Immune Mediators Important for a Protective Secondary Response to Babesia microti
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Parasite
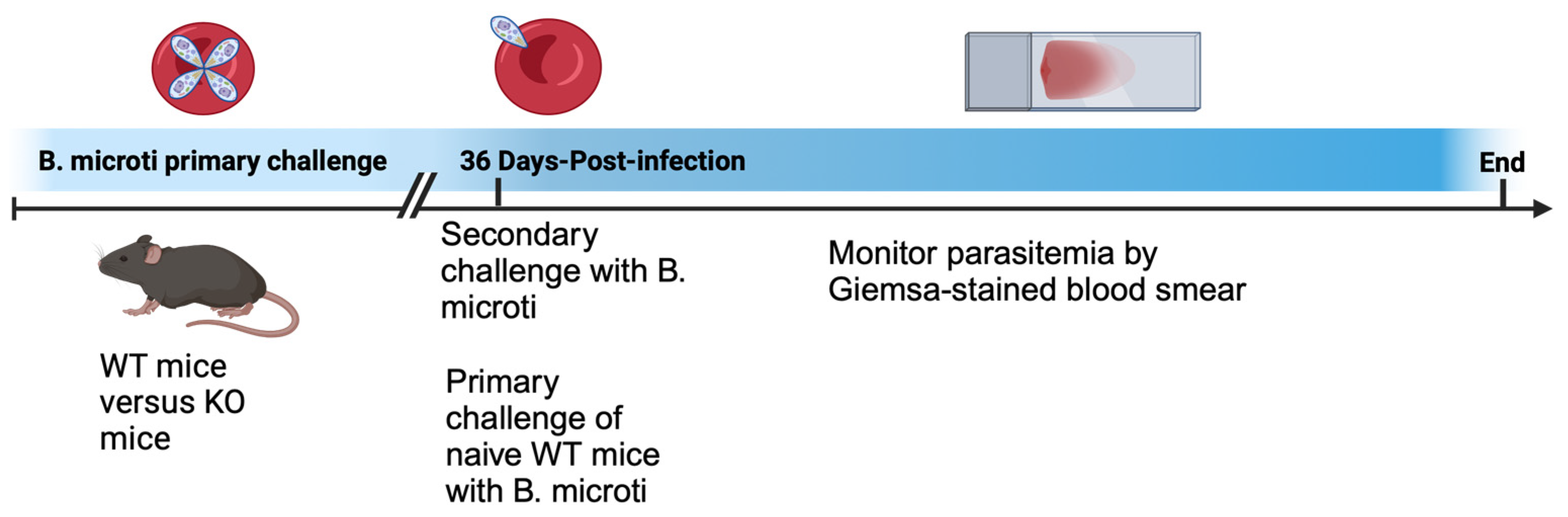
2.3. Primary and Secondary B. microti Challenge
2.4. Hematology and Analysis of Parasitemia
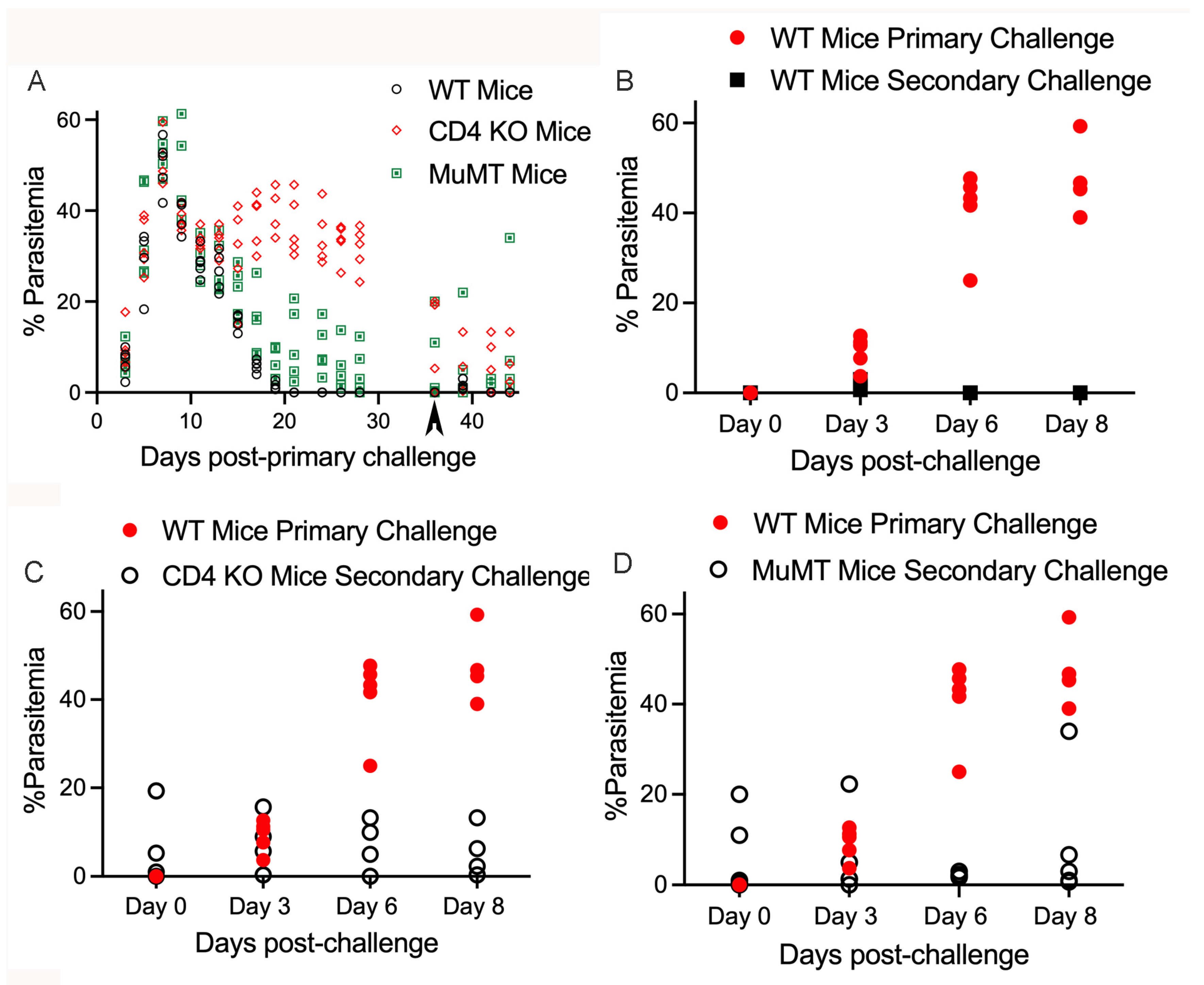
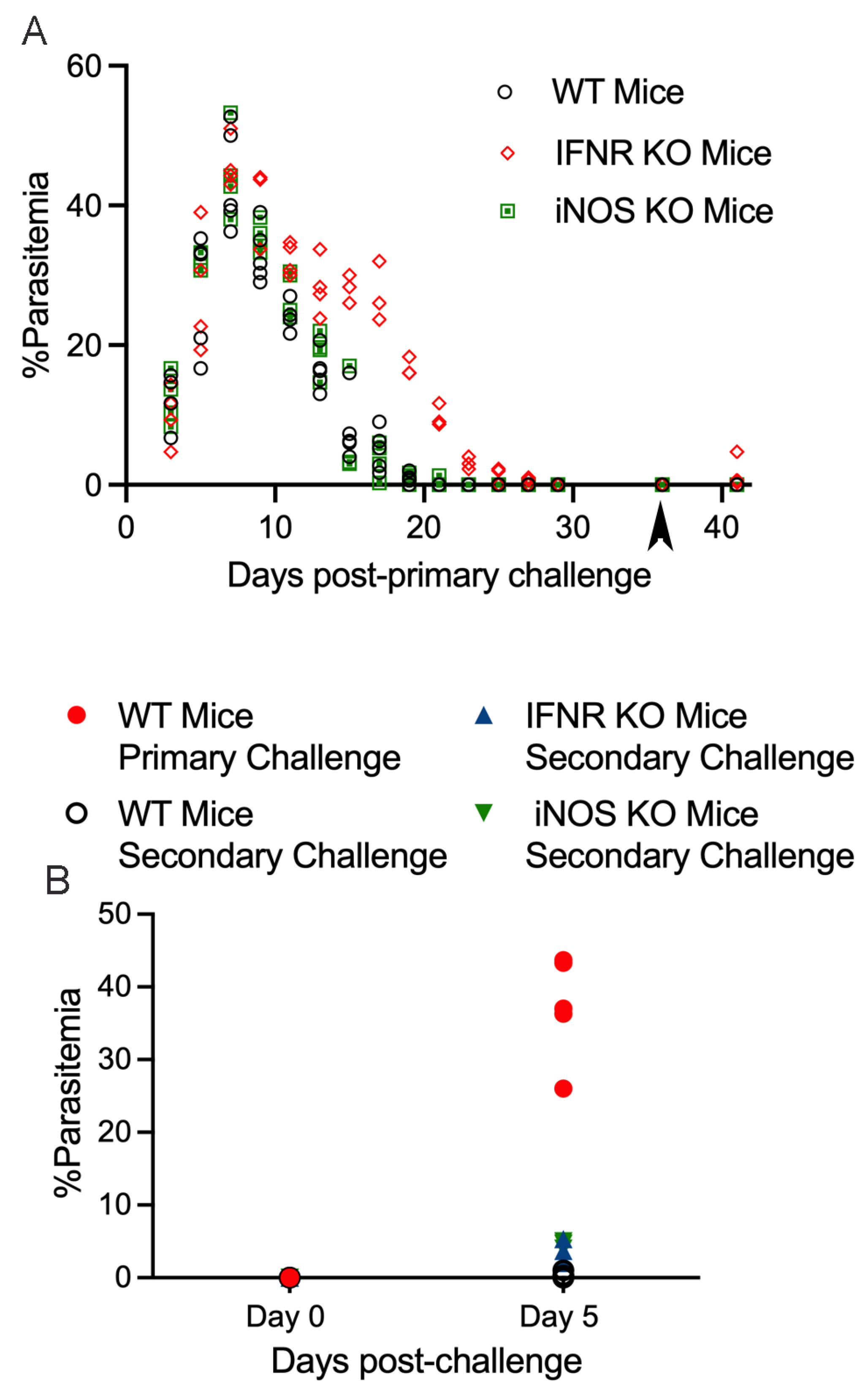
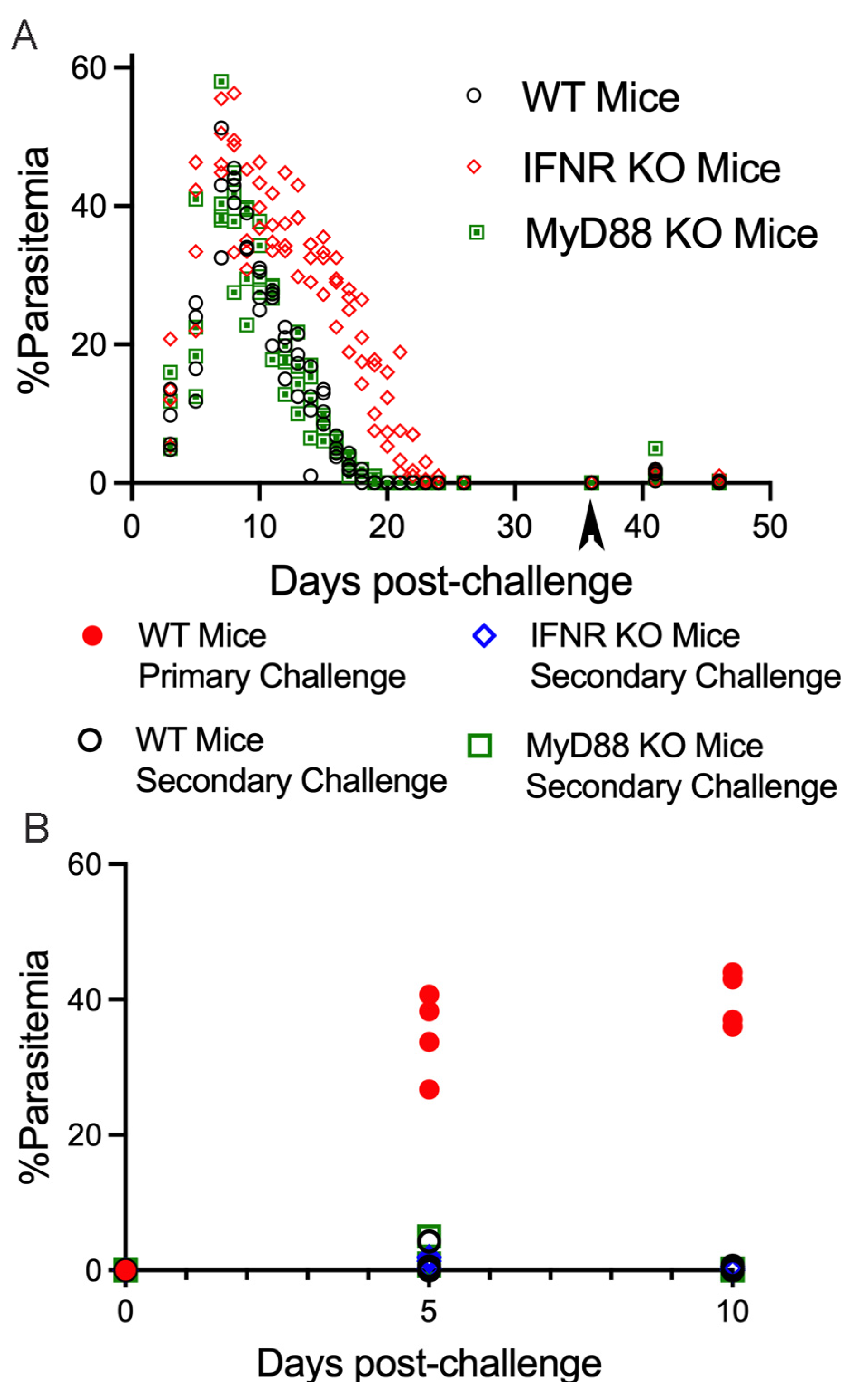
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lack, J.B.; Reichard, M.V.; Van Den Bussche, R.A. Phylogeny and evolution of the Piroplasmida as inferred from 18S rRNA sequences. Int. J. Parasitol. 2012, 42, 353–363. [Google Scholar] [CrossRef]
- Schnittger, L.; Rodriguez, A.E.; Florin-Christensen, M.; Morrison, D.A. Babesia: A world emerging. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2012, 12, 1788–1809. [Google Scholar] [CrossRef] [PubMed]
- Goethert, H.K.; Telford, S.R., 3rd. What is Babesia microti? Parasitology 2003, 127, 301–309. [Google Scholar] [CrossRef]
- Persing, D.H.; Conrad, P.A. Babesiosis: New insights from phylogenetic analysis. Infect. Agents Dis. 1995, 4, 182–195. [Google Scholar]
- Jalovecka, M.; Sojka, D.; Ascencio, M.; Schnittger, L. Babesia Life Cycle—When Phylogeny Meets Biology. Trends Parasitol. 2019, 35, 356–368. [Google Scholar] [CrossRef]
- Conrad, P.A.; Kjemtrup, A.M.; Carreno, R.A.; Thomford, J.; Wainwright, K.; Eberhard, M.; Quick, R.; Telford, S.R., 3rd; Herwaldt, B.L. Description of Babesia duncani n.sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int. J. Parasitol. 2006, 36, 779–789. [Google Scholar] [CrossRef]
