Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Infections: A Review of Epidemiology and Progress in Detection and Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Immune Response to OPV and Emergence of VDPVs
3.1.1. Immune Response in Immunocompetent Individuals
3.1.2. Vaccine-Associated Paralysis (VAPP) and Vaccine-Derived Poliovirus (VDPV)
3.1.3. Primary Immunodeficiencies
3.1.4. Immune Response to Poliovirus in Immunodeficient Individuals
3.2. Epidemiological and Clinical Presentation of Poliovirus Infection in Immunodeficient Patients
3.2.1. Demographic Characteristics of iVDPV Cases Reported to the WHO Registry
3.2.2. Risk of Poliomyelitis Among Patients with PID
3.2.3. Potential Risk of Polio Outbreaks Initiated by Individuals with iVDPV Infection
3.3. Detection and Management of Patients with iVDPV Infection
3.3.1. GPEI Efforts to Identify PID Individuals with iVDPV Infections
3.3.2. Management and Control Measures Recommended for PID Patients with iVDPV Infection
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Polio Eradication Initiative. List of Wild Poliovirus by Country and Year. Available online: http://polioeradication.org/polio-today/polio-now/wild-poliovirus-list (accessed on 20 November 2022).
- Estivariz, C.F.; Burns, C.C.; Macklin, G.R. Poliovirus Vaccine—Live. In Vaccines, 8th ed.; Orenstein, W.A., Offit, P.A., Edwards, K.M., Plotkin, S.A., Eds.; Elsevier: Philadelphia, PA, USA, 2023; pp. 914–968. [Google Scholar]
- Sutter, R.W.; Patriarca, P.A. Inactivated and live, attenuated poliovirus vaccines: Mucosal immunity. In Measles and Poliomyelitis; Kurstak, E., Ed.; Springer: Friesach, Austria, 1993; pp. 279–293. [Google Scholar]
- Agol, V.I. Vaccine-derived polioviruses. Biologicals 2006, 34, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.C.; Diop, O.M.; Sutter, R.W.; Kew, O.M. Vaccine-derived polioviruses. J. Infect. Dis. 2014, 210 (Suppl. S1), S283–S293. [Google Scholar] [CrossRef]
- Kew, O.M.; Wright, P.F.; Agol, V.I.; Delpeyroux, F.; Shimizu, H.; Nathanson, N.; Pallansch, M.A. Circulating vaccine-derived polioviruses: Current state of knowledge. Bull. World Health Organ. 2004, 82, 16–23. [Google Scholar]
- Alexander, L.N.; Seward, J.F.; Santibanez, T.A.; Pallansch, M.A.; Kew, O.M.; Prevots, D.R.; Strebel, P.M.; Cono, J.; Wharton, M.; Orenstein, W.A.; et al. Vaccine policy changes and epidemiology of poliomyelitis in the United States. JAMA 2004, 292, 1696–1701. [Google Scholar] [CrossRef]
- Platt, L.R.; Estivariz, C.F.; Sutter, R.W. Vaccine-associated paralytic poliomyelitis: A review of the epidemiology and estimation of the global burden. J. Infect. Dis. 2014, 210 (Suppl. S1), S380–S389. [Google Scholar] [CrossRef]
- Estivariz, C.F.; Watkins, M.A.; Handoko, D.; Rusipah, R.; Deshpande, J.; Rana, B.J.; Irawan, E.; Widhiastuti, D.; Pallansch, M.A.; Thapa, A.; et al. A large vaccine-derived poliovirus outbreak on Madura Island–Indonesia, 2005. J. Infect. Dis. 2008, 197, 347–354. [Google Scholar] [CrossRef]
- Jenkins, H.E.; Aylward, R.B.; Gasasira, A.; Donnelly, C.A.; Abanida, E.A.; Koleosho-Adelekan, T.; Grassly, N.C. Effectiveness of immunization against paralytic poliomyelitis in Nigeria. N. Engl. J. Med. 2008, 359, 1666–1674. [Google Scholar] [CrossRef]
- Global Polio Eradication Initiative. Responding to a Poliovirus Event or Outbreak Part 2: Protocol for Poliovirus Type 2 (V 2.4). Geneva: World Health Organization. Available online: https://terrance.who.int/mediacentre/data/sage/SAGE_Docs_Ppt_April2018/4_session_polio/April2018_session4_poliovirus_event_procedure_SOP.pdf (accessed on 5 June 2018).
