg. Infect. Dis. 2022, 28, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.C. Implications for New Zealand of potentially invasive ticks sympatric with Haemaphysalis longicornis Neumann, 1901 (Acari: Ixodidae). Syst. Appl. Acarol. 2013, 18, 1. [Google Scholar] [CrossRef]
- Rainey, T.; Occi, J.L.; Robbins, R.G.; Egizi, A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) Parasitizing a Sheep in New Jersey, United States. J. Med. Èntomol. 2018, 55, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Beard, C.B.; Occi, J.; Bonilla, D.L.; Egizi, A.M.; Fonseca, D.M.; Mertins, J.W.; Backenson, B.P.; Bajwa, W.I.; Barbarin, A.M.; Bertone, M.A.; et al. Multistate Infestation with the Exotic Disease-Vector Tick Haemaphysalis longicornis—United States, August 2017–September 2018. MMWR Morb. Mortal Wkly Rep. 2018, 67, 1310–1313. [Google Scholar] [CrossRef]
- Dinkel, K.D.; Herndon, D.R.; Noh, S.M.; Lahmers, K.K.; Todd, S.M.; Ueti, M.W.; Scoles, G.A.; Mason, K.L.; Fry, L.M. A U.S. isolate of Theileria orientalis, Ikeda genotype, is transmitted to cattle by the invasive Asian longhorned tick, Haemaphysalis longicornis. Parasites Vectors 2021, 14, 157. [Google Scholar] [CrossRef]
- Lakew, B.T.; Kheravii, S.K.; Wu, S.-B.; Eastwood, S.; Andrew, N.R.; Jenkins, C.; Walkden-Brown, S.W. Endemic infection of cattle with multiple genotypes of Theileria orientalis on the Northern Tablelands of New South Wales despite limited presence of ticks. Ticks Tick-Borne Dis. 2020, 12, 101645. [Google Scholar] [CrossRef]
- Fujisaki, K.; Kamio, T.; Kawazu, S.; Shimizu, S.; Shimura, K. Theileria sergenti: Experimental transmission by the long-nosed cattle louse, Linognathus vituli. Ann. Trop. Med. Parasitol. 1993, 87, 217–218. [Google Scholar] [CrossRef]
- Hammer, J.F.; Jenkins, C.; Bogema, D.; Emery, D. Mechanical transfer of Theileria orientalis: Possible roles of biting arthropods, colostrum and husbandry practices in disease transmission. Parasites Vectors 2016, 9, 34. [Google Scholar] [CrossRef]
- Onoe, S.; Sugimoto, C.; Tanaka, M.; Kubota, S.; Hirai, T.; Yonemichi, H.; Mori, K.; Onuma, M. Prenatal Infections with Theileria sergenti in Calves. J. Protozool. Res. 1994, 4, 119–123. [Google Scholar]
- Swilks, E.; Fell, S.A.; Hammer, J.F.; Sales, N.; Krebs, G.L.; Jenkins, C. Transplacental transmission of Theileria orientalis occurs at a low rate in field-affected cattle: Infection in utero does not appear to be a major cause of abortion. Parasites Vectors 2017, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.; Gedye, K.; McFadden, A.; Pulford, D.; Heath, A.; Pomroy, W. Review of the New Zealand Theileria orientalis Ikeda Type Epidemic and Epidemiological Research since 2012. Pathogens 2021, 10, 1346. [Google Scholar] [CrossRef] [PubMed]
- Espiritu, H.M.; Lee, H.-W.; Lee, S.-S.; Cho, Y.-I. A clinical case of bovine anemia due to Theileria orientalis group in a non-grazed dairy cow in the upper part of South Korea. Korean J. Vet. Res. 2021, 61, e33. [Google Scholar] [CrossRef]
- Lawrence, K.E.; Forsyth, S.F.; Vaatstra, B.L.; McFadden, A.M.J.; Pulford, D.J.; Govindaraju, K.; Pomroy, W.E. Cluster analysis of the clinical histories of cattle affected with bovine anaemia associated with Theileria orientalis Ikeda type infection. N. Z. Vet. J. 2017, 65, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Forshaw, D.; Alex, S.M.; Palmer, D.G.; Cotter, J.; Roberts, W.D.; Jenkins, C.; Hair, S. Theileria orientalis Ikeda genotype infection associated with anaemia, abortion and death in beef cattle in Western Australia. Aust. Vet. J. 2020, 98, 290–297. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Ohashi, K.; Sugimoto, C.; Onuma, M. Theileria orientalis: Cloning a cDNA encoding a protein similar to thiol protease with haemoglobin-binding activity. Exp. Parasitol. 2005, 111, 143–153. [Google Scholar] [CrossRef]
