Deletion of gE in Herpes Simplex Virus 1 Leads to Increased Extracellular Virus Production and Augmented Interferon Alpha Production by Peripheral Blood Mononuclear Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Viruses and Infections
2.3. PBMC Co-Incubation with HSV-1-Infected Cells and IFNalpha Production Assays
2.4. gE Complementation Assays
2.5. PBMC Stimulation with Cell-Free HSV-1 and IFNalpha Production Assays
2.6. PBMC Stimulation with Supernatant of HSV-1-Infected Cells and IFNalpha Production Assays
2.7. IFNalpha Production by PBMC upon Stimulation with WT HSV-1-Infected Cells in the Presence of Virus-Free Supernatant of gEnull HSV-1- or WT HSV-1-Infected Cells
2.8. Extracellular Virus Titers
2.9. Statistical Analysis
3. Results
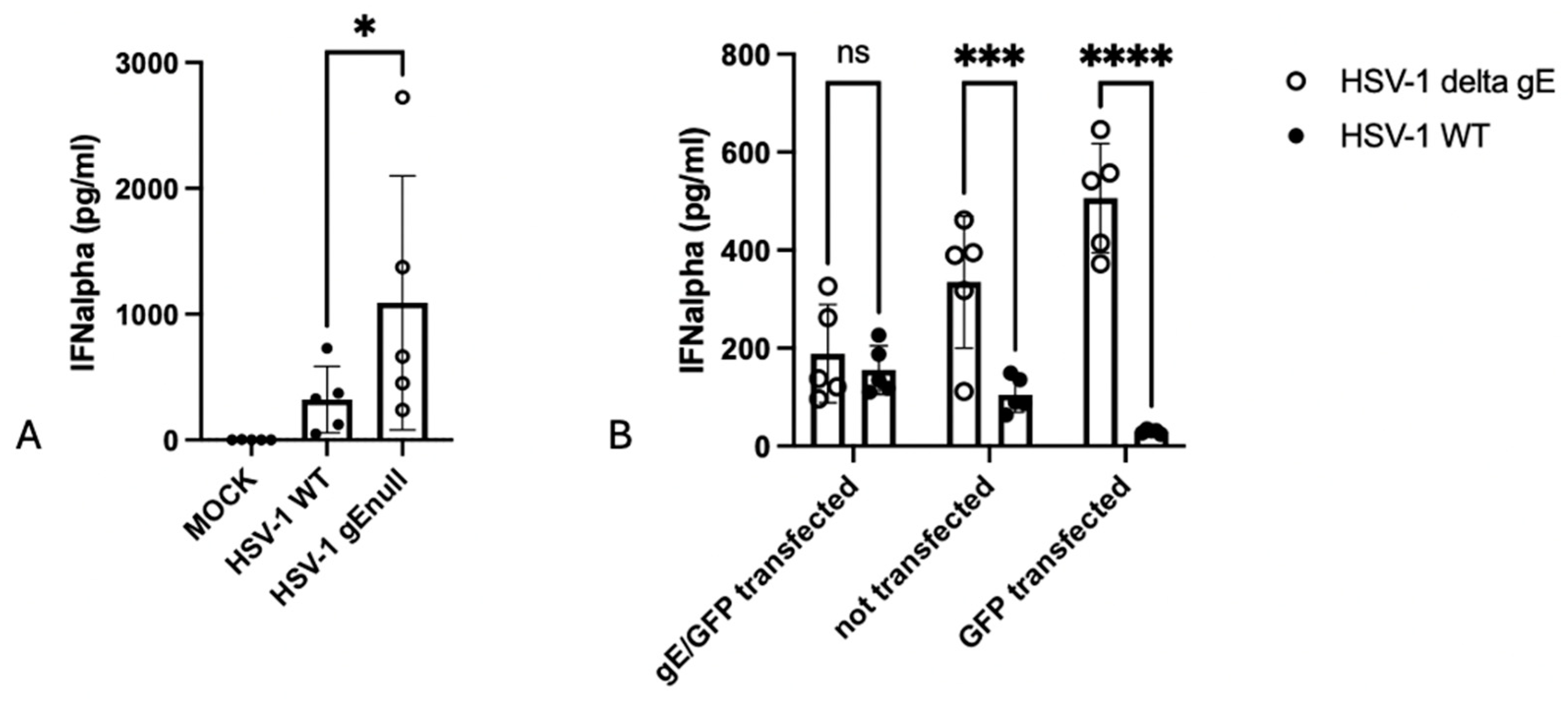
3.1. Lack of gE Results in Increased HSV-1-Induced IFNalpha Production by Human Peripheral Blood Mononuclear Cells
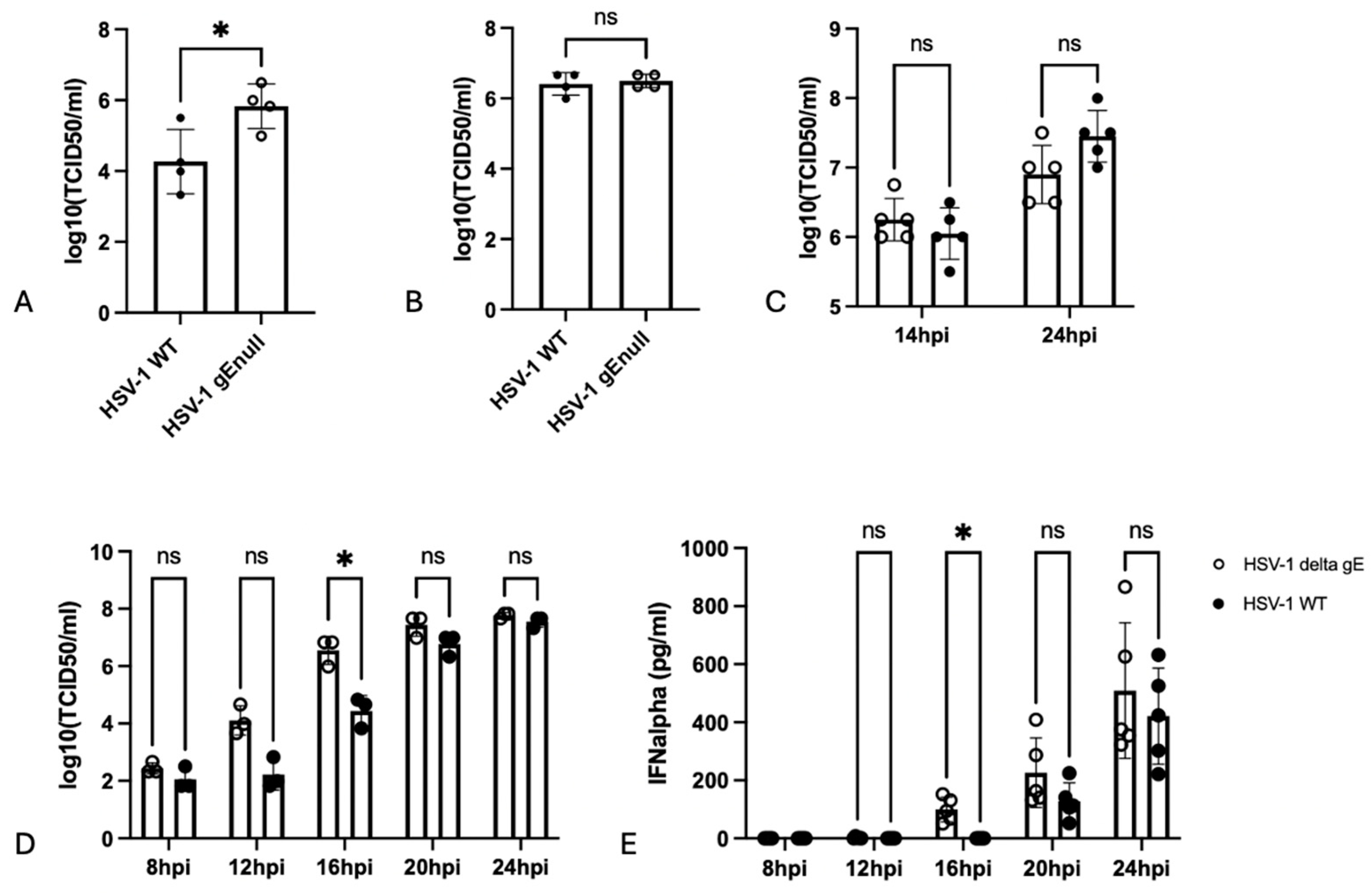
3.2. Lack of gE Results in Increased Extracellular Virus Titers in HSV-1-Infected Vero Cells
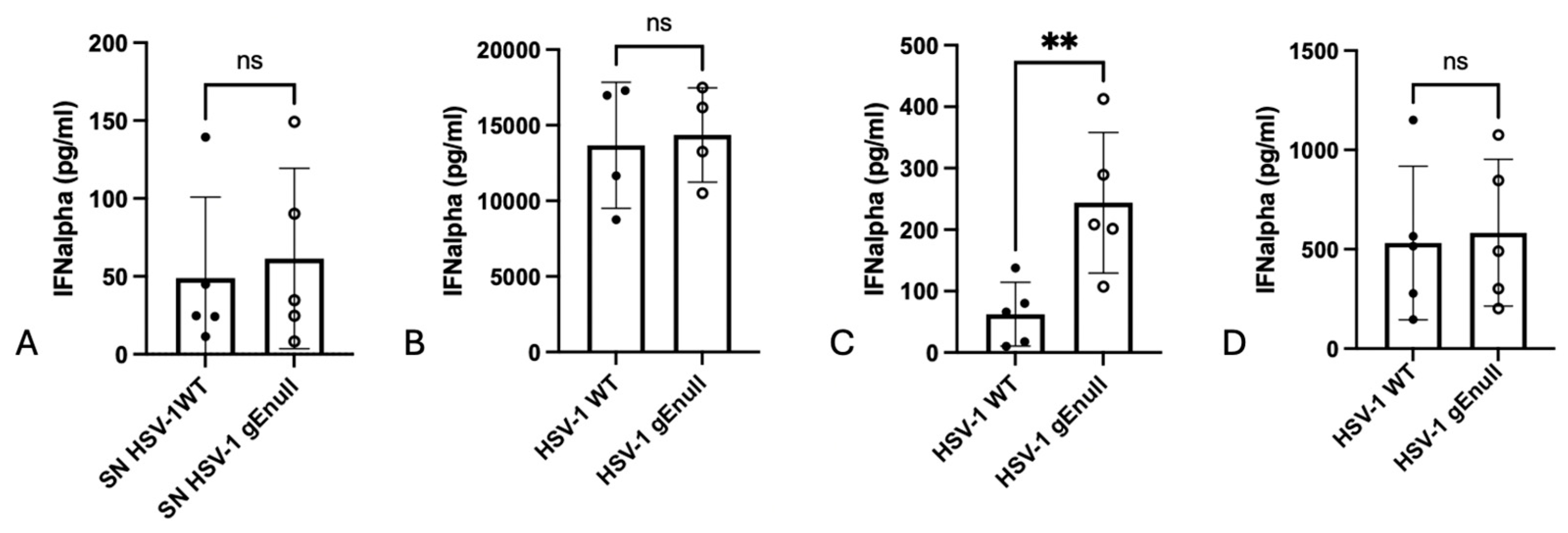
3.3. Stimulation of PBMC with Equal Amounts of HSV-1 WT or HSV-1 gEnull Virions Results in Similar IFNalpha Production
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and Virulence of Herpes Simplex Virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Manry, J.; Chen, J.; Rosain, J.; Seeleuthner, Y.; AbuZaitun, O.; Lorenzo, L.; Khan, T.; Hasek, M.; Hernandez, N.; et al. Herpes Simplex Encephalitis in a Patient with a Distinctive Form of Inherited IFNAR1 Deficiency. J. Clin. Investig. 2021, 131, e139980. [Google Scholar] [CrossRef]
- Reizis, B. Plasmacytoid Dendritic Cells: Development, Regulation, and Function. Immunity 2019, 50, 37–50. [Google Scholar] [CrossRef]
- Ye, Y.; Gaugler, B.; Mohty, M.; Malard, F. Plasmacytoid Dendritic Cell Biology and Its Role in Immune-mediated Diseases. Clin. Transl. Immunol. 2020, 9, e1139. [Google Scholar] [CrossRef] [PubMed]