- Global Polio Eradication Initiative. Polio Eradication & Endgame Strategic Plan 2013–2018. WHO/POLIO/13.02. Geneva, Switzerland. Available online: https://www.archive.polioeradication.org/who-we-are/strategic-plan-2013-2018/ (accessed on 12 December 2024).
- Sutter, R.W.; Prevots, D.R. Vaccine-Associated Paralytic Poliomyelitis Among Immunodeficient Persons. Infect. Med. 1994, 11, 426–438. [Google Scholar]
- Duintjer Tebbens, R.J.; Pallansch, M.A.; Thompson, K.M. Modeling the prevalence of immunodeficiency-associated long-term vaccine-derived poliovirus excretors and the potential benefits of antiviral drugs. BMC Infect. Dis. 2015, 15, 379. [Google Scholar] [CrossRef]
- Macklin, G.; Liao, Y.; Takane, M.; Dooling, K.; Gilmour, S.; Mach, O.; Kew, O.M.; Sutter, R.W.; The iVDPV Working Group. Prolonged Excretion of Poliovirus among Individuals with Primary Immunodeficiency Disorder: An Analysis of the World Health Organization Registry. Front. Immunol. 2017, 8, 1103. [Google Scholar] [CrossRef] [PubMed]
- Shaghaghi, M.; Soleyman-Jahi, S.; Abolhassani, H.; Yazdani, R.; Azizi, G.; Rezaei, N.; Barbouche, M.R.; McKinlay, M.A.; Aghamohammadi, A. New insights into physiopathology of immunodeficiency-associated vaccine-derived poliovirus infection; systematic review of over 5 decades of data. Vaccine 2018, 36, 1711–1719. [Google Scholar] [CrossRef]
- World Health Organization. Department of Immunization, Vaccines and Biologicals. In Polio laboratory Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: https://www.medbox.org/document/polio-laboratory-manual (accessed on 5 November 2024).
- Jorba, J.; Campagnoli, R.; De, L.; Kew, O. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J. Virol. 2008, 82, 4429–4440. [Google Scholar] [CrossRef] [PubMed]
- Hammon, W.M.; Coriell, L.L.; Ludwig, E.H.; Mc, A.R.; Greene, A.E.; Sather, G.E.; Wehrle, P.F. Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis. 5. Reanalysis of results based on laboratory-confirmed cases. J. Am. Med. Assoc. 1954, 156, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wahid, R.; Cannon, M.J.; Chow, M. Virus-specific CD4+ and CD8+ cytotoxic T-cell responses and long-term T-cell memory in individuals vaccinated against polio. J. Virol. 2005, 79, 5988–5995. [Google Scholar] [CrossRef] [PubMed]
- Ghendon, Y.; Sanakoyeva, I.I. Comparison of the resistance of the intestinal tract ot poliomyelitis virus (Sabin’s strains) in persons after naturally and experimentally acquired immunity. Acta Virol. 1961, 5, 265–273. [Google Scholar]
- Alexander, J.P., Jr.; Gary, H.E., Jr.; Pallansch, M.A. Duration of poliovirus excretion and its implications for acute flaccid paralysis surveillance: A review of the literature. J. Infect. Dis. 1997, 175 (Suppl. S1), S176–S182. [Google Scholar] [CrossRef]
- Onorato, I.M.; Modlin, J.F.; McBean, A.M.; Thoms, M.L.; Losonsky, G.A.; Bernier, R.H. Mucosal immunity induced by enhanced-potency inactivated and oral polio vaccines. J. Infect. Dis. 1991, 163, 1–6. [Google Scholar] [CrossRef]
- Ogra, P.L. Mucosal immune response to poliovirus vaccines in childhood. Rev. Infect. Dis. 1984, 6 (Suppl. S2), S361–S368. [Google Scholar] [CrossRef] [PubMed]
