Pathogens Causing Pediatric Community Acquired Urinary Tract Infections and Their Increasing Antimicrobial Resistance: A Nationwide Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Analysis
2.3. Statistical Analysis
3. Results
3.1. Demographic Characteristics
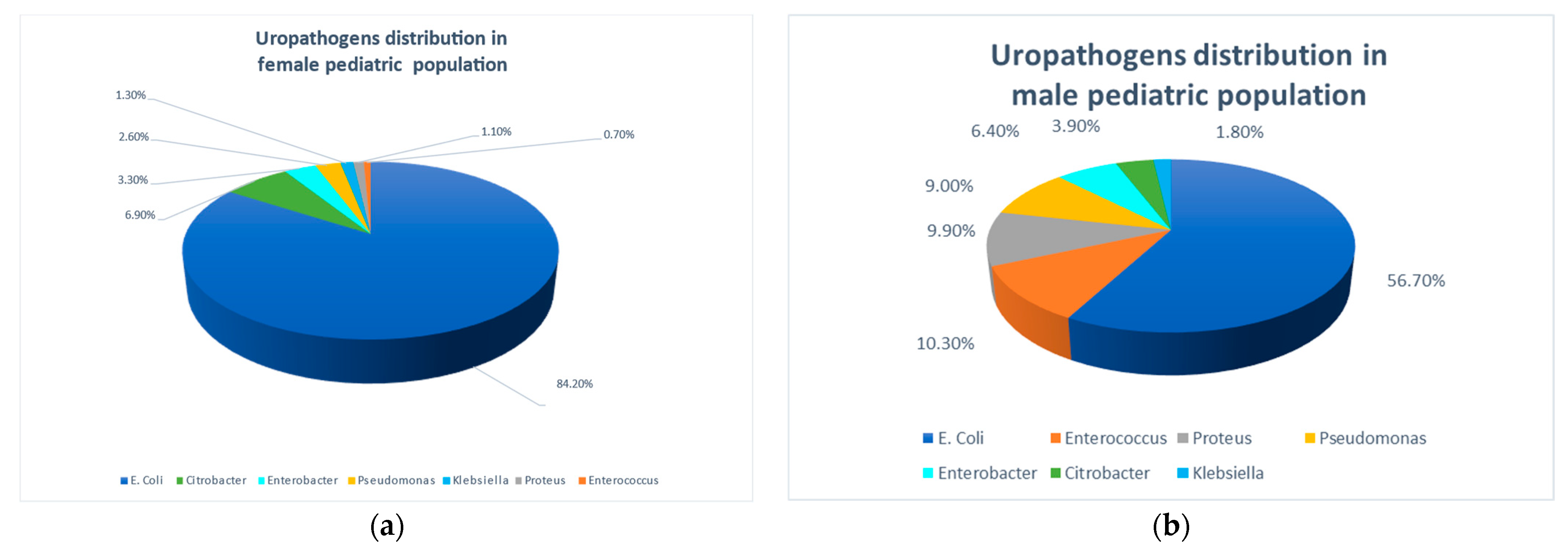
