Abstract
Staphylococcus pseudintermedius is an emergent zoonotic agent associated with multidrug resistance (MDR). This work aimed to describe the antibacterial activity of four essential oils (EOs) and silver nanoparticles (AgNPs) against 15 S. pseudintermedius strains isolated from pyoderma. The four EOs, namely Rosmarinus officinalis (RO), Juniperus communis (GI), Citrus sinensis (AR), and Abies alba (AB), and AgNPs were used alone and in combination to determine the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC). All strains were MDR and methicillin-resistant. Among the antibiotic cohort, only rifampicin, doxycycline, and amikacin were effective. EOs’ chemical analysis revealed 124 compounds belonging to various chemical classes. Of them, 35 were found in AR, 75 in AB, 77 in GI, and 57 in RO. The monoterpenic fraction prevailed over the sesquiterpenic in all EOs. When EOs were tested alone, AB showed the lowest MIC followed by GI, AR, and RO (with values ranging from 1:128 to 1:2048). MBC increased in the following order: AB, AR, GI, and RO (with values ranging from 1:512 to 1:2048). MIC and MBC values for AgNPs were 10.74 mg/L ± 4.23 and 261.05 mg/L ± 172.74. In conclusion, EOs and AgNPs could limit the use of antibiotics or improve the efficacy of conventional therapies.
1. Introduction
Over the last decade, Staphylococcus pseudintermedius has gained attention due to its zoonotic potential, directly linked to the genetic acquisition of antibiotic-resistant genes (ARGs) and virulence factors [1,2,3]. Up to 97.8% of Methicillin-resistant. pseudintermedius (MRSP) isolates have shown multidrug resistance to three or more antibiotics commonly administered in veterinary medicine [4,5]. The colonization of S. pseudintermedius is quite similar to that of S. aureus in humans, with human nares being the most prevalent site of colonization, in contrast to the pharynx and rectum in companion animals (e.g., cats, dogs, horses) [1,6]. S. pseudintermedius is noted for its opportunistic potential, particularly in immunocompromised hosts, and is a typical commensal of dogs. Furthermore, it has been linked to several cases of human colonization and infection, most of which were caused by intimate contact between companion dogs and people [7,8,9,10]. S. pseudintermedius is more likely to adapt in humans due to the intimate interaction between companion animals (particularly dogs and cats), owners, and other individuals, such as small-animal veterinarians, as reported in the literature [11]. There are limited studies that discuss the transmission, colonization, and infection of humans by S. pseudintermedius because it is often misidentified as S. aureus. Additionally, most diagnostic laboratories cannot afford to use advanced technologies like MALDI-TOF MS, PCR with species-specific gene targeting, MLST, and whole-genome sequencing. Consequently, accurately determining the true occurrence of zoonotic transmission, human colonization, and infection, and the present epidemiology of this pathogen poses some difficulties [12].
As a result, the need for alternatives to standard antibiotics is urgent. In this context, secondary metabolites of plants including essential oils (EOs), produced in response to environmental conditions such as herbivore assault, abiotic stress, or interspecific interactions, have emerged as significant potential choices [13]. The use of EOs as antibiotic alternatives has attracted substantial attention in recent years, indicating a paradigm change in the approach to bacterial diseases management [14]. EOs are the primary components of aromatherapy, and they are produced by up to 17,000 distinct plant species from 60 different families (e.g., Lamiaceae, Rutaceae, Myrtaceae, Zingiberaceae, and Asteraceae) [15]. The wide and complicated chemical composition of EOs, which often comprises many bioactive substances functioning synergistically, is one of their primary benefits [16]. Because of this complication, it is difficult for bacteria to evolve specific resistance, which is a major concern with standard antibiotics [17]. Further researches are necessary to comprehensively comprehend the mechanisms of EOs and their suitable use in clinical settings [18,19]. In recent years, various in vivo and in vitro investigations have been conducted focusing on the efficiency of several EOs against the etiological agents of pyoderma in dogs [15,20,21,22]. Many EOs may be used to treat various skin disorders; however, due to their bioactive chemical components, some are particularly efficient against Gram-positive and Gram-negative bacteria [23], in particular those with significant percentages of thymol and carvacrol have extraordinary membrane-damaging action in bacteria [24,25,26].
A second rapidly-growing field is nanotechnology, aiming to synthesize and characterize nanoparticles (NPs) with several applications in different scientific disciplines [27]. As known, silver (Ag) is a noble metal used for centuries as an antibacterial agent and its chemical properties made it a valuable candidate as a metallic precursor for the synthesis of NPs [5]. Due to their unique physical and chemical characteristics, AgNPs demonstrated formidable antimicrobial and anti-inflammatory capabilities, making them interesting in dermatology [28,29]. Wound recovery is one of the principal applications of AgNPs [30]. Antimicrobial properties aid in the prevention of infections, while wound healing is expedited by their capacity to stimulate cell proliferation and collagen synthesis [31,32,33].
This study investigates the in vitro inhibitory (MIC) and bactericidal (MBC) effects of four commercially available EOs in conjunction with chemically-synthesized silver nanoparticles against multi-drug resistant S. pseudintermedius. For the best knowledge of the authors this is the first in vitro study focused on the combined use of NPs and EOs.
2. Materials and Methods
2.1. Bacterial Strains, Identification, and Culture Conditions
Fifteen S. pseudintermedius strains were chosen from the Department of Biomedical, Surgical, and Dental Sciences’ (University of Milan) bacterial collection. Bacteria were isolated starting from 201 from deep canine cutaneous pyoderma and stored at −20 °C in 25% (v/v) glycerol (Carlo Erba, Cornaredo, Italy). Original isolation was performed using both phenotypic and molecular techniques. In brief, phenotypic identification was made using Mannitol Salt Agar (Microbiol, Macchiareddu, Italy) as a selective and differential medium after the first isolation on Columbia Base Agar (ThermoFisher, Monza, Italy) with 5% defibrinated sheep blood (Microbiol, Italy). Molecular identification was conducted, [34] coupled with a Restriction Fragment Length Polymorphism (RFLP) assay [35]. After identification, each sample was stored in filtered (0.22 µm) glycerol at −20 °C. The day before the experiment, each sample was thawed at room temperature and plated on Columbia Base Agar (ThermoFisher, Italy) with 5% defibrinated sheep blood (Microbiol, Italy) and incubated aerobically at 37 °C for 18–24 h.