- Singh, P.; Lonardi, S.; Liang, Q.; Vydyam, P.; Khabirova, E.; Fang, T.; Gihaz, S.; Thekkiniath, J.; Munshi, M.; Abel, S.; et al. Babesia duncani multi-omics identifies virulence factors and drug targets. Nat. Microbiol. 2023, 8, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Vannier, E.; Krause, P.J. Human babesiosis. N. Engl. J. Med. 2012, 366, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, A.; Zintl, A.; Montero, E.; Hunfeld, K.P.; Gray, J. Human babesiosis in Europe. Pathogens 2021, 10, 1165. [Google Scholar] [CrossRef]
- Krause, P.J.; McKay, K.; Gadbaw, J.; Christianson, D.; Closter, L.; Lepore, T.; Telford, S.R., 3rd; Sikand, V.; Ryan, R.; Persing, D.; et al. Increasing health burden of human babesiosis in endemic sites. Am. J. Trop. Med. Hyg. 2003, 68, 431–436. [Google Scholar] [CrossRef]
- Hatcher, J.C.; Greenberg, P.D.; Antique, J.; Jimenez-Lucho, V.E. Severe babesiosis in Long Island: Review of 34 cases and their complications. Clin. Infect. Dis. 2001, 32, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Gewurz, B.E.; Hill, D.; Marty, F.M.; Vannier, E.; Foppa, I.M.; Furman, R.R.; Neuhaus, E.; Skowron, G.; Gupta, S.; et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin. Infect. Dis. 2008, 46, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Prasad, A.; Neuhaus, E.; Joshi, S.; Nowakowski, J.; Nelson, J.; Mittleman, A.; Aguero-Rosenfeld, M.; Topal, J.; Krause, P.J. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin. Infect. Dis. 2010, 50, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Spielman, A.; Telford, S.R., 3rd; Sikand, V.K.; McKay, K.; Christianson, D.; Pollack, R.J.; Brassard, P.; Magera, J.; Ryan, R.; et al. Persistent parasitemia after acute babesiosis. N. Engl. J. Med. 1998, 339, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Raffalli, J.; Wormser, G.P. Persistence of babesiosis for >2 years in a patient on rituximab for rheumatoid arthritis. Diagn. Microbiol. Infect. Dis. 2016, 85, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Skariah, S.; Arnaboldi, P.; Dattwyler, R.J.; Sultan, A.A.; Gaylets, C.; Walwyn, O.; Mulhall, H.; Wu, X.; Dargham, S.R.; Mordue, D.G. Elimination of Babesia microti Is dependent on intraerythrocytic killing and CD4(+) T Cells. J. Immunol. 2017, 199, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Carey, E.; Carey, D.E.; Krause, P.J. Recurrence of human babesiosis caused by reinfection. Emerg. Infect. Dis. 2021, 27, 2658–2661. [Google Scholar] [CrossRef]
- Gleason, N.N.; Healy, G.R.; Western, K.A.; Benson, G.D.; Schultz, M.G. The “Gray” strain of Babesia microti from a human case established in laboratory animals. J. Parasitol. 1970, 56, 1256–1257. [Google Scholar] [CrossRef]
- Adachi, O.; Kawai, T.; Takeda, K.; Matsumoto, M.; Tsutsui, H.; Sakagami, M.; Nakanishi, K.; Akira, S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 1998, 9, 143–150. [Google Scholar] [CrossRef]
- Clark, I.A.; Allison, A.C.; Cox, F.E. Protection of mice against Babesia and Plasmodium with BCG. Nature 1976, 259, 309–311. [Google Scholar] [CrossRef]