- Eamens, G.J.; Gonsalves, J.R.; Jenkins, C.; Collins, D.; Bailey, G. Distribution and temporal prevalence of Theileria orientalis major piroplasm surface protein types in eastern Australian cattle herds. Aust. Vet. J. 2013, 91, 332–340. [Google Scholar] [CrossRef]
- Eamens, G. Meat and Livestock Australia Final Report: Bovine Theileriosis—Distribution and Significance of Major Piroplasm Surface Protein (MPSP) Types; NSW Department of Primary Industries: North Sydney, Australia, 2012; pp. 1–59. [Google Scholar]
- Bogema, D.; Fell, S.; O’Rourke, B.; Collins, D.; Eamens, G.; Jenkins, C. Development and validation of an inexpensive and efficient method for the extraction of Theileria orientalis DNA from blood. Vet. Parasitol. 2015, 212, 379–381. [Google Scholar] [CrossRef]
- Bogema, D.R.; Deutscher, A.T.; Fell, S.; Collins, D.; Eamens, G.J.; Jenkins, C. Development and Validation of a Quantitative PCR Assay Using Multiplexed Hydrolysis Probes for Detection and Quantification of Theileria orientalis Isolates and Differentiation of Clinically Relevant Subtypes. J. Clin. Microbiol. 2015, 53, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Burggraaf, W. Australian Meat Production and Exports by State; Department of Primary Industries and Regional Development: Perth, Australia, 2007; pp. 1–17.
- Hogan, J.; Shaw, I.; Berry, P. A Review of the Australian Dairy Industry; Australian Bureau of Agricultural and Resource Economics: Canberra, Australia, 2005; pp. 1–51. [Google Scholar]
- McFadden, A.; Gias, E.; Heuer, C.; McFadden, F.S.; Pulford, D. Prevalence and spatial distribution of cattle herds infected with Theileria orientalis in New Zealand between 2012 and 2013. N. Z. Vet. J. 2016, 64, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Gebrekidan, H.; Gasser, R.B.; Perera, P.K.; McGrath, S.; McGrath, S.; Stevenson, M.A.; Jabbar, A. Investigating the first outbreak of oriental theileriosis in cattle in South Australia using multiplexed tandem PCR (MT-PCR). Ticks Tick Borne Dis. 2015, 6, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Riek, R.F. Epidemiology and transmission of Theileria sp. of cattle in Australia. Aust. Vet. J. 1982, 59, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Bendixen, M. A Practical Guide to the Use of Correspondence Analysis in Marketing Research. Mark. Res. On-Line 2003, 1, 16–36. [Google Scholar]
- Jenkins, C. Bovine theileriosis in Australia: A decade of disease. Microbiol. Aust. 2018, 39, 215–219. [Google Scholar] [CrossRef]
- Islam, M.K.; Jabbar, A.; Campbell, B.E.; Cantacessi, C.; Gasser, R.B. Bovine theileriosis—An emerging problem in south-eastern Australia? Infect. Genet. Evol. 2011, 11, 2095–2097. [Google Scholar] [CrossRef]
- Eamens, G.J.; Bailey, G.; Jenkins, C.; Gonsalves, J.R. Significance of Theileria orientalis types in individual affected beef herds in New South Wales based on clinical, smear and PCR findings. Vet. Parasitol. 2013, 196, 96–105. [Google Scholar] [CrossRef]
- Perera, P.K.; Gasser, R.B.; Anderson, G.A.; Jeffers, M.; Bell, C.M.; Jabbar, A. Epidemiological survey following oriental theileriosis outbreaks in Victoria, Australia, on selected cattle farms. Vet. Parasitol. 2013, 197, 509–521. [Google Scholar] [CrossRef]
- Swilks, E.; Jenkins, C.; Poynting, A.; Collins, D.; Krebs, G. Prevalence and effect of Theileria orientalis infection in homebred calves in the Gloucester region of New South Wales, Australia. Aust. Vet. J. 2017, 95, 211–216. [Google Scholar] [CrossRef]
- Roberts, F. A systematic study of the Australian species of the genus Haemaphysalis Koch (Acarina: Ixodidae). Aust. J. Zool. 1963, 11, 35–80. [Google Scholar] [CrossRef]
- Roberts, F.H.S. Australian Ticks; Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia, 1970; p. 267.