2.2. Determination of Antimicrobial Profiles
The Kirby–Bauer Disk diffusion assay was used to investigate the susceptibility of the strains to 22 antibiotic molecules following the Clinical and Laboratory Standard Institute guidelines [36]. The antimicrobials (abbreviations and concentrations in µg) used were Oxacillin (OX; 5), Amoxicillin + clavulanic acid (AMC; 30), Amoxicillin (AMO; 30), Carbenicillin (CAR; 100), Cephalexin (CL; 30), Cefovecin (CVN; 30), Ceftiofur (EFT; 30), Ceftriaxone (CRO 30, Clindamycin (DA; 10), Lincomycin + Spectinomycin (LC-SP; 15), Doxycycline (DO; 30), Enrofloxacin (ENR; 5), Marbofloxacin (MAR; 5), Pradofloxacin (PRA; 5), Amikacin (AK; 30), Gentamicin (CN; 30), Neomycin (N; 30), Tobramycin (TOB; 10), Kanamycin (K; 30), Rifampicin (RD; 30), Azithromycin (AZM; 15), Erythromycin (E; 30).
ARGs were detected using two sets of mPCR targeting mecA, blaZ, aacA-aphD, tetM and tetK genes, as previously described [15].
2.3. Synthesis and Characterization of Silver Nanoparticles
AgNPs were chemically synthesized using silver nitrate (AgNO3, Carlo Erba, Italy) as the metal ion donor, as described [37]. Briefly, a 100 mM solution of AgNO3 was prepared by dissolving 8.49 g of salt in 500 mL of distilled water and heated at 90 °C for 5 min. By dripping, 12.5 mL of 1% Tri-Sodium Citrate (TSC) in water was added. The reduction of metal ions was ascertained by the color change of the solution from transparent to brown. The volume was transferred into a separatory funnel and darkened for 24 h. After eluting, the volume fraction containing the NPs (approximately 50 mL) was centrifuged (4000 RCF, 15 min), washed twice with distilled water, and freeze-dried (CoolSafe Basic, Labogene, Scandinavia) for 24 h at −54 °C.
The reduction from Ag+ to Ag0 was monitored by measuring the ultraviolet-visible absorption spectrum UV-Vis (SpectraMax 340 PC, Molecular Devices (Germany) GmbH, Munich, Germany) at wavelengths from 310 to 770 nm (with a 50 nm path). Readings were taken twice within 15 min. Using a transmission electron microscope (EFTEM Leo 912ab (Zeiss, Milan, Italy)) at a voltage of 100 kV, the nanoparticles’ morphology was determined. Samples were briefly sonicated (30 kHz, 15 s on and 45 s off), and immediately, a drop of the aqueous AgNPs’ suspension was mounted on a carbon grid and placed on a filter paper to absorb excess solvent. The morphological analysis (diameter and particle size distribution) was calculated with ImageJ2 software (v. 1.54h).
2.4. Characterization of Essential Oils
The four EOs, Rosmarinus officinalis (RO), Juniperus communis (GI), Citrus sinensis (AR), and Abies alba (AB), were purchased from an Italian company (Vitalis, Milan, Italy).
The EOs were diluted in methanol (as reported in the literature [38]), and characterization was performed using an Agilent 5975C Series GC/MS and FID as the detector. Volatiles were separated using an apolar capillary column Zebron–Semivolatiles (Zebron, Phenomenex, Torrance, CA, USA) of 30 m × 0.25 mm (ID) and a film thickness of 0.25 µm. The carrier gas was helium at a flow rate of 1 mL/min. Two µLs of each sample were injected in the GC MS using a CTC PAL system in triplicates. The injector was set at 230 °C under splitless mode. The temperature program was isothermal for 3.5 min at 40 °C, then, two different temperature ramps were conducted to reach 140 °C, 150 °C (hold time of 7 min), and 220 °C (hold time of 23 min) at rates of 7 °C/min and 20 °C/min, respectively. The transfer line to the mass spectrometer was maintained at 150 °C. The mass spectra were obtained by electronic impact at 70 eV, a multiplier voltage of 1294 V, collecting data at a m/z range of 35–500. The retention indices were determined in relation to a homologous series of n-alkanes (C7–C30, Sigma Aldrich, Milan, Italy) under the same operational conditions. The chromatograms were elaborated using the open-source MS-DIAL using the NIST14 library as a reference, considering a total score (TS) = 70% as the cutoff of the data, as suggested by the Metabolomics Standards Initiative of the International Metabolomics Society [39]. The TS was a quality index applied in Ms-DIAL, calculated using (i) retention index similarity, (ii) accurate mass similarity, (iii) spectra similarity, and (iv) isotope spectra similarity of each compound [40]. The score of each parameter was standardized (0 = no quality and 1 = perfect match) and mathematically elaborated to yield a TS range of 0–100. For the retained molecules (TS > 70%), the abundance data were expressed as a percentage of the sum of the total ion current (TIC). The results were expressed as average ± standard deviations.
2.5. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of AgNPs and EOs
The MIC of AgNPs and EOs was determined by following the microdilution method according to the Clinical and Laboratory Standard Institute guidelines [36]. As found in the literature, EOs were initially diluted (75%) in dimethyl sulfoxide (DMSO, Merck, Milan, Italy) to allow two-fold dilution in Mueller–Hinton broth (Microbiol, Italy), reaching a linear oil gradient from 18.75% (v/v) to 0.04% (v/v). AgNPs were dissolved in distilled water to reach an initial concentration of 2.048 mg/mL and create a gradient from 512 µg/mL to 1 µg/mL. When combined with EOs, AgNPs were diluted in DMSO to maintain the same antimicrobial gradient. Before testing EOs and AgNPs against field strains, ATCC cultures were used (S. aureus ATCC 6358, Escherichia coli ATCC 25922, Klebsiella pneumoniae subsp. ozaenae ATCC 11296, Micrococcus yunnanensis ATCC 7468, Enterococcus casseliflavus ATCC 12755, Providencia rettgeri ATCC 9250, Proteus vulgaris ATCC 7829, Streptococcus agalactiae ATCC 13813, Salmonella enterica subsp. enterica serovar Enteritidis ATCC 25928, see Supplemental Table S1). DMSO alone (100% v/v) was tested to rule out its potential antibacterial activity. Positive and negative controls were inserted into each 96-well plate consisting of bac-teria without tested molecules and broth alone, respectively. After 24 h of incubation at 37 °C, the MIC was visually determined as the lowest concentration that inhibited bacterial growth.
The MBC was determined by inoculating the entire volume of the wells (200 µL) into tubes with sterile Mueller–Hinton broth and incubating at 37 °C for 24 h. The MBC was indicated as the lowest concentration capable of killing the bacteria in the broth. The lack of growth in the tubes was verified with a densitometer (BioSan Densitometer, Riga, Latvia) and compared with the positive and negative controls.