- Clark, I.A.; Allison, A.C. Babesia microti and Plasmodium berghei yoelii infections in nude mice. Nature 1974, 252, 328–329. [Google Scholar] [CrossRef]
- Clark, I.A.; Cox, F.E.; Allison, A.C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology 1977, 74, 9–18. [Google Scholar] [CrossRef]
- Igarashi, I.; Suzuki, R.; Waki, S.; Tagawa, Y.; Seng, S.; Tum, S.; Omata, Y.; Saito, A.; Nagasawa, H.; Iwakura, Y.; et al. Roles of CD4(+) T cells and gamma interferon in protective immunity against Babesia microti infection in mice. Infect. Immun. 1999, 67, 4143–4148. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Delfin, I.; Homer, M.J.; Wettstein, P.J.; Persing, D.H. Innate resistance to Babesia infection is influenced by genetic background and gender. Infect. Immun. 2001, 69, 7955–7958. [Google Scholar] [CrossRef] [PubMed]
- Vannier, E.; Borggraefe, I.; Telford, S.R., 3rd; Menon, S.; Brauns, T.; Spielman, A.; Gelfand, J.A.; Wortis, H.H. Age-associated decline in resistance to Babesia microti is genetically determined. J. Infect. Dis. 2004, 189, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Clawson, M.L.; Paciorkowski, N.; Rajan, T.V.; La Vake, C.; Pope, C.; La Vake, M.; Wikel, S.K.; Krause, P.J.; Radolf, J.D. Cellular immunity, but not gamma interferon, is essential for resolution of Babesia microti infection in BALB/c mice. Infect. Immun. 2002, 70, 5304–5306. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Delfin, I.; Wettstein, P.J.; Persing, D.H. Resistance to acute babesiosis is associated with interleukin-12- and gamma interferon-mediated responses and requires macrophages and natural killer cells. Infect. Immun. 2003, 71, 2002–2008. [Google Scholar] [CrossRef]