- Siegal, F.P.; Kadowaki, N.; Shodell, M.; Fitzgerald-Bocarsly, P.A.; Shah, K.; Ho, S.; Antonenko, S.; Liu, Y.-J. The Nature of the Principal Type 1 Interferon-Producing Cells in Human Blood. Science 1999, 284, 1835–1837. [Google Scholar] [CrossRef] [PubMed]
- Baranek, T.; Zucchini, N.; Dalod, M. Plasmacytoid Dendritic Cells and the Control of Herpesvirus Infections. Viruses 2009, 1, 383–419. [Google Scholar] [CrossRef]
- Delva, J.L.; Van Waesberghe, C.; Klupp, B.G.; Mettenleiter, T.C.; Favoreel, H.W. Alphaherpesvirus-Induced Activation of Plasmacytoid Dendritic Cells Depends on the Viral Glycoprotein GD and Is Inhibited by Non-Infectious Light Particles. PLoS Pathog. 2021, 17, e1010117. [Google Scholar] [CrossRef] [PubMed]
- Swiecki, M.; Wang, Y.; Gilfillan, S.; Colonna, M. Plasmacytoid Dendritic Cells Contribute to Systemic but Not Local Antiviral Responses to HSV Infections. PLoS Pathog. 2013, 9, e1003728. [Google Scholar] [CrossRef]
- Donaghy, H.; Bosnjak, L.; Harman, A.N.; Marsden, V.; Tyring, S.K.; Meng, T.-C.; Cunningham, A.L. Role for Plasmacytoid Dendritic Cells in the Immune Control of Recurrent Human Herpes Simplex Virus Infection. J. Virol. 2009, 83, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Jamali, A.; Hu, K.; Sendra, V.G.; Blanco, T.; Lopez, M.J.; Ortiz, G.; Qazi, Y.; Zheng, L.; Turhan, A.; Harris, D.L.; et al. Characterization of Resident Corneal Plasmacytoid Dendritic Cells and Their Pivotal Role in Herpes Simplex Keratitis. Cell Rep. 2020, 32, 108099. [Google Scholar] [CrossRef] [PubMed]
- Bettahi, I.; Zhang, X.; Afifi, R.E.; Benmohamed, L. Protective Immunity to Genital Herpes Simplex Virus Type 1 and Type 2 Provided by Self-Adjuvanting Lipopeptides That Drive Dendritic Cell Maturation and Elicit a Polarized Th1 Immune Response. Viral Immunol. 2006, 19, 220–236. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Facchetti, F.; Lanzavecchia, A.; Colonna, M. Plasmacytoid Dendritic Cells Activated by Influenza Virus and CD40L Drive a Potent TH1 Polarization. Nat. Immunol. 2000, 1, 305–310. [Google Scholar] [CrossRef]