- Barker, S.C.; Walker, A.R. Ticks of Australia. The species that infest domestic animals and humans. Zootaxa 2014, 3816, 1–144. [Google Scholar] [CrossRef] [PubMed]
- Stewart, N.P.; Uilenberg, G.; de Vos, A.J. Review of Australian species of Theileria, with special reference to Theileria buffeli of cattle. Trop. Anim. Health Prod. 1996, 28, 81–90. [Google Scholar] [CrossRef]
- Laan, B.; Handasyde, K.; Beveridge, I. Occurence of the tick Haemaphysalis bancrofti Nuttall & Warburton, 1915 in Victoria with additional data on its distribution and with scanning electron micrographs of life cycle stages. Proc. R. Soc. Vic. 2011, 123, 189–199. [Google Scholar]
- Marendy, D.; Baker, K.; Emery, D.; Rolls, P.; Stutchbury, R. Haemaphysalis longicornis: The life-cycle on dogs and cattle, with confirmation of its vector status for Theileria orientalis in Australia. Vet. Parasitol. 2019, 277, 100022. [Google Scholar] [CrossRef] [PubMed]
- Stewart, N.P.; de Vos, A.J.; McGregor, W.; Shiels, I. Haemaphysalis humerosa, not H. longicornis, is the likely vector of Theileria buffeli in Australia. Aust. Vet. J. 1987, 64, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Stewart, N.P.; DE Vos, A.J.; Shiels, I.; McGregor, W. The experimental transmission of Theileria buffeli of cattle in Australia by Haemaphysalis humerosa. Aust. Vet. J. 1987, 64, 81–83. [Google Scholar] [CrossRef]
- Stewart, N.; Devos, A.; Shiels, I.; Jorgensen, W. Transmission of Theileria buffeli to cattle by Haemaphysalis bancrofti fed on Artificially Infected Mice. Vet. Parasitol. 1989, 34, 123–127. [Google Scholar] [CrossRef]
- Yam, J.; Bogema, D.R.; Micallef, M.L.; Djordjevic, S.P.; Jenkins, C. Complete Genomes of Theileria orientalis Chitose and Buffeli Genotypes Reveal within Species Translocations and Differences in ABC Transporter Content. Pathogens 2022, 11, 801. [Google Scholar] [CrossRef]