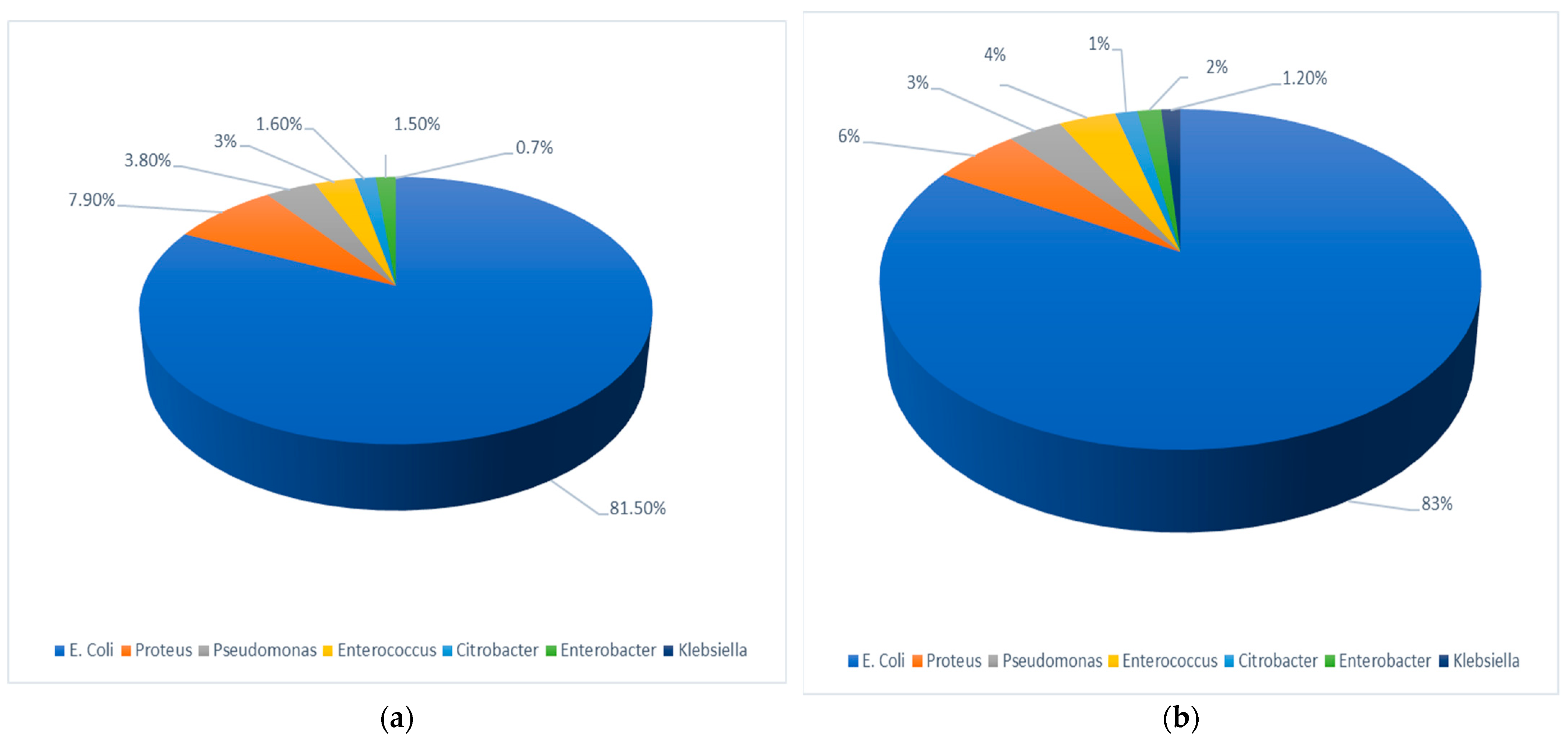
3.2. Isolated Urinary Pathogens
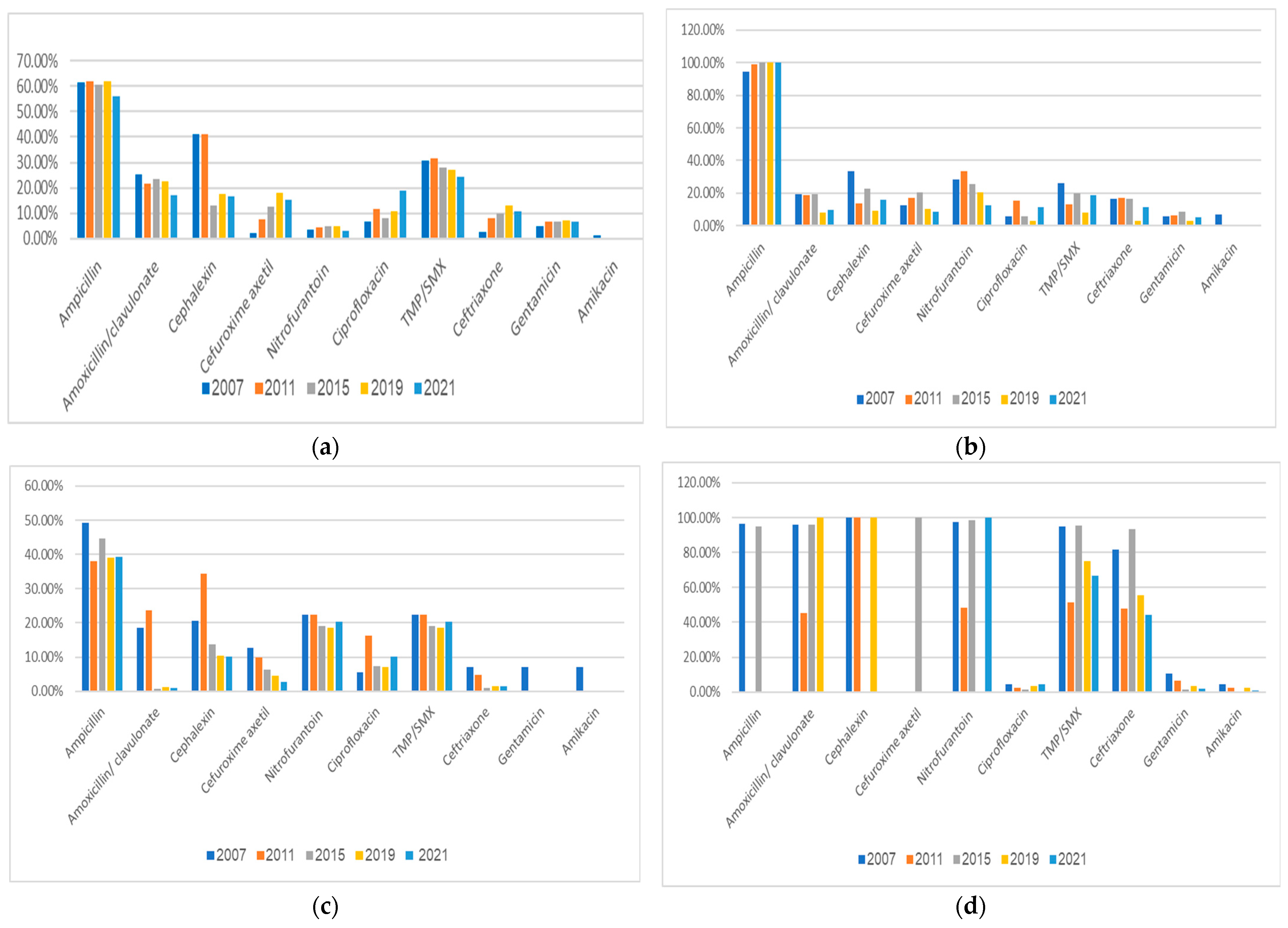
3.3. Antimicrobial Resistance Profiles of Bacterial Uropathogens
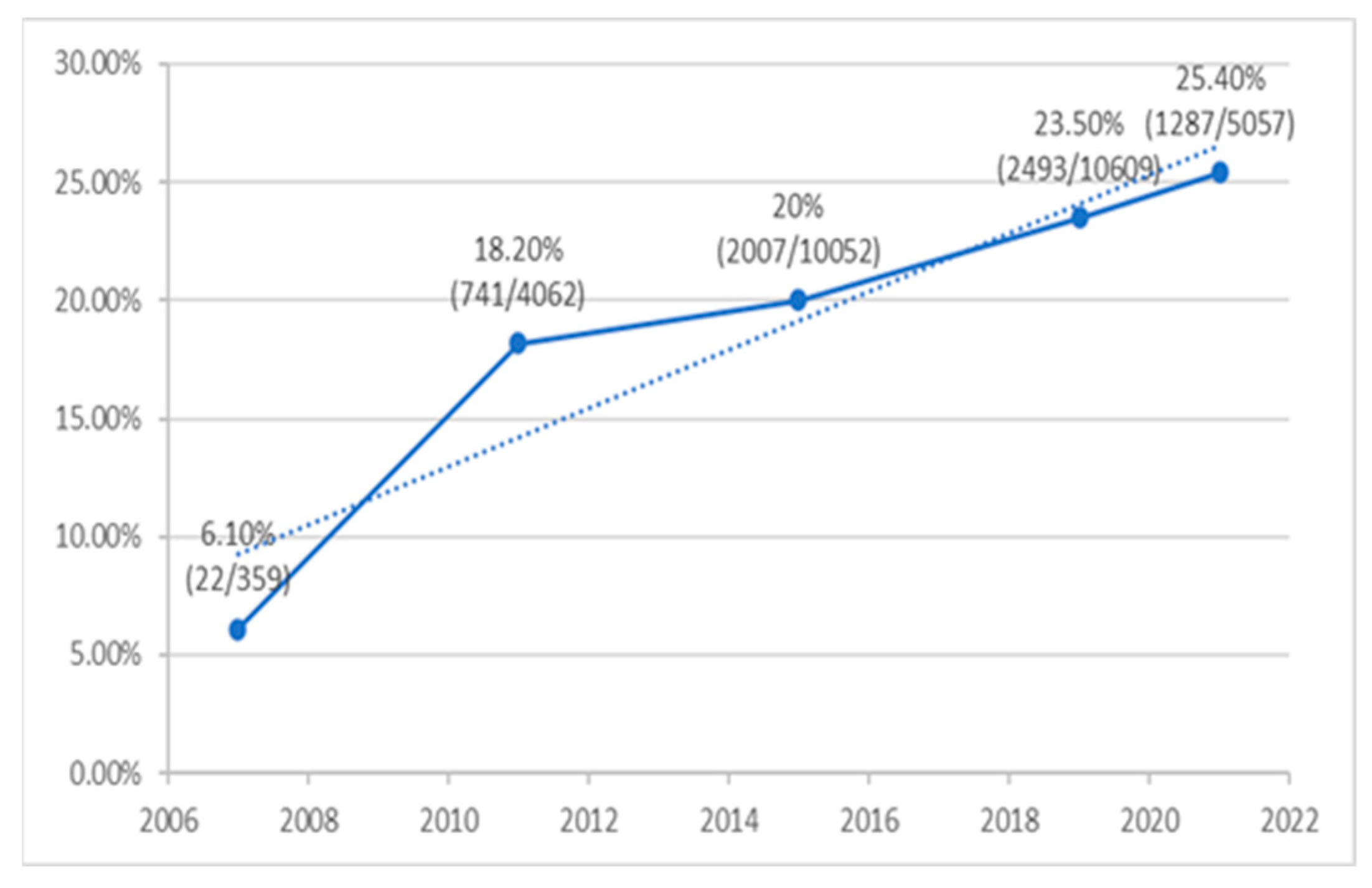
3.4. Frequency of ESBL-Producing E. coli
4. Discussion
- Gender and sector differences in uropathogens: a significantly higher relative prevalence of E. coli in females and the Arab population.
- A significantly increased resistance of E. coli isolates during the 15-year study period to ciprofloxacin, cefuroxime, ceftriaxone, and gentamicin, with most non-E. coli uropathogens resistant to the antimicrobial agents often used empirically to treat UTIs in children.
- The rate of ESBL-positive E. coli causing UTI increased considerably and significantly over the study years, from only 6.1% in 2007 to 25.4% in 2021.