3. Results
3.1. Phenotypic and Molecular Profiling of Antibiotic Resistance
All S. pseudintermedius strains were found to be resistant to at least three antibiotic classes tested (β-lactams [B-LAC], Lincosamides [LIN], Tetracyclines [TET], Fluoroquinolones [FLQ], Aminoglycosides [AMN], Rifamycins [RIF], Macrolides [MAC]) and are therefore classified as MDR. Furthermore, all were found to be resistant to methicillin, which makes them MRSP (Table 1).

Table 1.
Antibiotic resistance profiles of the S. pseudintermedius strains included in the study.
Resistance to penicillins and fluoroquinolones was 97.4% and 80.7%, respectively. The cephalosporins (all third-generation except for first-generation cephalexin) showed a slightly different trend, with a resistance of 89.5%. Interestingly, among the third-generation molecules tested, ceftriaxone showed the same resistance as cephalexin (78.9%), a first-generation cephalosporin. Penicillins were effective in about 2.6% of strains, while cephalosporins were effective in 6.6%. Lincosamides are the antibiotic category with the second-greatest resistance detected (around 95%). clindamycin, while only two strains were susceptible to the combination of lincomycin and spectinomycin. Approximately 47% of strains were susceptible to doxycycline. Fluoroquinolones are the second antibacterial category used in the treatment of pyoderma; among the three molecules, marbofloxacin is the most active (32%), followed by ENR (15.8%) and PRA (5%). Among aminoglycosides (which showed a sensitivity of 33%), amikacin was the molecule to which all strains were sensitive, followed by gentamicin and tobramycin with susceptibilities of 37% and 26%, respectively. In this study, rifampicin is the molecule to which all strains are susceptible, opposite of the macrolides trend (both AZM and E), which showed resistance to all S. pseudintermedius strains tested.
Genetically, all strains were positive for the mecA gene, conferring resistance to methicillin (and all penicillins and cephalosporins except ceftaroline), with 84% being positive for the blaZ gene, conferring resistance to β-lactams (in particular, ampicillin, amoxicillin, and amoxiclavulanate). The aacA–aphD gene (aminoglycoside resistance) was amplified in 78% of the bacteria, while the tetM and tetK genes were present in 5 and 7 strains, respectively.
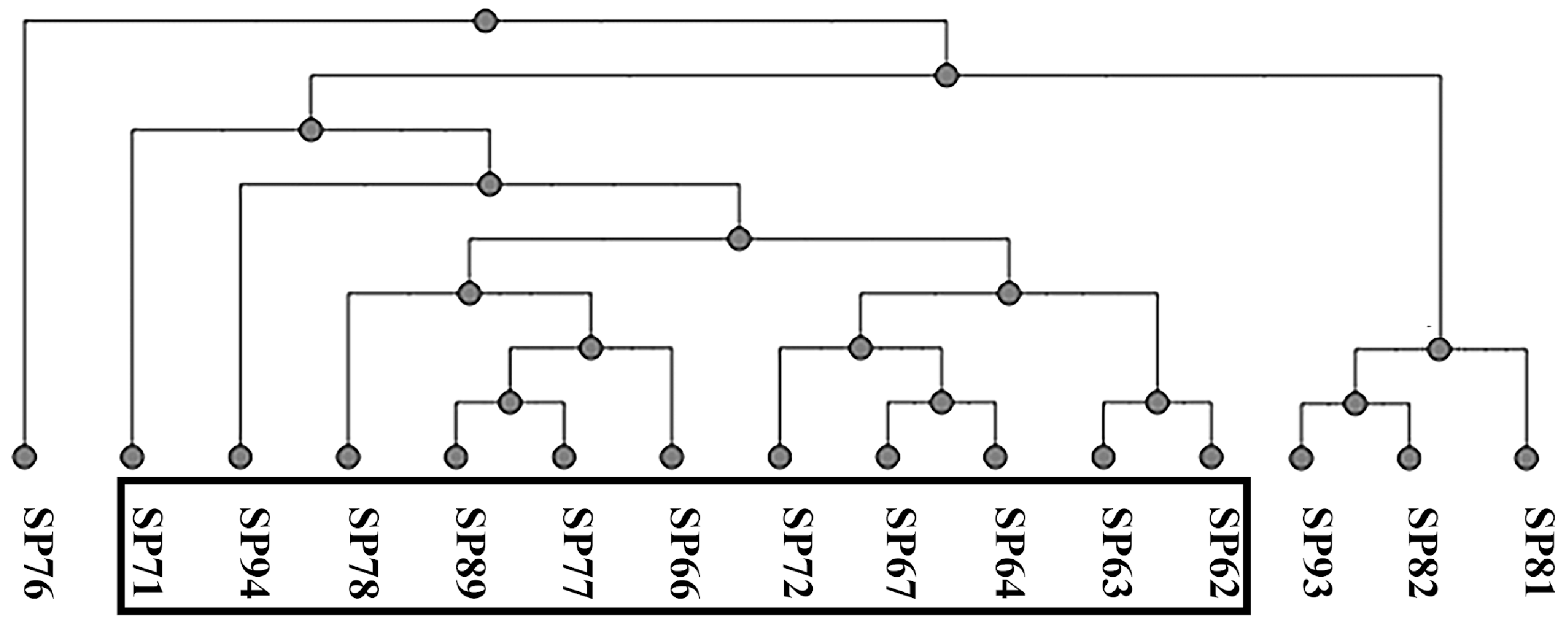
All the strains were full-length sequenced using a third-generation sequencing machine [41]. The Multi-locus Sequence Typing (MLST) analysis was conducted using an online tool (accessed on November 2023), and three different sequence types (STs) were found; ST71 was the most prevalent (11/15; 73%), followed by ST258 (3/15; 20%) and ST301 (1/15; 7%). Figure 1 represents the UPGMA cluster derived from the pangenome analysis with an online tool (accessed on November 2023). The black box includes the SP strains of ST71, which share the staphylococcal chromosome cassette (SCC)mec type II–III.
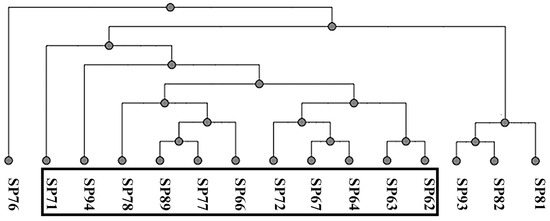
Figure 1.
Pangenome-based UPGMA cluster analysis.