- Moro, M.H.; David, C.S.; Magera, J.M.; Wettstein, P.J.; Barthold, S.W.; Persing, D.H. Differential effects of infection with a Babesia-like piroplasm, WA1, in inbred mice. Infect. Immun. 1998, 66, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Ruebush, M.J.; Hanson, W.L. Susceptibility of five strains of mice to Babesia microti of human origin. J. Parasitol. 1979, 65, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Menis, M.; Whitaker, B.I.; Wernecke, M.; Jiao, Y.; Eder, A.; Kumar, S.; Xu, W.; Liao, J.; Wei, Y.; MaCurdy, T.E.; et al. Babesiosis occurrence among United States Medicare beneficiaries, ages 65 and older, during 2006-2017: Overall and by state and county of residence. Open Forum Infect. Dis. 2021, 8, ofaa608. [Google Scholar] [CrossRef]
- Menis, M.; Forshee, R.A.; Kumar, S.; McKean, S.; Warnock, R.; Izurieta, H.S.; Gondalia, R.; Johnson, C.; Mintz, P.D.; Walderhaug, M.O.; et al. Babesiosis occurrence among the elderly in the United States, as recorded in large Medicare databases during 2006-2013. PLoS ONE 2015, 10, e0140332. [Google Scholar] [CrossRef] [PubMed]
- Menis, M.; Anderson, S.A.; Izurieta, H.S.; Kumar, S.; Burwen, D.R.; Gibbs, J.; Kropp, G.; Erten, T.; Macurdy, T.E.; Worrall, C.M.; et al. Babesiosis among elderly Medicare beneficiaries, United States, 2006–2008. Emerg. Infect. Dis. 2012, 18, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Marcos, L.A.; Wormser, G.P. Relapsing babesiosis With molecular evidence of resistance to certain antimicrobials commonly used to treat Babesia microti infections. Open Forum Infect. Dis. 2023, 10, ofad391. [Google Scholar] [CrossRef]