- Lakew, B.T.; Kheravii, S.K.; Wu, S.B.; Eastwood, S.; Andrew, N.R.; Nicholas, A.H.; Walkden-Brown, S.W. Detection and distribution of haematophagous flies and lice on cattle farms and potential role in the trans-mission of Theileria orientalis. Vet. Parasitol. 2021, 298, 109516. [Google Scholar] [CrossRef]
- Emery, D.L. Approaches to Integrated Parasite Management (IPM) for Theileria orientalis with an Emphasis on Immunity. Pathogens 2021, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K. The Epidemiology of Theileria orientalis Ikeda Type in New Zealand, in Veterinary Clinical Sciences; Massey University: Palmerston North, New Zealand, 2020; p. 522. [Google Scholar]
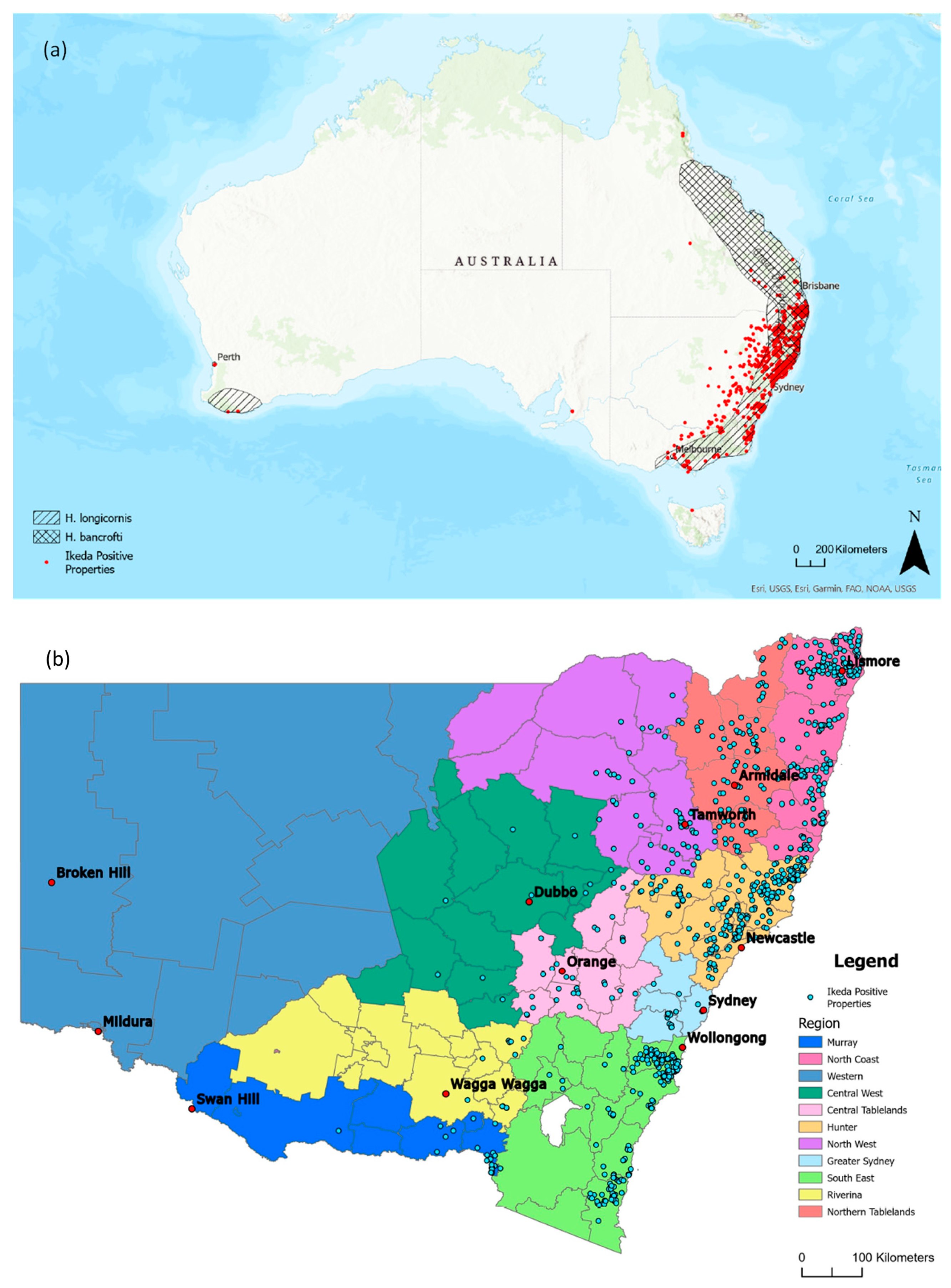
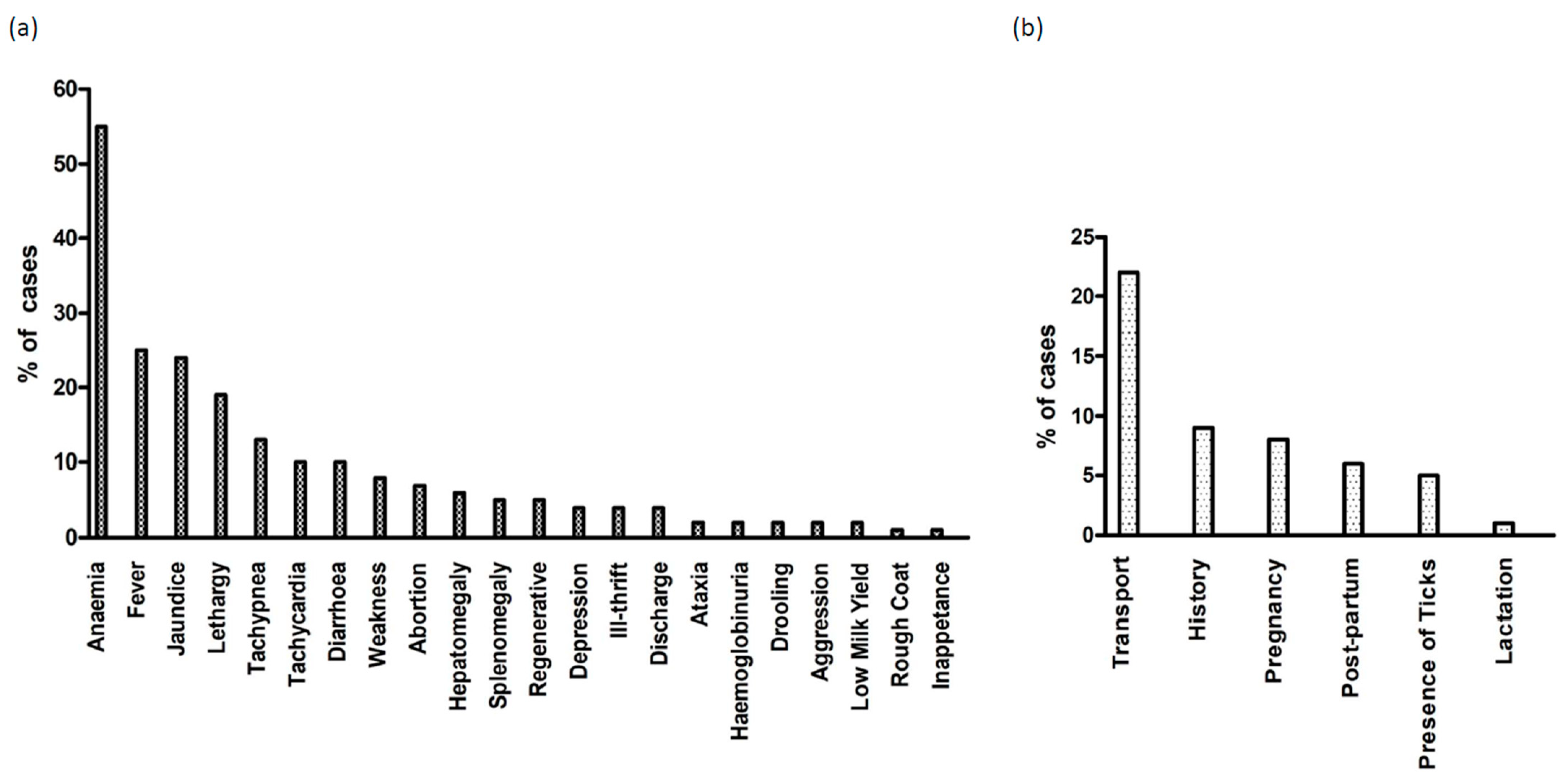
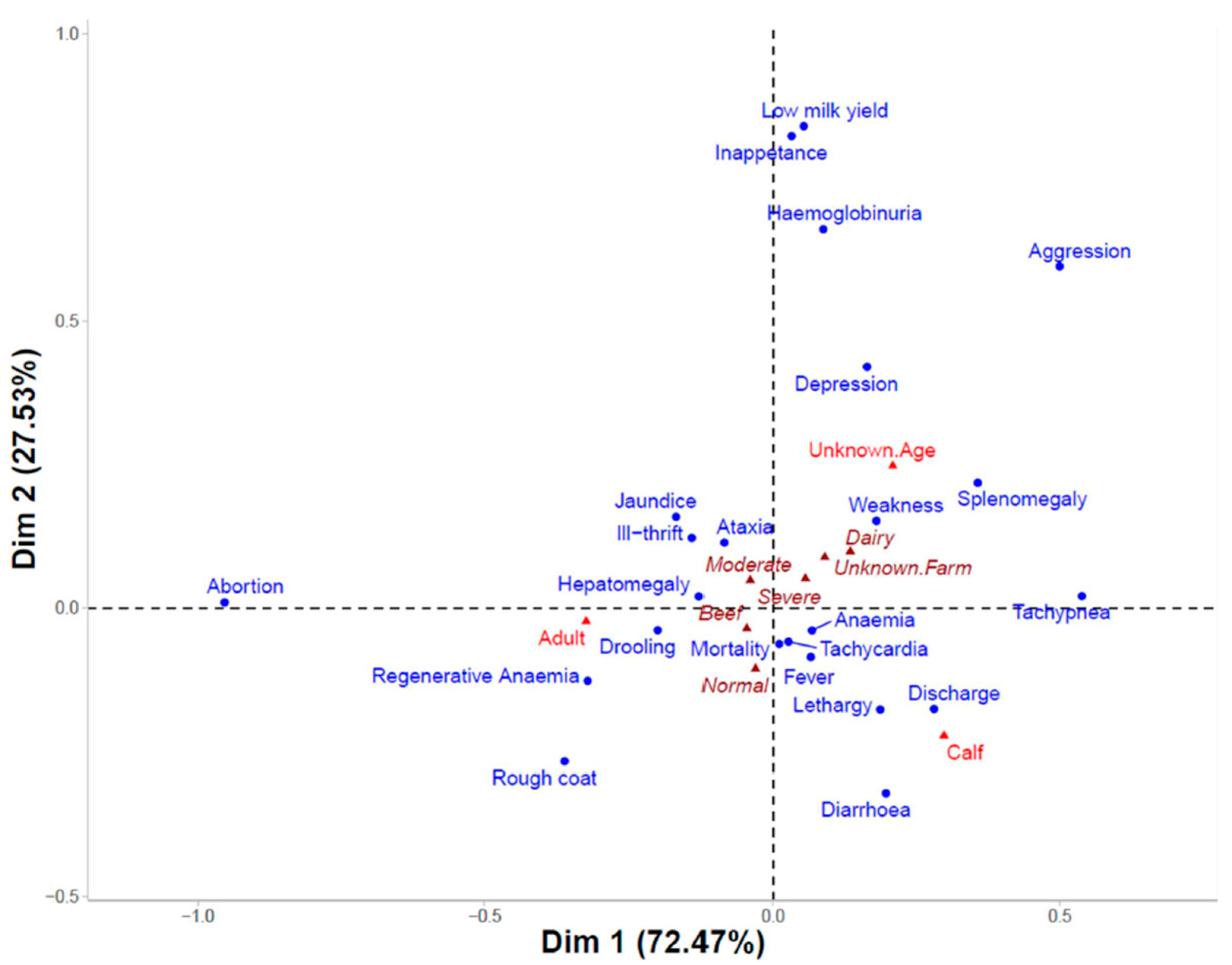
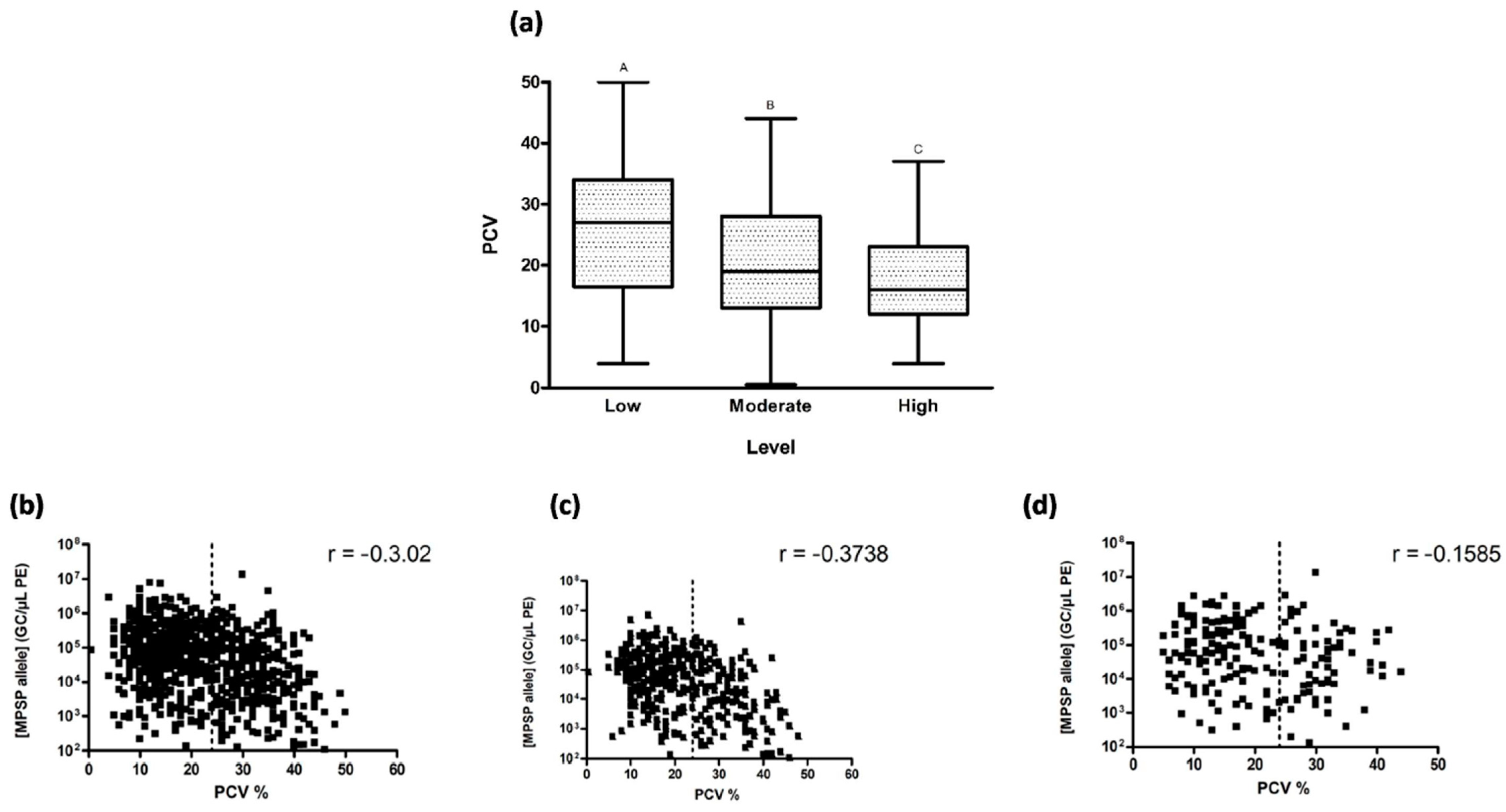