3.2. Characterization of Antimicrobial Molecules
3.2.1. AgNPs
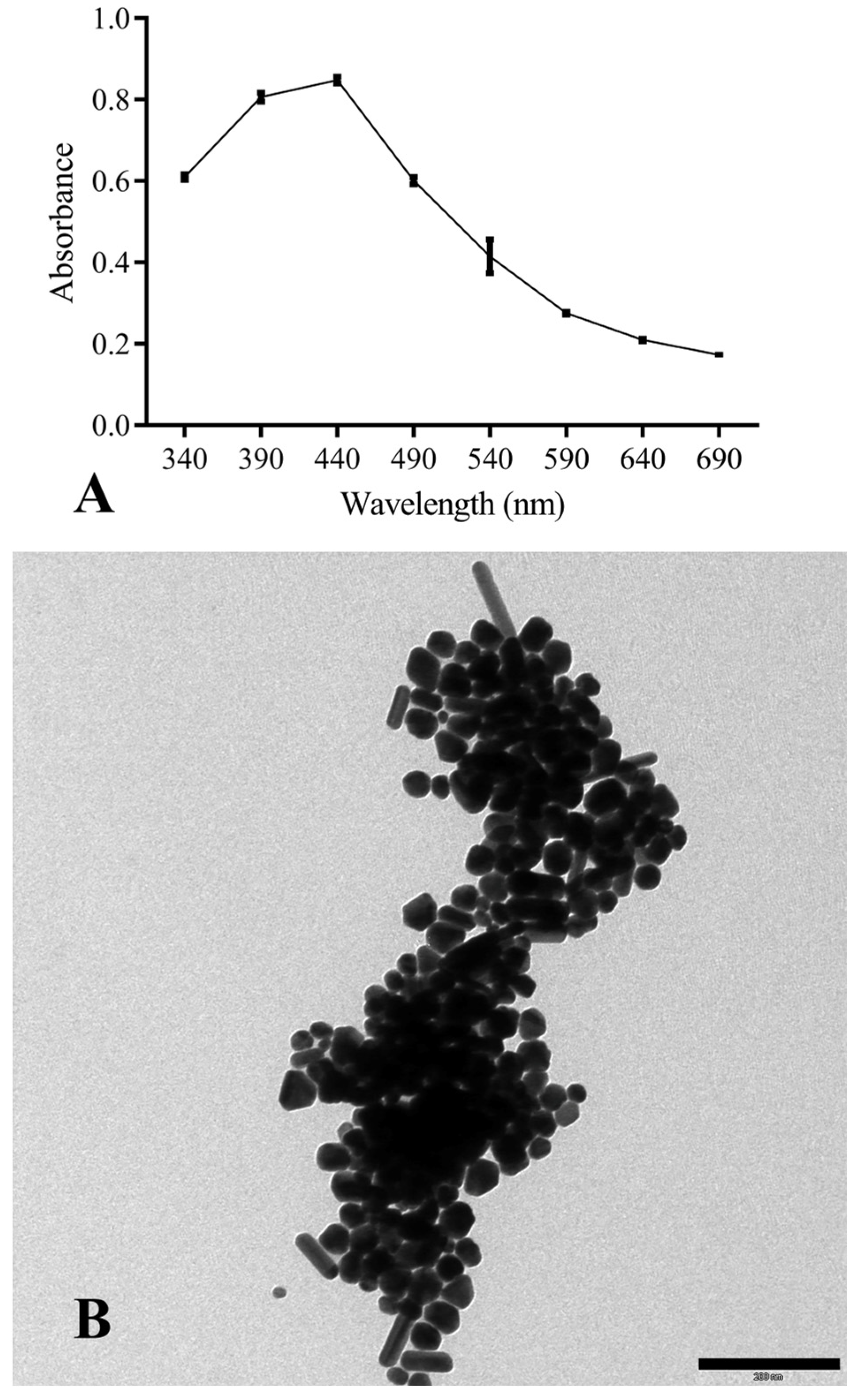
AgNPs were obtained by chemical synthesis from silver nitrate as the Ag+ donor and TSC as the reducing and stabilizing agent. The first macroscopic characterization indicative of the reduction of Ag+ to Ag0 was the observation (over time) of the solution’s color change, which progressively turned from transparent to dark brown. Once the synthesis was completed, ultraviolet-visible spectroscopy (UV-Vis spectroscopy) confirmed the presence of metal nanoparticles. This technique makes it possible to derive qualitative–quantitative information by exploiting the ability of different substances to absorb a given wavelength, which in the case of AgNPs is 440 nm (Figure 2A).
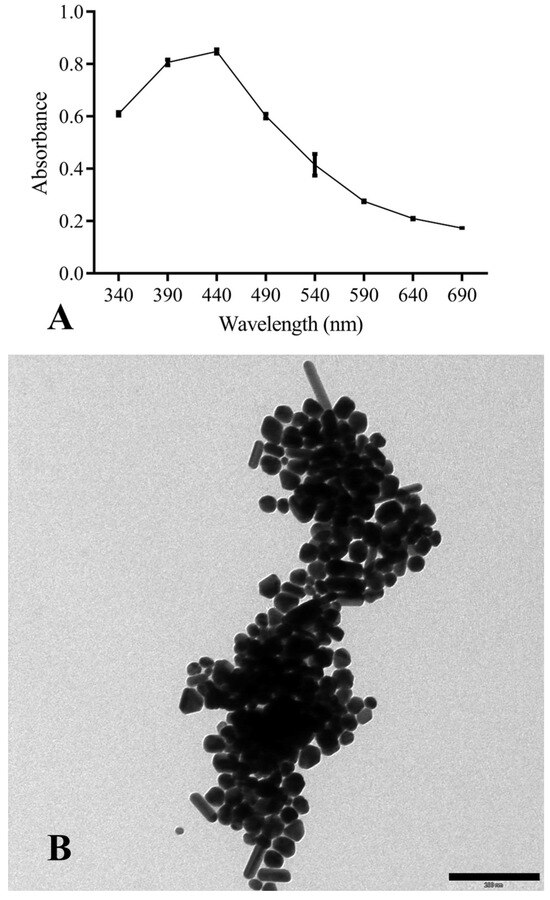
Figure 2.
(A) UV-Vis absorption spectra of chemically-synthesized AgNPs; (B) TEM morphological analysis of NPs (scale bar corresponds to 200 nm).
During the synthesis, the pH of the reaction was not controlled; consequently, the morphology of the particles (Figure 2B) was not homogeneous but had different symmetries: triangular, pentagonal, hexagonal, filiform, spherical, and cubic.