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simões E Silva, A.C.; Oliveira, E.A.; Mak, R.H. Urinary tract infection in pediatrics: An overview. J. Pediatr. (Rio J.) 2020, 96 (Suppl. S1), 65–79. [Google Scholar] [CrossRef]
- Al Lawati, H.; Blair, B.M.; Larnard, J. Urinary Tract Infections: Core Curriculum 2024. Am. J. Kidney Dis. 2024, 83, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Pothoven, R. Management of urinary tract infections in the era of antimicrobial resistance. Drug Target Insights 2023, 17, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Autore, G.; Bernardi, L.; Ghidini, F.; La Scola, C.; Berardi, A.; Biasucci, G.; Marchetti, F.; Pasini, A.; Capra, M.E.; Castellini, C.; et al. Antibiotic prophylaxis for the prevention of urinary tract infections in children: Guideline and recommendations from the Emilia-Romagna Pediatric Urinary Tract Infections (UTI-Ped-ER) study group. Antibiotics 2023, 12, 1040. [Google Scholar] [CrossRef]
- Nuutinen, M.; Uhari, M. Recurrence and follow-up after urinary tract infection under the age of 1 year. Pediatr. Nephrol. 2001, 16, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Beiraghdar, F.; Panahi, Y.; Einollahi, B.; Moharamzad, Y.; Nemati, E.; Amirsalari, S. Predisposing factors for renal scarring in children with urinary tract infection. Saudi J. Kidney Dis. Transpl. 2012, 23, 532–537. [Google Scholar] [PubMed]
- Kaufman, J.; Temple-Smith, M.; Sanci, L. Urinary tract infections in children: An overview of diagnosis and management. BMJ Paediatr. Open 2019, 3, e000487. [Google Scholar] [CrossRef]
- Tambekar, D.H.; Dhanorkar, D.V. The Prevalence and Antibiogram of Potential Bacterial Pathogens in Clinical Specimens. In Proceedings of the 46th Annual Conference of Association of Microbiologist of India, Department of Microbiology, Osmania University, Hyderabad, India, 8–10 December 2005. [Google Scholar]
- Bryce, A.; Hay, A.D.; Lane, I.F.; Thornton, H.V.; Wootton, M.; Costelloe, C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: Systematic review and meta-analysis. BMJ 2016, 352, i939. [Google Scholar] [CrossRef]
- Lukac, P.J.; Bonomo, R.A.; Logan, L.K. Extended-spectrum β-lactamase-producing Enterobacteriaceae in children: Old foe, emerging threat. Clin. Infect. Dis. 2015, 60, 1389–1397. [Google Scholar] [CrossRef]
- Subcommittee on Urinary Tract Infection. Reaffirmation of AAP Clinical Practice Guideline: The diagnosis and management of the initial urinary tract infection in febrile infants and young children 2–24 months of age. Pediatrics 2016, 138, e20163026. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Hindler, J.F. New consensus guidelines from the Clinical and Laboratory Standards Institute for antimicrobial susceptibility testing of infrequently isolated or fastidious bacteria. Clin. Infect. Dis. 2007, 44, 280–286. [Google Scholar] [CrossRef]
- Lee, B.; Kang, S.Y.; Kang, H.M.; Yang, N.R.; Kang, H.G.; Ha, I.S.; Cheong, H.I.; Lee, H.J.; Choi, E.H. Outcome of antimicrobial therapy of pediatric urinary tract infections caused by extended-spectrum β-lactamase-producing enterobacteriaceae. Infect. Chemother. 2013, 45, 415–421. [Google Scholar] [CrossRef]
- Leverstein-van Hall, M.A.; Fluit, A.C.; Paauw, A.; Box, A.T.; Brisse, S.; Verhoef, J. Evaluation of the Etest ESBL and the BD Phoenix, VITEK 1, and VITEK 2 automated instruments for detection of extended-spectrum beta-lactamases in multiresistant Escherichia coli and Klebsiella spp. J. Clin. Microbiol. 2002, 40, 3703–3711. [Google Scholar] [CrossRef]