- Marcos, L.A.; Leung, A.; Kirkman, L.; Wormser, G.P. Use of tafenoquine to treat a patient with relapsing babesiosis with clinical and molecular evidence of resistance to azithromycin and atovaquone. IDCases 2022, 27, e01460. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, J.; Gagliardi, T.; Arnaboldi, P.M.; Hale, S.J.; Skariah, S.; Sultan, A.A.; Mordue, D.G. Immune Mediators Important for a Protective Secondary Response to Babesia microti. Pathogens 2024, 13, 123. https://doi.org/10.3390/pathogens13020123
Conti J, Gagliardi T, Arnaboldi PM, Hale SJ, Skariah S, Sultan AA, Mordue DG. Immune Mediators Important for a Protective Secondary Response to Babesia microti. Pathogens. 2024; 13(2):123. https://doi.org/10.3390/pathogens13020123
Chicago/Turabian StyleConti, Joseph, Thomas Gagliardi, Paul M. Arnaboldi, Synthia J. Hale, Sini Skariah, Ali A. Sultan, and Dana G. Mordue. 2024. "Immune Mediators Important for a Protective Secondary Response to Babesia microti" Pathogens 13, no. 2: 123. https://doi.org/10.3390/pathogens13020123
APA StyleConti, J., Gagliardi, T., Arnaboldi, P. M., Hale, S. J., Skariah, S., Sultan, A. A., & Mordue, D. G. (2024). Immune Mediators Important for a Protective Secondary Response to Babesia microti. Pathogens, 13(2), 123. https://doi.org/10.3390/pathogens13020123






