Avian Influenza A Viruses Modulate the Cellular Cytoskeleton during Infection of Mammalian Hosts
Abstract
1. Introduction
2. Avian Influenza A Virus Infection Transmission to Humans
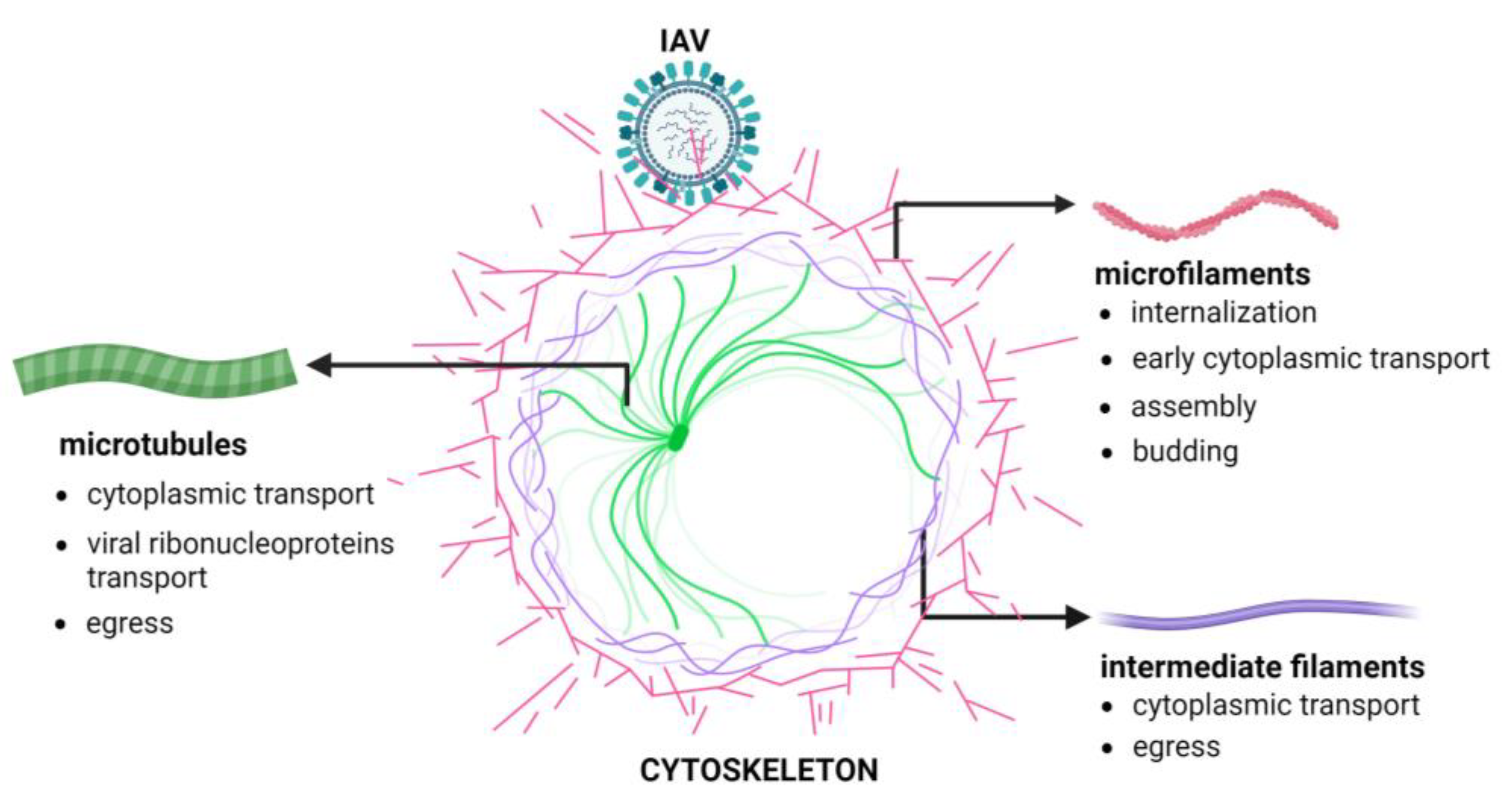
3. The Cellular Cytoskeleton
4. The Involvement of the Cellular Cytoskeleton in Influenza Virus Infection
5. Mammalian Cytoskeletal Proteins Are Modulated during Highly Pathogenic AIV Infection
6. The Cellular Cytoskeleton Is a Mediator of Host Defense Responses during Influenza Virus Infection
7. Conclusions
Funding
Conflicts of Interest
References
- Fukuyama, S.; Kawaoka, Y. The pathogenesis of influenza virus infections: The contributions of virus and host factors. Curr. Opin. Immunol. 2011, 23, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Doran, Á.; Colvin, C.L.; McLaughlin, E. What can we learn from historical pandemics? A systematic review of the literature. Soc. Sci. Med. 2024, 342, 116534. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Influenza Fact Sheet. Available online: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 3 October 2023).