| State | New South Wales (NSW) | Victoria (VIC) | Queensland (QLD) | Western Australia (WA) | South Australia (SA) | Tasmania (TAS) |
|---|---|---|---|---|---|---|
| No. of jobs received | 1457 | 76 | 55 | 10 | 4 | 3 |
| Clinical Sign | p-Value | OR | CI |
|---|---|---|---|
| Calves vs. adults | |||
| Lethargy | <0.0001 | 2.50 | 1.61–3.90 |
| Tachypnea | <0.0001 | 5.63 | 3.11–10.67 |
| Jaundice | 0.01 | 0.59 | 0.39–0.88 |
| Diarrhoea | <0.0005 | 2.67 | 1.56–4.61 |
| Fever | <0.005 | 1.79 | 1.22–2.62 |
| Anaemia | <0.0001 | 2.79 | 1.95–4.03 |
| Discharge | 0.02 | 2.71 | 1.12–6.83 |
| Mortality | <0.0005 | 1.87 | 1.33–2.63 |
| Beef vs. dairy cattle | |||
| Depression | 0.04 | 2.40 | 1.08–6.46 |
| Tachycardia | 0.02 | 2.58 | 1.16–6.90 |
| Fever | <0.0001 | 2.52 | 1.63–4.03 |
| Abortion | <0.0001 | 6.40 | 3.14–15.49 |
| Ill-thrift | <0.0001 | 0.20 | 0.06–0.54 |
| Mortality | <0.0001 | 2.98 | 2.12–4.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onizawa, E.; Jenkins, C. Epidemiology, Clinical Signs, and Risk Factors Associated with Theileriosis in Australian Cattle (2006–2022). Pathogens 2024, 13, 253. https://doi.org/10.3390/pathogens13030253
Onizawa E, Jenkins C. Epidemiology, Clinical Signs, and Risk Factors Associated with Theileriosis in Australian Cattle (2006–2022). Pathogens. 2024; 13(3):253. https://doi.org/10.3390/pathogens13030253
Chicago/Turabian StyleOnizawa, Emily, and Cheryl Jenkins. 2024. "Epidemiology, Clinical Signs, and Risk Factors Associated with Theileriosis in Australian Cattle (2006–2022)" Pathogens 13, no. 3: 253. https://doi.org/10.3390/pathogens13030253
APA StyleOnizawa, E., & Jenkins, C. (2024). Epidemiology, Clinical Signs, and Risk Factors Associated with Theileriosis in Australian Cattle (2006–2022). Pathogens, 13(3), 253. https://doi.org/10.3390/pathogens13030253