- Auray, G.; Talker, S.C.; Keller, I.; Python, S.; Gerber, M.; Liniger, M.; Ganges, L.; Bruggmann, R.; Ruggli, N.; Summerfield, A. High-Resolution Profiling of Innate Immune Responses by Porcine Dendritic Cell Subsets in Vitro and in Vivo. Front. Immunol. 2020, 11, 1429. [Google Scholar] [CrossRef]
- Rothenfusser, S.; Tuma, E.; Endres, S.; Hartmann, G. Plasmacytoid Dendritic Cells: The Key to CpG. Hum. Immunol. 2002, 63, 1111–1119. [Google Scholar] [CrossRef]
- Delva, J.L.; Nauwynck, H.J.; Mettenleiter, T.C.; Favoreel, H.W. The Attenuated Pseudorabies Virus Vaccine Strain Bartha K61: A Brief Review on the Knowledge Gathered during 60 Years of Research. Pathogens 2020, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Delva, J.L.; Van Waesberghe, C.; Van Den Broeck, W.; Lamote, J.A.; Vereecke, N.; Theuns, S.; Couck, L.; Favoreel, H.W. The Attenuated Pseudorabies Virus Vaccine Strain Bartha Hyperactivates Plasmacytoid Dendritic Cells by Generating Large Amounts of Cell-Free Virus in Infected Epithelial Cells. J. Virol. 2022, 96, e02199-21. [Google Scholar] [CrossRef]
- Lamote, J.A.S.; Kestens, M.; Van Waesberghe, C.; Delva, J.; De Pelsmaeker, S.; Devriendt, B.; Favoreel, H.W. The Pseudorabies Virus Glycoprotein GE/GI Complex Suppresses Type I Interferon Production by Plasmacytoid Dendritic Cells. J. Virol. 2017, 91, e02276-16. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, A.; Goldsmith, K.; Johnson, D.C. Herpes Simplex Virus Glycoproteins GD and GE/GI Serve Essential but Redundant Functions during Acquisition of the Virion Envelope in the Cytoplasm. J. Virol. 2003, 77, 8481–8494. [Google Scholar] [CrossRef]
- Rasmussen, S.B.; Sørensen, L.N.; Malmgaard, L.; Ank, N.; Baines, J.D.; Chen, Z.J.; Paludan, S.R. Type I Interferon Production during Herpes Simplex Virus Infection Is Controlled by Cell-Type-Specific Viral Recognition through Toll-Like Receptor 9, the Mitochondrial Antiviral Signaling Protein Pathway, and Novel Recognition Systems. J. Virol. 2007, 81, 13315–13324. [Google Scholar] [CrossRef] [PubMed]
- Dingwell, K.S.; Doering, L.C.; Johnson, D.C. Glycoproteins E and I Facilitate Neuron-to-Neuron Spread of Herpes Simplex Virus. J. Virol. 1995, 69, 7087–7098. [Google Scholar] [CrossRef] [PubMed]