- Mostafa, A.; Abdelwhab, E.M.; Mettenleiter, T.C.; Pleschka, S. Zoonotic Potential of Influenza A Viruses: A Comprehensive Overview. Viruses 2018, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, H.; Li, C. Advances in deciphering the interactions between viral proteins of influenza A virus and host cellular proteins. Cell Insight 2023, 2, 100079. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/flu/avianflu/avian-in-humans.htm (accessed on 31 January 2024).
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife-threats to biodiversity and human health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Scheibner, D.; Salaheldin, A.H.; Bagato, O.; Zaeck, L.M.; Mostafa, A.; Blohm, U.; Müller, C.; Eweas, A.F.; Franzke, K.; Karger, A.; et al. Phenotypic effects of mutations observed in the neuraminidase of human origin H5N1 influenza A viruses. PLoS Pathog 2023, 19, e1011135. [Google Scholar] [CrossRef]
- Dey, P.; Ahuja, A.; Panwar, J.; Choudhary, P.; Rani, S.; Kaur, M.; Sharma, A.; Kaur, J.; Yadav, A.K.; Sood, V.; et al. Immune Control of Avian Influenza Virus Infection and Its Vaccine Development. Vaccines 2023, 11, 593. [Google Scholar] [CrossRef]
- Swayne, D.E.; Suarez, D.L. Highly pathogenic avian influenza. Rev. Sci. Tech. 2000, 19, 463–482. [Google Scholar] [CrossRef]
- Chen, J.; Lee, K.H.; Steinhauer, D.A.; Stevens, D.J.; Skehel, J.J.; Wiley, D.C. Structure of the hemagglutinin precursor cleavage site, a determinant of influenza pathogenicity and the origin of the labile conformation. Cell 1998, 95, 409–417. [Google Scholar] [CrossRef]
- Escalera-Zamudio, M.; Golden, M.; Gutiérrez, B.; Thézé, J.; Keown, J.R.; Carrique, L.; Bowden, T.A.; Pybus, O.G. Parallel evolution in the emergence of highly pathogenic avian influenza A viruses. Nat Commun 2020, 11, 5511. [Google Scholar] [CrossRef]
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W.; Chen, J.; Jie, Z.; Qiu, H.; Xu, K.; et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013, 368, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G. Predictions for future human influenza pandemics. J. Infect. Dis. 1997, 176, S14–S19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Webster, R.G. Influenza virus: Transmission between species and relevance to emergence of the next human pandemic. Arch. Virol. Suppl. 1997, 13, 105–113. [Google Scholar] [PubMed]
- Webby, R.J.; Webster, R.G. Are we ready for pandemic influenza? Science 2003, 302, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, M.; Fouchier, R.A. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J. 2014, 33, 823–841. [Google Scholar] [CrossRef] [PubMed]
- Steel, J.; Lowen, A.C.; Mubareka, S.; Palese, P. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog. 2009, 5, e1000252. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zheng, M.; Wang, P.; Mok, B.W.; Liu, S.; Lau, S.Y.; Chen, P.; Liu, Y.C.; Liu, H.; Chen, Y.; et al. An NS-segment exonic splicing enhancer regulates influenza A virus replication in mammalian cells. Nat. Commun. 2017, 8, 14751. [Google Scholar] [CrossRef]
- Du, W.; de Vries, E.; van Kuppeveld, F.J.M.; Matrosovich, M.; de Haan, C.A.M. Second sialic acid-binding site of influenza A virus neuraminidase: Binding receptors for efficient release. FEBS J. 2021, 288, 5598–5612. [Google Scholar] [CrossRef]
- Vourc’h, G.; Moutou, F.; Morand, S.; Jourdain, E. Zoonoses the Ties that Bind Humans to Animals [Internet]; Éditions Quae: Versailles, France, 2022. [Google Scholar]
- Chen, H.; Smith, G.J.; Li, K.S.; Wang, J.; Fan, X.H.; Rayner, J.M.; Vijaykrishna, D.; Zhang, J.X.; Zhang, L.J.; Guo, C.T.; et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: Implications for pandemic control. Proc. Natl. Acad. Sci. USA 2006, 103, 2845–2850. [Google Scholar] [CrossRef]
- Yuen, K.Y.; Chan, P.K.; Peiris, M.; Tsang, D.N.; Que, T.L.; Shortridge, K.F.; Cheung, P.T.; To, W.K.; Ho, E.T.; Sung, R.; et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 1998, 351, 467–471. [Google Scholar] [CrossRef]
- Claas, E.C.; de Jong, J.C.; van Beek, R.; Rimmelzwaan, G.F.; Osterhaus, A.D. Human influenza virus A/HongKong/156/97 (H5N1) infection. Vaccine 1998, 16, 977–978. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.B.; Lim, W.; Hu-Primmer, J.; Sims, L.; Fukuda, K.; Mak, K.H.; Rowe, T.; Thompson, W.W.; Conn, L.; Lu, X.; et al. Risk of influenza A (H5N1) infection among poultry workers, Hong Kong, 1997–1998. J. Infect. Dis. 2002, 185, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.M.; Lim, W.; Bridges, C.B.; Rowe, T.; Hu-Primmer, J.; Lu, X.; Abernathy, R.A.; Clarke, M.; Conn, L.; Kwong, H.; et al. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J. Infect. Dis. 1999, 180, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Bahgat, M.M.; Kutkat, M.A.; Nasraa, M.H.; Mostafa, A.; Webby, R.; Bahgat, I.M.; Ali, M.A. Characterization of an avian influenza virus H5N1 Egyptian isolate. J. Virol. Methods 2009, 159, 244–250. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cumulative Number of Confirmed Human Cases for Avian Influenza A (H5N1) Reported to WHO, 2003–2023. 2023. Available online: https://www.who.int/publications/m/item/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who-2003-2022-5-jan-2023 (accessed on 26 January 2024).