- Graif, N.; Abozaid, S.; Peretz, A. Trends in distribution and antibiotic resistance of bacteria isolated from urine cultures of children in Northern Israel between 2010 and 2017. Microb. Drug Resist. 2020, 26, 1342–1349. [Google Scholar] [CrossRef]
- Eremenko, R.; Barmatz, S.; Lumelsky, N.; Colodner, R.; Strauss, M.; Alkan, Y. Urinary tract infection in outpatient children and adolescents: Risk analysis of antimicrobial resistance. Isr. Med. Assoc. J. 2020, 22, 236–240. [Google Scholar]
- Konca, C.; Tekin, M.; Uckardes, F.; Akgun, S.; Almis, H.; Bucak, I.H.; Genc, Y.; Turgut, M. Antibacterial resistance patterns of pediatric community-acquired urinary infection: Overview. Pediatr. Int. 2017, 59, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Kazanasmaz, H. Uropathogens and antibiotic resistance in the community and hospital-induced urinary tract infected children. J. Glob. Antimicrob. Resist. 2020, 20, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Long, W.N.W.; Javed, S.; Reynolds, T. Rising resistance of urinary tract pathogens in children: A cause for concern. Ir. J. Med. Sci. 2022, 191, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Pouladfar, G.; Basiratnia, M.; Anvarinejad, M.; Abbasi, P.; Amirmoezi, F.; Zare, S. The antibiotic susceptibility patterns of uropathogens among children with urinary tract infection in Shiraz. Medicine 2017, 96, e7834. [Google Scholar] [CrossRef]
- Gupta, P.; Mandal, J.; Krishnamurthy, S.; Barathi, D.; Pandit, N. Profile of urinary tract infections in paediatric patients. Indian J. Med. Res. 2015, 141, 473–477, Erratum in Indian J. Med. Res. 2015, 141, 850. [Google Scholar] [CrossRef] [PubMed]
- Sharef, S.W.; El-Naggari, M.; Al-Nabhani, D.; Al Sawai, A.; Al Muharrmi, Z.; Elnour, I. Incidence of antibiotics resistance among uropathogens in Omani children presenting with a single episode of urinary tract infection. J. Infect. Public Health 2015, 8, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Hisanaga, T.L.; Laing, N.M.; DeCorby, M.R.; Nichol, K.A.; Weshnoweski, B.; Johnson, J.; Noreddin, A.; Low, D.E.; Karlowsky, J.A.; et al. Antibiotic resistance in Escherichia coli outpatient urinary isolates: Final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int. J. Antimicrob. Agents 2006, 27, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Mirsoleymani, S.R.; Salimi, M.; Shareghi Brojeni, M.; Ranjbar, M.; Mehtarpoor, M. Bacterial pathogens and antimicrobial resistance patterns in pediatric urinary tract infections: A four-year surveillance study (2009–2012). Int. J. Pediatr. 2014, 2014, 126142. [Google Scholar] [CrossRef] [PubMed]
- Alsubaie, M.A.; Alsuheili, A.Z.; Aljehani, M.N.; Alothman, A.A.; Alzahrani, A.S.; Mohammedfadel, H.A.; Alshehry, M.A.; Alnajjar, A.A. Antibiotic resistance patterns of pediatric community-acquired urinary tract infections in a tertiary care center in Jeddah, Saudi Arabia. J. Infect. Dev. Ctries. 2023, 17, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Edlin, R.S.; Copp, H.L. Antibiotic resistance in pediatric urology. Ther. Adv. Urol. 2014, 6, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Marcus, N.; Ashkenazi, S.; Yaari, A.; Samra, Z.; Livni, G. Non-Escherichia coli versus Escherichia coli community-acquired urinary tract infections in children hospitalized in a tertiary center: Relative frequency, risk factors, antimicrobial resistance and outcome. Pediatr. Infect. Dis. J. 2005, 24, 581–585. [Google Scholar] [CrossRef]