- Polcicova, K.; Biswas, P.S.; Banerjee, K.; Wisner, T.W.; Rouse, B.T.; Johnson, D.C. Herpes Keratitis in the Absence of Anterograde Transport of Virus from Sensory Ganglia to the Cornea. Proc. Natl. Acad. Sci. USA 2005, 102, 11462–11467. [Google Scholar] [CrossRef]
- McGraw, H.M.; Awasthi, S.; Wojcechowskyj, J.A.; Friedman, H.M. Anterograde Spread of Herpes Simplex Virus Type 1 Requires Glycoprotein E and Glycoprotein I but Not Us9. J. Virol. 2009, 83, 8315–8326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomishima, M.J.; Enquist, L.W. A Conserved α-Herpesvirus Protein Necessary for Axonal Localization of Viral Membrane Proteins. J. Cell Biol. 2001, 154, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Scherer, J.; Hogue, I.B.; Yaffe, Z.A.; Tanneti, N.S.; Winer, B.Y.; Vershinin, M.; Enquist, L.W. A Kinesin-3 Recruitment Complex Facilitates Axonal Sorting of Enveloped Alpha Herpesvirus Capsids. PLoS Pathog. 2020, 16, e1007985. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.C.; Feenstra, V. Identification of a Novel Herpes Simplex Virus Type 1-Induced Glycoprotein Which Complexes with GE and Binds Immunoglobulin. J. Virol. 1987, 61, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.C.; Frame, M.C.; Ligas, M.W.; Cross, A.M.; Stow, N.D. Herpes Simplex Virus Immunoglobulin G Fc Receptor Activity Depends on a Complex of Two Viral Glycoproteins, GE and GI. J. Virol. 1988, 62, 1347–1354. [Google Scholar] [CrossRef]
- Favoreel, H.W.; Nauwynck, H.J.; Van Oostveldt, P.; Mettenleiter, T.C.; Pensaert, M.B. Antibody-Induced and Cytoskeleton-Mediated Redistribution and Shedding of Viral Glycoproteins, Expressed on Pseudorabies Virus-Infected Cells. J. Virol. 1997, 71, 8254–8261. [Google Scholar] [CrossRef]