- Xie, R.; Edwards, K.M.; Wille, M.; Wei, X.; Wong, S.S.; Zanin, M.; El-Shesheny, R.; Ducatez, M.; Poon, L.L.M.; Kayali, G.; et al. The episodic resurgence of highly pathogenic avian influenza H5 virus. Nature 2023, 622, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Monne, I.; Yamage, M.; Dauphin, G.; Claes, F.; Ahmed, G.; Giasuddin, M.; Salviato, A.; Ormelli, S.; Bonfante, F.; Schivo, A.; et al. Reassortant avian influenza A (H5N1) viruses with H9N2-PB1 gene in poultry, Bangladesh. Emerg. Infect. Dis. 2013, 19, 1630–1634. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, N.A.; Khan, S.U.; Balish, A.; Shanta, I.S.; Simpson, N.; Berman, L.; Haider, N.; Poh, M.K.; Islam, A.; Gurley, E.; et al. Multiple reassortment events among highly pathogenic avian influenza A(H5N1) viruses detected in Bangladesh. Virology 2014, 450–451, 297–307. [Google Scholar] [CrossRef]
- Li, Y.; Shi, J.; Zhong, G.; Deng, G.; Tian, G.; Ge, J.; Zeng, X.; Song, J.; Zhao, D.; Liu, L.; et al. Continued evolution of H5N1 influenza viruses in wild birds, domestic poultry, and humans in China from 2004 to 2009. J. Virol. 2010, 84, 8389–8397. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.; Jiao, P.; Deng, G.; Tian, G.; Li, Y.; Hoffmann, E.; Webster, R.G.; Matsuoka, Y.; Yu, K. Molecular basis of replication of duck H5N1 influenza viruses in a mammalian mouse model. J. Virol. 2005, 79, 12058–12064. [Google Scholar] [CrossRef]
- Hatta, M.; Gao, P.; Halfmann, P.; Kawaoka, Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 2001, 293, 1840–1842. [Google Scholar] [CrossRef]
- Pan, M.; Gao, R.; Lv, Q.; Huang, S.; Zhou, Z.; Yang, L.; Li, X.; Zhao, X.; Zou, X.; Tong, W.; et al. Human infection with a novel, highly pathogenic avian influenza A (H5N6) virus: Virological and clinical findings. J. Infect. 2016, 72, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Matrosovich, M.N.; Krauss, S.; Webster, R.G. H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology 2001, 281, 156–162. [Google Scholar] [CrossRef]
- Liu, N.; Song, W.; Wang, P.; Lee, K.; Chan, W.; Chen, H.; Cai, Z. Proteomics analysis of differential expression of cellular proteins in response to avian H9N2 virus infection in human cells. Proteomics 2008, 8, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Butt, K.M.; Smith, G.J.; Chen, H.; Zhang, L.J.; Leung, Y.H.; Xu, K.M.; Lim, W.; Webster, R.G.; Yuen, K.Y.; Peiris, J.S.; et al. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J. Clin. Microbiol. 2005, 43, 5760–5767. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Stevens, D.J.; Skehel, J.J.; Wiley, D.C. X-ray structures of H5 avian and H9 swine influenza virus hemagglutinins bound to avian and human receptor analogs. Proc. Natl. Acad. Sci. USA 2001, 98, 11181–11186. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Liu, J.; Liang, W.; Wang, A.; Liu, Z.; Yang, Y.; Lv, J.; Bao, Y.; Gao, Y.; Miao, Z.; et al. Response profiles of cytokines and chemokines against avian H9N2 influenza virus within the mouse lung. Med. Microbiol. Immunol. 2014, 203, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Ungchusak, K.; Auewarakul, P.; Dowell, S.F.; Kitphati, R.; Auwanit, W.; Puthavathana, P.; Uiprasertkul, M.; Boonnak, K.; Pittayawonganon, C.; Cox, N.J.; et al. Probable person-to-person transmission of avian influenza A (H5N1). N. Engl. J. Med. 2005, 352, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Kandun, I.N.; Wibisono, H.; Sedyaningsih, E.R.; Yusharmen; Hadisoedarsuno, W.; Purba, W.; Santoso, H.; Septiawati, C.; Tresnaningsih, E.; Heriyanto, B.; et al. Three Indonesian clusters of H5N1 virus infection in 2005. N. Engl. J. Med. 2006, 355, 2186–2194. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Z.; Shu, Y.; Yu, H.; Zhou, L.; Zu, R.; Huai, Y.; Dong, J.; Bao, C.; Wen, L.; et al. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet 2008, 371, 1427–1434. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, L.; Zhou, M.; Chen, Z.; Li, F.; Wu, H.; Xiang, N.; Chen, E.; Tang, F.; Wang, D.; et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N. Engl. J. Med. 2014, 370, 520–532. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Meng, L.S.; Kong, D.H.; Liu, J.; Li, S.Z.; Zhou, C.; Sun, J.; Song, R.J.; Wu, J.J. A Suspected Person-to-person Transmission of Avian Influenza A (H7N9) Case in Ward. Chin. Med. J. 2017, 130, 1255–1256. [Google Scholar] [CrossRef] [PubMed]