- Elnasasra, A.; Alnsasra, H.; Smolyakov, R.; Riesenberg, K.; Nesher, L. ethnic diversity and increasing resistance patterns of hospitalized community-acquired urinary tract infections in Southern Israel: A prospective study. Isr. Med. Assoc. J. 2017, 19, 538–542. [Google Scholar]
- Choe, H.S.; Lee, S.J.; Cho, Y.H.; Çek, M.; Tandoğdu, Z.; Wagenlehner, F.; Bjerklund-Johansen, T.E.; Naber, K.; GPIU Asian Investigators. Aspects of urinary tract infections and antimicrobial resistance in hospitalized urology patients in Asia: 10-year results of the Global Prevalence Study of Infections in Urology (GPIU). J. Infect. Chemother. 2018, 24, 278–283. [Google Scholar] [CrossRef]
- Moya-Dionisio, V.; Díaz-Zabala, M.; Ibáñez-Fernández, A.; Suárez-Leiva, P.; Martínez-Suárez, V.; Ordóñez-Álvarez, F.A.; Santos-Rodríguez, F. Uropathogen pattern and antimicrobial susceptibility in positive urinary cultures isolates from paediatric patients. Rev. Esp. Quimioter. 2016, 29, 146–150. (In Spanish) [Google Scholar]
- Alsubaie, M.A.; Alsuheili, A.Z.; Aljehani, M.N.; Alothman, A.A.; Alzahrani, A.S.; Mohammedfadel, H.A.; Alnajjar, A.A. Pediatric community acquired urinary tract infections due to extended-spectrum beta-lactamase versus non-extended-spectrum beta-lactamase producing bacteria. Pediatr. Int. 2023, 65, e15620. [Google Scholar] [CrossRef]
- Marcus, N.; Ashkenazi, S.; Samra, Z.; Cohen, A.; Livni, G. Community-acquired Pseudomonas aeruginosa urinary tract infections in children hospitalized in a tertiary center: Relative frequency, risk factors, antimicrobial resistance and treatment. Infection 2008, 36, 421–426. [Google Scholar] [CrossRef]
- Marcus, N.; Ashkenazi, S.; Samra, Z.; Cohen, A.; Livni, G. Community-acquired enterococcal urinary tract infections in hospitalized children. Pediatr. Nephrol. 2012, 27, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi-Hoffnung, L.; Livni, G.; Scheuerman, O.; Berger, I.; Eden, E.; Oved, K.; Shani, L.; Kronenfeld, G.; Simon, E.; Boico, O.; et al. Differential serum and urine CRP, IP-10, and TRAIL levels in pediatric urinary tract infection. Front Pediatr. 2021, 9, 771118. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, T.; Basu, U.; Lutz, K.C.; Gadhvi, J.; Komarovsky, J.V.; Li, Q.; Zimmern, P.E.; De Nisco, N.J. Inflammatory markers for improved recurrent UTI diagnosis in postmenopausal women. Life Sci. Alliance 2024, 7, e202302323. [Google Scholar] [CrossRef] [PubMed]
- Abobakr, M.; Uzun, B.; Uzun Ozsahin, D.; Sanlidag, T.; Arikan, A. Assessment of UTI Diagnostic Techniques Using the Fuzzy-PROMETHEE Model. Diagnostics 2023, 13, 3421. [Google Scholar] [CrossRef]
- The Israeli Medical Association Guidelines for Pediatric UTI. 2022. Hebrew. Available online: https://ima-contentfiles.s3.amazonaws.com/clinical_112_UrinaryTractInfectioninChildren.pdf (accessed on 10 January 2024).
- Frimodt-Møller, N.; Bjerrum, L. Treating urinary tract infections in the era of antibiotic resistance. Expert Rev. Anti. Infect. Ther. 2023, 21, 1301–1308. [Google Scholar] [CrossRef]
- Aryee, A.; Rockenschaub, P.; Robson, J.; Ahmed, Z.; Nic Fhogartaigh, C.; Ball, D.; Hayward, A.; Shallcross, L.J. Assessing the impact of discordant antibiotic treatment on adverse outcomes in community-onset UTI: A retrospective cohort study. Antimicrob. Chemother. 2024, 79, 134–142. [Google Scholar] [CrossRef]