The size analysis was performed with ImageJ2 software (v. 1.54h), which allowed the area of NPs with spherical symmetry to be derived (for the other symmetries, although it was possible to determine the area, the volume could not be calculated). The synthesized AgNPs had a size of 15 ± 2.7 nm. A factor affecting their antibacterial capacity is the surface area/volume ratio (S/V), which is linked to the contact surface area between the particles and the pathogen. In this work, the S/V ratio was 0.56 ± 0.09 nm2/nm3.
3.2.2. EOs
The constituents of the four EOs are reported in Table 2, listed in ascending order of their linear retention indices (LRIs) on the apolar column. A total of 124 compounds belonging to different chemical classes have been identified. Of these, 35 were detected in AR, representing 96.68% of EO, 75 in AB (98.09%), 77 in GI (91.75%), and 57 in RO (98.65%). In all EOs, the monoterpenic fraction (40.55%, 39.68%, 39.56%, and 60.17%, respectively) prevailed over the sesquiterpenic fraction (6.96%, 23.54%, 30.64%, and 17.73%). Oxygenated monoterpenes were more abundant than monoterpene hydrocarbons in AR (22:1), lower in AB (1:2) and GI (1:1.2), and in similar percentages in RO (1:1). In detail, cis- or trans-p-menth-2-en-1-ol characterized AR and AB (36.14% and 6.1%, respectively), GI (5.96%) together with 1,6-octadien-3-ol, 3,7-dimethyl- (6.12%), and RO (7.22%) together with cis-β-terpineol (8.1%). Among the sesquiterpenoids, oxygenated sesquiterpenes were the main components in AR, AB, and GI (6.62%, 12.57%, and 21.98%) with α-sinensal (1.91%), α-santalene (3.71%), and guaiol (3.98%) as the major compounds, respectively. Sesquiterpene hydrocarbons predominated in RO (12.41%), and (+)-sativene was the most abundant (5.71%). Diterpenoids were present in very low percentages (0.55% to 3.58%) in three of the four samples, and absent in RO.

Table 2.
Chemical composition (%) of Citrus sinensis (AR), Abies alba (AB), Juniperus communis (GI), and Rosmarinus officinalis (RO) essential oils determined by GC/MS.
3.3. Antibacterial Activity of EOs and NPs
In this work, the antibacterial action of AgNPs and EOs of Spruce (AB), Orange (AR), Juniper (GI), and Rosemary (RO) was investigated by determining the MIC and MBC (Table 3 and Table 4). In all the tests performed, the viability control with DMSO alone (100% v/v) showed that this substance (used to make the oils soluble and disperse the nanoparticles) did not interfere with the biological action observed by the tested molecules.

Table 3.
Minimum inhibitory concentration (MIC) of the tested EOs and NPs against S. pseudintermedius strains.

Table 4.
Minimum bactericidal concentration (MBC) of the tested EOs and NPs against S. pseudintermedius strains.
The results reported in Table 3 and Table 4 show that both AgNPs and EOs succeed in inhibiting and killing S. pseudintermedius strains. With 18/19 (94.73%) strains exhibiting MIC values greater than 1/128 v/v, AB produced the greatest inhibitory effects against all strains of S. pseudintermedius among the investigated essential oils. This was followed by GI (17/19; 89.47%), AR (9/19; 47.36%), and RO (9/17; 52.94%), respectively. AB had the highest level of bactericidal activity, as it was able to eliminate 13/18 (72.2%) strains with an EO concentration that was more than 1/32 v/v. This was followed by GI (13/19; %), AR (6/18; 33.3%), and RO (1/17; 5.8%), depending on the strain.
When AgNPs were introduced to EOs, the same pattern that was seen for EOs that were evaluated on their own was discovered. Following the incorporation of NPs, a decrease in the values of both the MIC and MBC was found for each and every EOs that was examined. More specifically, the MIC values for AB ranged from 1:256 to 1:2048 v/v, which led to the inhibition of 17/19 (89.4%) S. pseudintermedius strains at concentrations equal to or higher than 1:1024. This was followed by the inhibition of GI (15/19; 78.9%), AR (13/19; 68.4%), and RO (13/19; 78.9%), respectively. A distinct pattern was seen in the bactericidal efficacy of the combination when compared to the minimum inhibitory concentration. To be more specific, 17/19 (89.4%) of the strains that were examined were eliminated by AR oil at a concentration that was equal to or greater than 1:512. This was followed by GI (16/19; 84.2%), AB (14/19; 73.68%), and RO (12/19; 63.1%), in that order.
4. Discussion
The global increase in the prevalence of MDR, and more broadly, MRSP strains isolated from both cases of canine pyoderma and human infections, is of growing interest in the public health landscape. If this fact is also associated with the limited pharmacological choice resulting from the lack of new antibiotic molecules, developing alternatives to classic antimicrobial therapy must be considered. Within the realm of novel therapeutic possibilities, the rediscovery of alternative treatments based on the use of natural ingredients and the creation and characterization of nanotechnologies are piquing the scientific community’s attention.
The antibiogram findings suggest a scenario of widespread resistance, making it difficult for the veterinarian to select the optimal antibiotic to administer. Because all strains were mecA-positive, no β-lactam antibiotics may be used to treat infection with this disease. The only two compounds all strains were susceptible to were amikacin (aminoglycoside) and rifampicin (rifamycins), both nephrotoxic and hepatotoxic. The resistance profile found in this work is consistent with that obtained in another Italian investigation that examined MRSP and MSSP strains isolated from dogs in Milan and Naples [42].
The role of EOs against the most common pathogens has been investigated extensively [43,44,45,46,47,48,49]. On the other hand, data on their efficacy against S. pseudintermedius are scarce. The results of this work are quite promising and confirmed those of the limited available literature. Indeed, in different studies, the MIC value of RO towards S. pseudintermedius was 0.5% [50]. For S. aureus, multiple results are present: 1.5% [51]; 1.5–3.6% [52]; 2% [53]. Fu et al. found an MIC value for S. aureus and S. epidermidis of 0.125% and 0.250%, respectively [54]. No data from similar studies using the same pathogen are available for other EOs used in this work. Only one Italian study (Nocera et al., 2020) demonstrated that MRSP strains isolated from dogs are susceptible to the action of some EOs, albeit different ones [55]. As regards the main constituents of the four EOs tested, although their specific activity against SP has not yet been documented, some of them, such as p-menthenols, have already shown a significant antimicrobial potential against some pathogenic strains [56] or have been recognized (e.g., 1,6-octadien-3-ol, 3,7-dimethyl-, cis-β-terpineol, α-santalene, or guaiol) as responsible for the antibacterial activity of the EOs in which they were present in greater quantities [57,58,59,60]. However, it is now well-established that minor active compounds coexisting in essential oils can contribute to their registered antimicrobial activity [61]
In the present study, NPs were synthesized using a chemical method, which allowed the obtaining of particles of different symmetries. This leads to the second limitation found in this work that concerns the lack of homogeneity in the morphology of the particles obtained, as described in the results (and confirmed by other authors). The failure to control the pH of the reaction favored the synthesis of multiple morphologies which, nevertheless, exerted a bactericidal action certainly superior to one-dimensional colloidal solutions. This approach is certainly not free from potential errors, but it offers better results when compared to plant- or bacteria-mediated biological synthesis [5].