- Du Ry van Beest Holle, M.; Meijer, A.; Koopmans, M.; de Jager, C.M. Human-to-human transmission of avian influenza A/H7N7, The Netherlands, 2003. Eurosurveillance 2005, 10, 3–4. [Google Scholar] [CrossRef]
- Parry, J. H7N9 avian flu infects humans for the first time. BMJ 2013, 346, f2151. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Tsang, A.K.; Chan, J.F.; Cheng, V.C.; Chen, H.; Yuen, K.Y. Emergence in China of human disease due to avian influenza A(H10N8)—Cause for concern? J. Infect. 2014, 68, 205–215. [Google Scholar] [CrossRef]
- Shi, W.; Shi, Y.; Wu, Y.; Liu, D.; Gao, G.F. Origin and molecular characterization of the human-infecting H6N1 influenza virus in Taiwan. Protein Cell 2013, 4, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Su, C.; Wang, D.; Peng, Y.; Liu, M.; Hua, S.; Li, T.; Gao, G.F.; Tang, H.; Chen, J.; et al. Sequential reassortments underlie diverse influenza H7N9 genotypes in China. Cell Host Microbe 2013, 14, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhou, X.; Shi, W.; Huang, L.; Xia, W.; Liu, D.; Li, H.; Chen, S.; Lei, F.; Cao, L.; et al. Genesis of the novel human-infecting influenza A(H10N8) virus and potential genetic diversity of the virus in poultry, China. Eurosurveillance 2014, 19, 20841. [Google Scholar] [CrossRef]
- Li, M.; Peng, D.; Cao, H.; Yang, X.; Li, S.; Qiu, H.J.; Li, L.F. The Host Cytoskeleton Functions as a Pleiotropic Scaffold: Orchestrating Regulation of the Viral Life Cycle and Mediating Host Antiviral Innate Immune Responses. Viruses 2023, 15, 1354. [Google Scholar] [CrossRef]
- Mostowy, S.; Cossart, P. Septins: The fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2012, 13, 183–194. [Google Scholar] [CrossRef]
- Liu, L.; Luo, Q.; Sun, J.; Song, G. Nucleus and nucleus-cytoskeleton connections in 3D cell migration. Exp. Cell Res. 2016, 348, 56–65. [Google Scholar] [CrossRef]
- Fuchs, E.; Cleveland, D.W. A structural scaffolding of intermediate filaments in health and disease. Science 1998, 279, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Słońska, A.; Polowy, R.; Golke, A.; Cymerys, J. Role of cytoskeletal motor proteins in viral infection. Postepy Hig. Med. Dosw. 2012, 66, 810–817. [Google Scholar] [CrossRef] [PubMed]
- MacTaggart, B.; Kashina, A. Posttranslational modifications of the cytoskeleton. Cytoskeleton 2021, 78, 142–173. [Google Scholar] [CrossRef] [PubMed]
- Buxboim, A.; Kronenberg-Tenga, R.; Salajkova, S.; Avidan, N.; Shahak, H.; Thurston, A.; Medalia, O. Scaffold, mechanics and functions of nuclear lamins. FEBS Lett. 2023, 597, 2791–2805. [Google Scholar] [CrossRef]
- Buchwalter, A. Intermediate, but not average: The unusual lives of the nuclear lamin proteins. Curr. Opin. Cell Biol. 2023, 84, 102220. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, T.; Dehghani, F. The Cytoskeleton-A Complex Interacting Meshwork. Cells 2019, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Cooper, J.A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu. Rev. Biochem. 1986, 55, 987–1035. [Google Scholar] [CrossRef]
- Carlier, M.F.; Shekhar, S. Global treadmilling coordinates actin turnover and controls the size of actin networks. Nat. Rev. Mol. Cell Biol. 2017, 18, 389–401. [Google Scholar] [CrossRef]