- Neto, A.; Sage, H.; Patel, A.K.; Rivera-Sepulveda, A. Antibiotic Stewardship and Treatment of Uncomplicated Urinary Tract Infection (UTI) in Children and Adolescents in the Emergency Department of a Community Hospital. Clin. Pediatr. 2023, 24, 99228231175471. [Google Scholar] [CrossRef]
- Madhi, F.; Rybak, A.; Basmaci, R.; Romain, A.S.; Werner, A.; Biscardi, S.; Dubos, F.; Faye, A.; Grimprel, E.; Raymond, J.; et al. Antimicrobial treatment of urinary tract infections in children. Infect. Dis. Now 2023, 53, 104786. [Google Scholar] [CrossRef]
- Parry, C.M.; Taylor, A.; Williams, R.; Lally, H.; Corbett, H.J. Antimicrobial resistance of breakthrough urinary tract infections in young children receiving continual antibiotic prophylaxis. Eur. J. Pediatr. 2023, 182, 4087–4093. [Google Scholar] [CrossRef]
| Year | Total | ||||||
|---|---|---|---|---|---|---|---|
| 2007 | 2011 | 2015 | 2019 | 2021 | |||
| No. of Patients | 9936 | 10,726 | 12,113 | 11,015 | 9413 | 53,203 | |
| Age (years) | Mean ± SD | 7.2 ± 5.6 | 7.1 ± 5.5 | 7.0 ± 5.5 | 7.0 ± 5.4 | 7.3 ± 5.4 | 7.1 ± 5.5 |
| Median | 5.7 | 5.6 | 5.5 | 5.5 | 5.9 | 5.6 | |
| <3 years | N (%) | 2979 (30%) | 3145 (29.3%) | 3649 (30.1%) | 3223 (29.3%) | 2548 (27.1%) | 15,544 (29.2%) |
| ≥3 years | N (%) | 6957 (70%) | 7581 (70.7%) | 8464 (69.9%) | 7792 (70.7%) | 6865 (72.9%) | 37,659 (70.8%) |
| Gender | 8966 (90.2%) | 9871 (92%) | 11,181 (92.3%) | 10,375 (94.2%) | 8881 (94.4%) | 49,274 (92.6%) | |
| Female | N (%) | ||||||
| Male | N (%) | 970 (9.8%) | 855 (8%) | 932 (7.7%) | 640 (5.8%) | 532 (5.6%) | 3929 (7.4%) |
| Sector | 6698 (67.4%) | 6992 (65.2%) | 8443 (69.7%) | 7871 (71.5%) | 6812 (72.4%) | 36,816 (69.2%) | |
| Jewish | N (%) | ||||||
| Arab | N (%) | 3238 (32.6%) | 3734 (34.8%) | 3670 (30.3%) | 3144 (28.5%) | 2601 (27.6%) | 16,387 (30.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkalim Zemer, V.; Ashkenazi, S.; Levinsky, Y.; Richenberg, Y.; Jacobson, E.; Nathanson, S.; Shochat, T.; Kushnir, S.; Cohen, M.; Cohen, A.H. Pathogens Causing Pediatric Community Acquired Urinary Tract Infections and Their Increasing Antimicrobial Resistance: A Nationwide Study. Pathogens 2024, 13, 201. https://doi.org/10.3390/pathogens13030201
Shkalim Zemer V, Ashkenazi S, Levinsky Y, Richenberg Y, Jacobson E, Nathanson S, Shochat T, Kushnir S, Cohen M, Cohen AH. Pathogens Causing Pediatric Community Acquired Urinary Tract Infections and Their Increasing Antimicrobial Resistance: A Nationwide Study. Pathogens. 2024; 13(3):201. https://doi.org/10.3390/pathogens13030201
Chicago/Turabian StyleShkalim Zemer, Vered, Shai Ashkenazi, Yoel Levinsky, Yael Richenberg, Eyal Jacobson, Shay Nathanson, Tzippy Shochat, Shiri Kushnir, Moriya Cohen, and Avner Herman Cohen. 2024. "Pathogens Causing Pediatric Community Acquired Urinary Tract Infections and Their Increasing Antimicrobial Resistance: A Nationwide Study" Pathogens 13, no. 3: 201. https://doi.org/10.3390/pathogens13030201
APA StyleShkalim Zemer, V., Ashkenazi, S., Levinsky, Y., Richenberg, Y., Jacobson, E., Nathanson, S., Shochat, T., Kushnir, S., Cohen, M., & Cohen, A. H. (2024). Pathogens Causing Pediatric Community Acquired Urinary Tract Infections and Their Increasing Antimicrobial Resistance: A Nationwide Study. Pathogens, 13(3), 201. https://doi.org/10.3390/pathogens13030201