5. Conclusions
Our investigation has discovered, for the first time and for the best of our knowledge, the antibacterial effects of our chosen essential oils against methicillin-resistant Staphylococcus pseudintermedius strains obtained from dogs with pyoderma. Among the EOs tested, AR showed the most promising results both tested alone and in combination with AgNPs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens13020156/s1, Table S1: Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the tested EOs against bacterial reference strains.
Author Contributions
Conceptualization, G.M., P.A.M. and K.T.; methodology, G.M., G.L. and A.T.; formal analysis, G.M., G.L., S.V. and B.S.; investigation, A.S., M.I. and P.A.M.; resources, A.S., L.B. and M.I.; data curation, G.L., B.S., A.S., K.T. and S.V.; writing—original draft preparation, G.M., G.L., S.V. and A.T.; writing—review and editing, L.B., P.A.M. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented in this study are available from the corresponding author upon request.
Acknowledgments
The authors extend their gratitude to Laura Rota for her invaluable assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Somayaji, R.; Priyantha, M.A.R.; Rubin, J.E.; Church, D. Human Infections Due to Staphylococcus Pseudintermedius, an Emerging Zoonosis of Canine Origin: Report of 24 Cases. Diagn. Microbiol. Infect. Dis. 2016, 85, 471–476. [Google Scholar] [CrossRef]
- Ference, E.H.; Danielian, A.; Kim, H.W.; Yoo, F.; Kuan, E.C.; Suh, J.D. Zoonotic Staphylococcus Pseudintermedius Sinonasal Infections: Risk Factors and Resistance Patterns. Int. Forum Allergy Rhinol. 2019, 9, 724–729. [Google Scholar] [CrossRef]
- Kmieciak, W.; Szewczyk, E.M. Are Zoonotic Staphylococcus Pseudintermedius Strains a Growing Threat for Humans? Folia Microbiol. 2018, 63, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Meroni, G.; Filipe, J.F.S.; Drago, L.; Martino, P.A. Investigation on Antibiotic-Resistance, Biofilm Formation and Virulence Factors in Multi Drug Resistant and Non Multi Drug Resistant Staphylococcus Pseudintermedius. Microorganisms 2019, 7, 702. [Google Scholar] [CrossRef]
- Meroni, G.; Filipe, J.F.S.; Martino, P.A. In Vitro Antibacterial Activity of Biological-Derived Silver Nanoparticles: Preliminary Data. Vet. Sci. 2020, 7, 12. [Google Scholar] [CrossRef]
- Rubin, J.E.; Chirino-Trejo, M. Prevalence, Sites of Colonization, and Antimicrobial Resistance Among Staphylococcus Pseudintermedius Isolated from Healthy Dogs in Saskatoon, Canada. J. Vet. Diagn. Investig. 2011, 23, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Small, C.; Beatty, N.; Helou, G.E.; Small, C.; Beatty, N.; Helou, G.E. Staphylococcus Pseudintermedius Bacteremia in a Lung Transplant Recipient Exposed to Domestic Pets. Cureus 2021, 13, e14895. [Google Scholar] [CrossRef] [PubMed]
- Asleh, M.; Feinstein, Y.; Lazar, I.; Rokney, A.; Baum, M.; Sagi, O.; Leibovitz, E.; Danino, D. Severe Pneumonia Caused by Methicillin-Resistant Staphylococcus Pseudintermedius in an Oncology Patient: Case Report and Literature Review. Microb. Drug Resist. Larchmt. N 2022, 28, 222–228. [Google Scholar] [CrossRef]
- Blondeau, L.D.; Rubin, J.E.; Deneer, H.; Kanthan, R.; Sanche, S.; Beshard, N.; Mpofu, C.; Blondeau, J.M. Bacteremia with Staphylococcus Pseudintermedius in a 4 Month Old Pediatric Oncology Patient. J. Chemother. 2020, 32, 260–262. [Google Scholar] [CrossRef]
- Chuang, C.-Y.; Yang, Y.-L.; Hsueh, P.-R.; Lee, P.-I. Catheter-Related Bacteremia Caused by Staphylococcus Pseudintermedius Refractory to Antibiotic-Lock Therapy in a Hemophilic Child with Dog Exposure. J. Clin. Microbiol. 2010, 48, 1497–1498. [Google Scholar] [CrossRef]
- Paul, N.; Moodley, A.; Ghibaudo, G.; Guardabassi, L. Carriage of Methicillin-Resistant Staphylococcus Pseudintermedius in Small Animal Veterinarians: Indirect Evidence of Zoonotic Transmission. Zoonoses Public Health 2011, 58, 533–539. [Google Scholar] [CrossRef]
- Moses, I.B.; Santos, F.F.; Gales, A.C. Human Colonization and Infection by Staphylococcus Pseudintermedius: An Emerging and Underestimated Zoonotic Pathogen. Microorganisms 2023, 11, 581. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 90, 153626. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Meroni, G.; Cardin, E.; Rendina, C.; Herrera Millar, V.R.; Soares Filipe, J.F.; Martino, P.A. In Vitro Efficacy of Essential Oils from Melaleuca alternifolia and Rosmarinus officinalis, Manuka Honey-Based Gel, and Propolis as Antibacterial Agents against Canine Staphylococcus Pseudintermedius Strains. Antibiotics 2020, 9, 344. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory Activities of Selected Essential Oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef]
- Farrar, A.J.; Farrar, F.C. Clinical Aromatherapy. Nurs. Clin. N. Am. 2020, 55, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Veiskaramian, A.; Gholami, M.; Yarahmadi, S.; Amanolahi Baharvand, P.; Birjandi, M. Effect of Aromatherapy with Melissa Essential Oil on Stress and Hemodynamic Parameters in Acute Coronary Syndrome Patients: A Clinical Trial in the Emergency Department. Complement. Ther. Clin. Pract. 2021, 44, 101436. [Google Scholar] [CrossRef] [PubMed]