- Davidson, P.M.; Cadot, B. Actin on and around the Nucleus. Trends Cell Biol. 2021, 31, 211–223. [Google Scholar] [CrossRef]
- Kloc, M.; Wilk, K.; Vargas, D.; Shirato, Y.; Bilinski, S.; Etkin, L.D. Potential structural role of non-coding and coding RNAs in the organization of the cytoskeleton at the vegetal cortex of Xenopus oocytes. Development 2005, 132, 3445–3457. [Google Scholar] [CrossRef]
- Simpson, C.; Yamauchi, Y. Microtubules in Influenza Virus Entry and Egress. Viruses 2020, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Rieder, C.L.; Faruki, S.; Khodjakov, A. The centrosome in vertebrates: More than a microtubule-organizing center. Trends Cell Biol. 2001, 11, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Gudimchuk, N.B.; McIntosh, J.R. Regulation of microtubule dynamics, mechanics and function through the growing tip. Nat. Rev. Mol. Cell. Biol. 2021, 22, 777–795. [Google Scholar] [CrossRef] [PubMed]
- Guru, A.; Saravanan, S.; Sharma, D.; Narasimha, M. The microtubule end-binding proteins EB1 and Patronin modulate the spatiotemporal dynamics of myosin and pattern pulsed apical constriction. Development 2022, 149, dev199759. [Google Scholar] [CrossRef]
- Ramaekers, F.C.; Bosman, F.T. The cytoskeleton and disease. J. Pathol. 2004, 204, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shen, Y.; Sivagurunathan, S.; Weber, M.S.; Adam, S.A.; Shin, J.H.; Fredberg, J.J.; Medalia, O.; Goldman, R.; Weitz, D.A. Vimentin intermediate filaments and filamentous actin form unexpected interpenetrating networks that redefine the cell cortex. Proc. Natl. Acad. Sci. USA 2022, 119, e2115217119. [Google Scholar] [CrossRef] [PubMed]
- Redmond, C.J.; Coulombe, P.A. Intermediate filaments as effectors of differentiation. Curr. Opin. Cell Biol. 2021, 68, 155–162. [Google Scholar] [CrossRef]
- Odell, J.; Lammerding, J. Lamins as structural nuclear elements through evolution. Curr. Opin. Cell Biol. 2023, 85, 102267. [Google Scholar] [CrossRef]
- Aebi, U.; Cohn, J.; Buhle, L.; Gerace, L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature 1986, 323, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Maly, I.V.; Hofmann, W.A. Myosins in the Nucleus. Adv. Exp. Med. Biol. 2020, 1239, 199–231. [Google Scholar]
- da Silva, E.S.; Naghavi, M.H. Microtubules and viral infection. Adv. Virus Res. 2023, 115, 87–134. [Google Scholar] [PubMed]
- Horníková, L.; Bruštíková, K.; Huérfano, S.; Forstová, J. Nuclear Cytoskeleton in Virus Infection. Int. J. Mol. Sci. 2022, J23, 578. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.H.; Burckhardt, C.J.; Yakimovich, A.; Greber, U.F. Imaging, Tracking and Computational Analyses of Virus Entry and Egress with the Cytoskeleton. Viruses 2018, 10, 166. [Google Scholar] [CrossRef]
- Radtke, K.; Döhner, K.; Sodeik, B. Viral interactions with the cytoskeleton: A hitchhiker’s guide to the cell. Cell. Microbiol. 2006, 8, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Whittaker, G.R. Role of the actin cytoskeleton during influenza virus internalization into polarized epithelial cells. Cell. Microbiol. 2007, 9, 1672–1682. [Google Scholar] [CrossRef]
- Wu, W.; Panté, N. Vimentin plays a role in the release of the influenza A viral genome from endosomes. Virology 2016, 497, 41–52. [Google Scholar] [CrossRef]
- De Conto, F.; Di Lonardo, E.; Arcangeletti, M.C.; Chezzi, C.; Medici, M.C.; Calderaro, A. Highly dynamic microtubules improve the effectiveness of early stages of human influenza A/NWS/33 virus infection in LLC-MK2 cells. PLoS ONE 2012, 7, e41207. [Google Scholar] [CrossRef]
- Lakadamyali, M.; Rust, M.J.; Babcock, H.P.; Zhuang, X. Visualizing infection of individual influenza viruses. Proc. Natl. Acad. Sci. USA 2003, 100, 9280–9285. [Google Scholar] [CrossRef]