- Van de Walle, G.R.; Favoreel, H.W.; Nauwynck, H.J.; Pensaert, M.B. Antibody-Induced Internalization of Viral Glycoproteins and GE–GI Fc Receptor Activity Protect Pseudorabies Virus-Infected Monocytes from Efficient Complement-Mediated Lysis. J. Gen. Virol. 2003, 84, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Ndjamen, B.; Farley, A.H.; Lee, T.; Fraser, S.E.; Bjorkman, P.J. The Herpes Virus Fc Receptor GE-GI Mediates Antibody Bipolar Bridging to Clear Viral Antigens from the Cell Surface. PLoS Pathog. 2014, 10, e1003961. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.C.; Webb, M.; Wisner, T.W.; Brunetti, C. Herpes Simplex Virus GE/GI Sorts Nascent Virions to Epithelial Cell Junctions, Promoting Virus Spread. J. Virol. 2001, 75, 821–833. [Google Scholar] [CrossRef]
- Mingo, R.M.; Han, J.; Newcomb, W.W.; Brown, J.C. Replication of Herpes Simplex Virus: Egress of Progeny Virus at Specialized Cell Membrane Sites. J. Virol. 2012, 86, 7084–7097. [Google Scholar] [CrossRef]
- Feutz, E.; McLeland-Wieser, H.; Ma, J.; Roller, R.J. Functional Interactions between Herpes Simplex Virus PUL51, PUL7 and GE Reveal Cell-Specific Mechanisms for Epithelial Cell-to-Cell Spread. Virology 2019, 537, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Heilingloh, C.S.; Krawczyk, A. Role of L-Particles during Herpes Simplex Virus Infection. Front. Microbiol. 2017, 8, 2565. [Google Scholar] [CrossRef]
- Kruse, M.; Rosorius, O.; Krätzer, F.; Bevec, D.; Kuhnt, C.; Steinkasserer, A.; Schuler, G.; Hauber, J. Inhibition of CD83 Cell Surface Expression during Dendritic Cell Maturation by Interference with Nuclear Export of CD83 MRNA. J. Exp. Med. 2000, 191, 1581–1589. [Google Scholar] [CrossRef]
- Heilingloh, C.S.; Kummer, M.; Mühl-Zürbes, P.; Drassner, C.; Daniel, C.; Klewer, M.; Steinkasserer, A. L Particles Transmit Viral Proteins from Herpes Simplex Virus 1-Infected Mature Dendritic Cells to Uninfected Bystander Cells, Inducing CD83 Downmodulation. J. Virol. 2015, 89, 11046–11055. [Google Scholar] [CrossRef]
- Chouljenko, D.V.; Kim, I.-J.; Chouljenko, V.N.; Subramanian, R.; Walker, J.D.; Kousoulas, K.G. Functional Hierarchy of Herpes Simplex Virus 1 Viral Glycoproteins in Cytoplasmic Virion Envelopment and Egress. J. Virol. 2012, 86, 4262–4270. [Google Scholar] [CrossRef]