- Szewczuk, M.A.; Zych, S.; Oster, N.; Karakulska, J. Activity of Patchouli and Tea Tree Essential Oils against Staphylococci Isolated from Pyoderma in Dogs and Their Synergistic Potential with Gentamicin and Enrofloxacin. Animals 2023, 13, 1279. [Google Scholar] [CrossRef] [PubMed]
- Duangkaew, L.; Larsuprom, L.; Lekcharoensuk, C.; Chen, C. Effect of a Mixture of Essential Oils and a Plant-Based Extract for the Management of Localized Superficial Pyoderma in Dogs: An Open-Label Clinical Trial. Thai J. Vet. Med. 2017, 47, 513–522. [Google Scholar] [CrossRef]
- Aiemsaard, J.; Aiyaranoi, K.; Thongkham, E.; Borlace, G.; Senaphan, K. In Vivo Efficacy of Clove Essential Oil Spray Formulation on Canine Superficial Pyoderma. Songklanakarin J. Sci. Technol. 2022, 44, 308–315. [Google Scholar] [CrossRef]
- Patterson, J.E.; McElmeel, L.; Wiederhold, N.P. In Vitro Activity of Essential Oils Against Gram-Positive and Gram-Negative Clinical Isolates, Including Carbapenem-Resistant Enterobacteriaceae. Open Forum Infect. Dis. 2019, 6, ofz502. [Google Scholar] [CrossRef]
- Hajibonabi, A.; Yekani, M.; Sharifi, S.; Nahad, J.S.; Dizaj, S.M.; Memar, M.Y. Antimicrobial Activity of Nanoformulations of Carvacrol and Thymol: New Trend and Applications. OpenNano 2023, 13, 100170. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Fu, J.; Liao, S.; Yang, S.; Jia, X.; Gong, G. Evaluation of the Membrane Damage Mechanism of Thymol against Bacillus Cereus and Its Application in the Preservation of Skim Milk. Food Control 2022, 131, 108435. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, O.; Puiu, R.A.; Bîrcă, A.C.; Burdușel, A.-C.; Grumezescu, A.M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.T.J.; Nyam, K.L. Evaluation of Silver Nanoparticles in Cosmeceutical and Potential Biosafety Complications. Saudi J. Biol. Sci. 2022, 29, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Chindamo, G.; Chirio, D.; Morel, S.; Peira, E.; Vercelli, C.; Gallarate, M. Nanocarriers in Veterinary Medicine: A Challenge for Improving Osteosarcoma Conventional Treatments. Nanomaterials 2022, 12, 4501. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Thakur, V.; Kumar, V.; Raj, M.; Gupta, S.; Devi, N.; Upadhyay, S.K.; Macho, M.; Banerjee, A.; Ewe, D.; et al. Silver Nanoparticles and Its Mechanistic Insight for Chronic Wound Healing: Review on Recent Progress. Molecules 2022, 27, 5587. [Google Scholar] [CrossRef]
- Löfdahl, A.; Jern, A.; Flyman, S.; Kåredal, M.; Karlsson, H.L.; Larsson-Callerfelt, A.-K. Silver Nanoparticles Alter Cell Viability Ex Vivo and in Vitro and Induce Proinflammatory Effects in Human Lung Fibroblasts. Nanomaterials 2020, 10, 1868. [Google Scholar] [CrossRef]
- Franková, J.; Pivodová, V.; Vágnerová, H.; Juráňová, J.; Ulrichová, J. Effects of Silver Nanoparticles on Primary Cell Cultures of Fibroblasts and Keratinocytes in a Wound-Healing Model. J. Appl. Biomater. Funct. Mater. 2016, 14, 137–142. [Google Scholar] [CrossRef]
- Sasaki, T.; Tsubakishita, S.; Tanaka, Y.; Sakusabe, A.; Ohtsuka, M.; Hirotaki, S.; Kawakami, T.; Fukata, T.; Hiramatsu, K. Multiplex-PCR Method for Species Identification of Coagulase-Positive Staphylococci. J. Clin. Microbiol. 2010, 48, 765–769. [Google Scholar] [CrossRef]
- Bannoehr, J.; Franco, A.; Iurescia, M.; Battisti, A.; Fitzgerald, J.R. Molecular Diagnostic Identification of Staphylococcus pseudintermedius. J. Clin. Microbiol. 2009, 47, 469–471. [Google Scholar] [CrossRef]
- CLSI; Dolinsky, A.L.; Ohiro, R.K.; Fan, W.; Xiao, C.; Wu, F. National Committee for Clinical Laboratory Standards. 2000. Performance Standard for Antimicrobial Susceptibility Testing. Document M100–S10. J. Int. Med. Res. 2017, 46, 18. [Google Scholar] [CrossRef]
- Yerragopu, P.S.; Hiregoudar, S.; Nidoni, U.; Ramappa, K.T.; Sreenivas, A.; Doddagoudar, S. Chemical Synthesis of Silver Nanoparticles Using Tri-Sodium Citrate, Stability Study and Their Characterization. Int. Res. J. Pure Appl. Chem. 2020, 21, 37–50. [Google Scholar] [CrossRef]
- Garzoli, S.; Masci, V.L.; Caradonna, V.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Liquid and Vapor Phase of Four Conifer-Derived Essential Oils: Comparison of Chemical Compositions and Antimicrobial and Antioxidant Properties. Pharmaceuticals 2021, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Taglienti, A.; Donati, L.; Ferretti, L.; Tomassoli, L.; Sapienza, F.; Sabatino, M.; Di Massimo, G.; Fiorentino, S.; Vecchiarelli, V.; Nota, P.; et al. In Vivo Antiphytoviral Activity of Essential Oils and Hydrosols from Origanum vulgare, Thymus vulgaris, and Rosmarinus officinalis to Control Zucchini Yellow Mosaic Virus and Tomato Leaf Curl New Delhi Virus in Cucurbita pepo L. Front. Microbiol. 2022, 13, 840893. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data Independent MS/MS Deconvolution for Comprehensive Metabolome Analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, L.; García-Fonticoba, R.; Pérez, D.; Viñes, J.; Fàbregas, N.; Madroñero, S.; Meroni, G.; Martino, P.A.; Martínez, S.; Maté, M.L.; et al. Whole Genome Sequencing and de Novo Assembly of Staphylococcus Pseudintermedius: A Pangenome Approach to Unravelling Pathogenesis of Canine Pyoderma. Vet. Dermatol. 2021, 32, 654–663. [Google Scholar] [CrossRef]
- Nocera, F.P.; Meroni, G.; Fiorito, F.; De Martino, L.; Martino, P.A. Occurrence and Antimicrobial Susceptibility Patterns of Canine Staphylococcus Pseudintermedius Strains Isolated from Two Different Italian University Veterinary Hospitals. Vet. Ital. 2020, 56, 263–269. [Google Scholar] [CrossRef]