- de Vries, E.; Tscherne, D.M.; Wienholts, M.J.; Cobos-Jiménez, V.; Scholte, F.; García-Sastre, A.; Rottier, P.J.; de Haan, C.A. Dissection of the influenza A virus endocytic routes reveals macropinocytosis as an alternative entry pathway. PLoS Pathog. 2011, 7, e1001329. [Google Scholar] [CrossRef]
- Huang, X.; Yin, G.; Zhou, B.; Cai, Y.; Hu, J.; Huang, J.; Chen, Z.; Liu, Q.; Feng, X. KRT10 plays an important role in the release of viral genome from endosomes during H9N2 subtype AIV replication in HeLa cells. Vet. Microbiol. 2023, 284, 109824. [Google Scholar] [CrossRef]
- Zhang, L.J.; Xia, L.; Liu, S.L.; Sun, E.Z.; Wu, Q.M.; Wen, L.; Zhang, Z.L.; Pang, D.W. A “Driver Switchover” Mechanism of Influenza Virus Transport from Microfilaments to Microtubules. ACS Nano 2018, 12, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Greber, U.F.; Way, M. A superhighway to virus infection. Cell 2006, 124, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.J.; Lakadamyali, M.; Zhang, F.; Zhuang, X. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat. Struct. Mol. Biol. 2004, 11, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, L.; Verkade, P.; Yamauchi, Y. Correlative Light and Electron Microscopy of Influenza Virus Entry and Budding. Methods Mol. Biol. 2018, 1836, 237–260. [Google Scholar] [PubMed]
- Ramos, I.; Stamatakis, K.; Oeste, C.L.; Pérez-Sala, D. Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections. Int. J. Mol. Sci. 2020, 21, 4675. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Yang, J.; Huang, X.; Huang, X.; Yin, G.; Cai, Y.; Feng, X.; Zhang, X.; Li, Y.; Liu, Q. The Function behind the Relation between Lipid Metabolism and Vimentin on H9N2 Subtype AIV Replication. Viruses 2022, 14, 1814. [Google Scholar] [CrossRef] [PubMed]
- Arcangeletti, M.C.; De Conto, F.; Ferraglia, F.; Pinardi, F.; Gatti, R.; Orlandini, G.; Covan, S.; Motta, F.; Rodighiero, I.; Dettori, G.; et al. Host-cell-dependent role of actin cytoskeleton during the replication of a human strain of influenza A virus. Arch. Virol. 2008, 153, 1209–1221. [Google Scholar] [CrossRef]
- Simpson-Holley, M.; Ellis, D.; Fisher, D.; Elton, D.; McCauley, J.; Digard, P. A functional link between the actin cytoskeleton and lipid rafts during budding of filamentous influenza virions. Virology 2002, 301, 212–225. [Google Scholar] [CrossRef]
- Kumakura, M.; Kawaguchi, A.; Nagata, K. Actin-myosin network is required for proper assembly of influenza virus particles. Virology 2015, 476, 141–150. [Google Scholar] [CrossRef]
- De Conto, F.; Fazzi, A.; Razin, S.V.; Arcangeletti, M.C.; Medici, M.C.; Belletti, S.; Chezzi, C.; Calderaro, A. Mammalian Diaphanous-related formin-1 restricts early phases of influenza A/NWS/33 virus (H1N1) infection in LLC-MK2 cells by affecting cytoskeleton dynamics. Mol. Cell. Biochem. 2018, 437, 185–201. [Google Scholar] [CrossRef]
- Bedi, S.; Ono, A. Friend or Foe: The Role of the Cytoskeleton in Influenza A Virus Assembly. Viruses 2019, 11, 46. [Google Scholar] [CrossRef]
- De Conto, F.; Conversano, F.; Razin, S.V.; Belletti, S.; Arcangeletti, M.C.; Chezzi, C.; Calderaro, A. Host-cell dependent role of phosphorylated keratin 8 during influenza A/NWS/33 virus (H1N1) infection in mammalian cells. Virus Res. 2021, 295, 198333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, Y.; Xuan, Z.; Zhang, S.; Wang, X.; Hao, Y.; Wu, J.; Zhang, S. p38MAPK, Rho/ROCK and PKC pathways are involved in influenza-induced cytoskeletal rearrangement and hyperpermeability in PMVEC via phosphorylating ERM. Virus Res. 2014, 192, 6–15. [Google Scholar] [CrossRef]