- Leylek, R.; Idoyaga, J. The Versatile Plasmacytoid Dendritic Cell: Function, Heterogeneity, and Plasticity. Int. Rev. Cell Mol. Biol. 2019, 349, 177–211. [Google Scholar] [PubMed]
- Mancini, M.; Vidal, S.M. Insights into the Pathogenesis of Herpes Simplex Encephalitis from Mouse Models. Mamm. Genome 2018, 29, 425–445. [Google Scholar] [CrossRef] [PubMed]
- García-Sastre, A.; Biron, C.A. Type 1 Interferons and the Virus-Host Relationship: A Lesson in Détente. Science 2006, 312, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, G.; Pellegry, C.M.; Yasui, F.; Li, F.; Zurawski, S.M.; Zurawski, G.; Levy, Y.; Ting, J.P.-Y.; Su, L. TLR9- and CD40-Targeting Vaccination Promotes Human B Cell Maturation and IgG Induction via PDC-Dependent Mechanisms in Humanized Mice. Front. Immunol. 2021, 12, 672143. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claeys, M.; Delva, J.; Jacqmotte, C.; Waesberghe, C.V.; Favoreel, H.W. Deletion of gE in Herpes Simplex Virus 1 Leads to Increased Extracellular Virus Production and Augmented Interferon Alpha Production by Peripheral Blood Mononuclear Cells. Pathogens 2024, 13, 1138. https://doi.org/10.3390/pathogens13121138
Claeys M, Delva J, Jacqmotte C, Waesberghe CV, Favoreel HW. Deletion of gE in Herpes Simplex Virus 1 Leads to Increased Extracellular Virus Production and Augmented Interferon Alpha Production by Peripheral Blood Mononuclear Cells. Pathogens. 2024; 13(12):1138. https://doi.org/10.3390/pathogens13121138
Chicago/Turabian StyleClaeys, Manon, Jonas Delva, Cedric Jacqmotte, Cliff Van Waesberghe, and Herman W. Favoreel. 2024. "Deletion of gE in Herpes Simplex Virus 1 Leads to Increased Extracellular Virus Production and Augmented Interferon Alpha Production by Peripheral Blood Mononuclear Cells" Pathogens 13, no. 12: 1138. https://doi.org/10.3390/pathogens13121138
APA StyleClaeys, M., Delva, J., Jacqmotte, C., Waesberghe, C. V., & Favoreel, H. W. (2024). Deletion of gE in Herpes Simplex Virus 1 Leads to Increased Extracellular Virus Production and Augmented Interferon Alpha Production by Peripheral Blood Mononuclear Cells. Pathogens, 13(12), 1138. https://doi.org/10.3390/pathogens13121138