- Meenu, M.; Padhan, B.; Patel, M.; Patel, R.; Xu, B. Antibacterial Activity of Essential oils from Different Parts of Plants against Salmonella and Listeria spp. Food Chem. 2023, 404, 134723. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Antibacterial Activity and Mechanism of Ginger Essential Oil against Escherichia Coli and Staphylococcus Aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Rodríguez-Lázaro, D.; Domínguez, R.; Zhong, J.; Lorenzo, J.M. The Role of Essential Oils against Pathogenic Escherichia coli in Food Products. Microorganisms 2020, 8, 924. [Google Scholar] [CrossRef]
- Yang, S.-K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Akseer, R.; Lim, S.-H.E.; Lai, K.-S. Disruption of KPC-Producing Klebsiella Pneumoniae Membrane via Induction of Oxidative Stress by Cinnamon Bark (Cinnamomum verum J. Presl) Essential Oil. PLoS ONE 2019, 14, e0214326. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, O.; Sharifi, A.; Ahmadi, A.; Nayeri Fasaei, B. Antibacterial and Anti-PmrA Activity of Plant Essential Oils against Fluoroquinolone-Resistant Streptococcus Pneumoniae Clinical Isolates. Lett. Appl. Microbiol. 2018, 67, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Baek, K.-H.; Kang, S.C. Control of Salmonella in Foods by Using Essential Oils: A Review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Zouhir, A.; Jridi, T.; Nefzi, A.; Ben Hamida, J.; Sebei, K. Inhibition of Methicillin-Resistant Staphylococcus Aureus (MRSA) by Antimicrobial Peptides (AMPs) and Plant Essential Oils. Pharm. Biol. 2016, 54, 3136–3150. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.O.; Barreto, H.M.; Lima, E.d.O.; de Souza, E.L.; de Siqueira Júnior, J.P. Antimicrobial Effect of the Essential Oil from Rosmarinus officinalis L. against Staphylococcus Pseudintermedius Isolated from Dogs. Rev. Bras. Biociênc. 2013, 11, 280–283. [Google Scholar]
- Ait-Ouazzou, A.; Lorán, S.; Bakkali, M.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagán, R.; Conchello, P. Chemical Composition and Antimicrobial Activity of Essential Oils of Thymus algeriensis, Eucalyptus globulus and Rosmarinus officinalis from Morocco. J. Sci. Food Agric. 2011, 91, 2643–2651. [Google Scholar] [CrossRef]
- Mekonnen, A.; Yitayew, B.; Tesema, A.; Taddese, S. In Vitro Antimicrobial Activity of Essential Oil of Thymus schimperi, Matricaria chamomilla, Eucalyptus globulus, and Rosmarinus officinalis. Int. J. Microbiol. 2016, 2016, 9545693. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, L.; Flamini, G.; Cioni, P.L.; Morelli, I. Composition and Antimicrobial Properties of Essential Oils of Four Mediterranean Lamiaceae. J. Ethnopharmacol. 1993, 39, 167–170. [Google Scholar] [CrossRef]
- Fu, Y.; Zu, Y.; Chen, L.; Shi, X.; Wang, Z.; Sun, S.; Efferth, T. Antimicrobial Activity of Clove and Rosemary Essential Oils Alone and in Combination. Phytother. Res. PTR 2007, 21, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Nocera, F.P.; Mancini, S.; Najar, B.; Bertelloni, F.; Pistelli, L.; De Filippis, A.; Fiorito, F.; De Martino, L.; Fratini, F. Antimicrobial Activity of Some Essential Oils against Methicillin-Susceptible and Methicillin-Resistant Staphylococcus Pseudintermedius-Associated Pyoderma in Dogs. Anim. Open Access J. 2020, 10, 1782. [Google Scholar] [CrossRef] [PubMed]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Goswami, P.; Singh, V.R.; Verma, S.K.; Singh, N.; Kurmi, A.; Darokar, M.P.; Saikia, D. P-Menthenols Chemotype of Cymbopogon Distans from India: Composition, Antibacterial and Antifungal Activity of the Essential Oil against Pathogens. J. Essent. Oil Res. 2018, 30, 40–46. [Google Scholar] [CrossRef]
- El-Massry, K.F.; El-Ghorab, A.H.; Shaaban, H.A.; Shibamoto, T. Chemical Compositions and Antioxidant/Antimicrobial Activities of Various Samples Prepared from Schinus Terebinthifolius Leaves Cultivated in Egypt. J. Agric. Food Chem. 2009, 57, 5265–5270. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.M.; Pinto, J.E.B.P.; Bertolucci, S.K.V.; Alvarenga, A.A.; Alves, M.N.; Duarte, M.C.T.; Sartoratto, A. Chemical Composition and Antimicrobial Activity of the Essential Oil from the Leaves and Flowers of Aloysia Gratissima. Rev. Bras. Plantas Med. 2013, 15, 583–588. [Google Scholar] [CrossRef]
- Martins, R.L.; Simões, R.C.; Rabelo, É.d.M.; Farias, A.L.F.; Rodrigues, A.B.L.; da S. Ramos, R.; Fernandes, J.B.; da S. Santos, L.; Almeida, S.S.M.d.S. Chemical Composition, an Antioxidant, Cytotoxic and Microbiological Activity of the Essential Oil from the Leaves of Aeollanthus Suaveolens Mart. Ex Spreng. PLoS ONE 2016, 11, e0166684. [Google Scholar] [CrossRef]
- Demir, H.; Kalaycı, S. Chemical Composition and Antimicrobial Activity of Essential Oils of Ocimum basilicum Var. Album (L.) Benth, Lavandula Angustifolia Subsp. Angustifolia, Melissa Officinalis Belonging to Lamiaceae Family. J. Food Sci. Eng. 2017, 7. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Ikeda, N.Y.; Miano, A.C.; Saldaña, E.; Moreno, A.M.; Stashenko, E.; Contreras-Castillo, C.J.; Da Gloria, E.M. Unraveling the Selective Antibacterial Activity and Chemical Composition of Citrus Essential Oils. Sci. Rep. 2019, 9, 17719. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).