- Seo, D.; Gammon, D.B. Manipulation of the Host Cytoskeleton by Viruses: Insights and Mechanisms. Viruses 2022, 14, 1586. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bankhead, A., 3rd; Eisfeld, A.J.; Hatta, Y.; Jeng, S.; Chang, J.H.; Aicher, L.D.; Proll, S.; Ellis, A.L.; Law, G.L.; et al. Host regulatory network response to infection with highly pathogenic H5N1 avian influenza virus. J. Virol. 2011, 85, 10955–10967. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Liang, W.; Liu, J.; Meng, D.; Wei, L.; Chai, T.; Cai, Y. Proteomic Analysis of Differential Expression of Cellular Proteins in Response to Avian H9N2 Virus Infection of A549 Cells. Front. Microbiol. 2016, 7, 1962. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Lu, J.; Yu, R.; Wang, X.; Wang, T.; Dong, F.; Peng, B.; Wu, W.; Liu, H.; Geng, Y.; et al. Preliminary Proteomic Analysis of A549 Cells Infected with Avian Influenza Virus H7N9 and Influenza A Virus H1N1. PLoS ONE 2016, 11, e0156017. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Yang, C.; Fang, F.; Wang, Y.; Chang, H.; Chen, Z.; Chen, P. Differential mitochondrial proteomic analysis of A549 cells infected with avian influenza virus subtypes H5 and H9. Virol. J. 2021, 18, 39. [Google Scholar] [CrossRef]
- Qi, W.; Tian, J.; Su, S.; Huang, L.; Li, H.; Liao, M. Identification of potential virulence determinants associated H9N2 avian influenza virus PB2 E627K mutation by comparative proteomics. Proteomics 2015, 15, 1512–1524. [Google Scholar] [CrossRef]
- Zhao, D.; Liang, L.; Li, Y.; Liu, L.; Guan, Y.; Jiang, Y.; Chen, H. Proteomic analysis of the lungs of mice infected with different pathotypes of H5N1 avian influenza viruses. Proteomics 2012, 12, 1970–1982. [Google Scholar] [CrossRef]
- Li, Y.; Ming, F.; Huang, H.; Guo, K.; Chen, H.; Jin, M.; Zhou, H. Proteome Response of Chicken Embryo Fibroblast Cells to Recombinant H5N1 Avian Influenza Viruses with Different Neuraminidase Stalk Lengths. Sci. Rep. 2017, 7, 40698. [Google Scholar] [CrossRef]
- Sharma, S.; Mayank, A.K.; Nailwal, H.; Tripathi, S.; Patel, J.R.; Bowzard, J.B.; Gaur, P.; Donis, R.O.; Katz, J.M.; Cox, N.J.; et al. Influenza A viral nucleoprotein interacts with cytoskeleton scaffolding protein α-actinin-4 for viral replication. FEBS J. 2014, 281, 2899–2914. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Reis, R.; Volcic, M.; Liu, G.; Wang, M.K.; Chia, B.S.; Nchioua, R.; Groß, R.; Münch, J.; Kirchhoff, F.; et al. Actin cytoskeleton remodeling primes RIG-I-like receptor activation. Cell 2022, 185, 3588–3602. [Google Scholar] [CrossRef]
- Liu, H.; Ye, G.; Liu, X.; Xue, M.; Zhou, Q.; Zhang, L.; Zhang, K.; Huang, L.; Weng, C. Vimentin inhibits type I interferon production by disrupting the TBK1-IKKε-IRF3 axis. Cell Rep. 2022, 41, 111469. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Borg, N.A. The multifaceted roles of NLRP3-modulating proteins in virus infection. Front. Immunol. 2022, 13, 987453. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T.; Akira, S. Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity. Immunol. Rev. 2011, 243, 61–73. [Google Scholar] [CrossRef]
- Tolar, P. Cytoskeletal control of B cell responses to antigens. Nat. Rev. Immunol. 2017, 17, 621–634. [Google Scholar] [CrossRef]
- McShane, A.N.; Malinova, D. The Ins and Outs of Antigen Uptake in B cells. Front. Immunol. 2022, 13, 892169. [Google Scholar] [CrossRef]
- Ridge, K.M.; Eriksson, J.E.; Pekny, M.; Goldman, R.D. Roles of vimentin in health and disease. Genes Dev. 2022, 36, 391–407. [Google Scholar] [CrossRef]
- Cheng, F.; Eriksson, J.E. Intermediate Filaments and the Regulation of Cell Motility during Regeneration and Wound Healing. Cold Spring Harb. Perspect. Biol. 2017, 9, a022046. [Google Scholar] [CrossRef]
- Ruggieri, A.; Dazert, E.; Metz, P.; Hofmann, S.; Bergeest, J.P.; Mazur, J.; Bankhead, P.; Hiet, M.S.; Kallis, S.; Alvisi, G.; et al. Dynamic oscillation of translation and stress granule formation mark the cellular response to virus infection. Cell Host Microbe 2012, 12, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.M.; Anekalla, K.R.; Hu, Y.S.; Davis, J.M.; Ciesielski, M.; Gadhvi, G.; Chen, S.Y.; Turner, M.; Cheng, Y.; Coates, B.M.; et al. Influenza-induced activation of recruited alveolar macrophages during the early inflammatory phase drives lung injury and lethality. bioRxiv 2022. [Google Scholar] [CrossRef]
- Yu, M.B.; Guerra, J.; Firek, A.; Langridge, W.H.R. Extracellular vimentin modulates human dendritic cell activation. Mol. Immunol. 2018, 104, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wen, Z.; Shi, X.; Liu, Y.J.; Eriksson, J.E.; Jiu, Y. The diverse roles and dynamic rearrangement of vimentin during viral infection. J Cell Sci 2020, 134, jcs250597. [Google Scholar] [CrossRef] [PubMed]
- Arrindell, J.; Desnues, B. Vimentin: From a cytoskeletal protein to a critical modulator of immune response and a target for infection. Front. Immunol. 2023, 14, 1224352. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, Y.; Ren, C.; Yang, S.; Tian, S.; Chen, H.; Jin, M.; Zhou, H. Influenza A virus protein PB1-F2 impairs innate immunity by inducing mitophagy. Autophagy 2021, 17, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Mostowy, S.; Shenoy, A.R. The cytoskeleton in cell-autonomous immunity: Structural determinants of host defence. Nat. Rev. Immunol. 2015, 15, 559–573. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Chies, J.A.B. Zoonotic spillover: Understanding basic aspects for better prevention. Genet. Mol. Biol. 2021, 44 (Suppl. 1), e20200355. [Google Scholar] [CrossRef]
| Cytoskeletal Proteins | Main Roles | AIV Strain | Mammalian Host | Effects | References |
|---|---|---|---|---|---|
| actin | signaling, transcription, endocytosis, viral budding | H9N2 | A549 cells human | upregulation | [100] |
| AGS cells human | cleaved into fragments to reprogram host protein synthesis | [37] | |||
| F-actin capping protein | growth/polymerization of actin filaments | H7N9 | A549 cells human | downregulation | [101] |
| keratin | structural support, communication between the plasma and nuclear membranes, interaction with small nuclear ribonucleoprotein bodies, metabolism, cell proliferation, apoptotic pathways, shaping host response | H5N1 | Calu-3 cells human | upregulation | [99] |
| keratin 1,2,10 | A549 cells human | downregulation | [102] | ||
| keratin 9,73,78 | upregulation | [102] | |||
| keratin type I and II | H9N2 | AGS cells human | cleaved into fragments | [37] | |
| keratin 10 | A549 cells human | upregulation | [100] | ||
| vimentin isoform 1 | cell proliferation, differentiation, migration, signal transduction, and tissue remodeling | H5N1 | A549 cells human | upregulation | [102] |
| dynein 1, ezrin, LIM, SH3 1, moesin | cytoskeleton regulation, cell shape control, modulation of signaling pathways, modulation of endothelial permeability | H9N2 | mice | downregulation | [103] |
| myosin light polypeptide 3,4,9 and myosin light chain-2 | motor proteins | ||||
| gamma-actin, keratin 78, septin-2 b, coronin actin-binding protein 1A, lamin B, tropomyosin alpha-1 | gene transcription regulation, cell scaffolding | H5N1 | mice | upregulation | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Conto, F. Avian Influenza A Viruses Modulate the Cellular Cytoskeleton during Infection of Mammalian Hosts. Pathogens 2024, 13, 249. https://doi.org/10.3390/pathogens13030249
De Conto F. Avian Influenza A Viruses Modulate the Cellular Cytoskeleton during Infection of Mammalian Hosts. Pathogens. 2024; 13(3):249. https://doi.org/10.3390/pathogens13030249
Chicago/Turabian StyleDe Conto, Flora. 2024. "Avian Influenza A Viruses Modulate the Cellular Cytoskeleton during Infection of Mammalian Hosts" Pathogens 13, no. 3: 249. https://doi.org/10.3390/pathogens13030249
APA StyleDe Conto, F. (2024). Avian Influenza A Viruses Modulate the Cellular Cytoskeleton during Infection of Mammalian Hosts. Pathogens, 13(3), 249. https://doi.org/10.3390/pathogens13030249