- Valtanen, S.; Roivainen, M.; Piirainen, L.; Stenvik, M.; Hovi, T. Poliovirus-Specific Intestinal Antibody Responses Coincide with Decline of Poliovirus Excretion. J. Infect. Dis. 2000, 182, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ogra, P.L.; Fishaut, M.; Gallagher, M.R. Viral vaccination via the mucosal routes. Rev. Infect. Dis. 1980, 2, 352–369. [Google Scholar] [CrossRef]
- Ghendon, Y.; Robertson, S.E. Interrupting the transmission of wild polioviruses with vaccines: Immunological considerations. Bull. World Health Organ. 1994, 72, 973–983. [Google Scholar]
- Abbink, F.; Buisman, A.M.; Doornbos, G.; Woldman, J.; Kimman, T.G.; Conyn-van Spaendonck, M.A. Poliovirus-specific memory immunity in seronegative elderly people does not protect against virus excretion. J. Infect. Dis. 2005, 191, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Faden, H.; Duffy, L.; Sun, M.; Shuff, C. Long-term immunity to poliovirus in children immunized with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines. J. Infect. Dis. 1993, 168, 452–454. [Google Scholar] [CrossRef]
- Nathanson, N.; Kew, O.M. From emergence to eradication: The epidemiology of poliomyelitis deconstructed. Am. J. Epidemiol. 2010, 172, 1213–1229. [Google Scholar] [CrossRef] [PubMed]
- Macadam, A.J.; Pollard, S.R.; Ferguson, G.; Skuce, R.; Wood, D.; Almond, J.W.; Minor, P.D. Genetic basis of attenuation of the Sabin type 2 vaccine strain of poliovirus in primates. Virology 1993, 192, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, M.M.; Balanant, J.; Macadam, A.; Otelea, D.; Combiescu, M.; Combiescu, A.A.; Crainic, R.; Delpeyroux, F. Evolution of the Sabin type 1 poliovirus in humans: Characterization of strains isolated from patients with vaccine-associated paralytic poliomyelitis. J. Virol. 1997, 71, 7758–7768. [Google Scholar] [CrossRef]
- Global Polio Eradication Initiative. Classification and Reporting of Vaccine-Derived Polioviruses (VDPVs). Available online: http://polioeradication.org/wp-content/uploads/2016/09/Reporting-and-Classification-of-VDPVs_Aug2016_EN.pdf (accessed on 4 April 2021).
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 42, 1473–1507. [Google Scholar] [CrossRef]
- Abolhassani, H.; Azizi, G.; Sharifi, L.; Yazdani, R.; Mohsenzadegan, M.; Delavari, S.; Sohani, M.; Shirmast, P.; Chavoshzadeh, Z.; Mahdaviani, S.A.; et al. Global systematic review of primary immunodeficiency registries. Expert Rev. Clin. Immunol. 2020, 16, 717–732. [Google Scholar] [CrossRef]
- Yong, P.F.K.; Grimbacher, B.; Thaventhiran, J.E.D. “A Rose is a Rose is a Rose,” but CVID is Not CVID. Adv. Immunol. 2011, 111, 47–107. [Google Scholar] [PubMed]
- Rosen, F.S.; Cooper, M.D.; Wedgwood, R.J. The primary immunodeficiencies. N. Eng. J. Med. 1995, 333, 431–440. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, F.O. Hypogammaglobulinaemia in the United Kingdom. VII. The role of humoral antibodies in protection against and recovery from bacterial and virus infections in hypogammaglobulinaemia. Spec. Rep. Ser. Med. Res. Counc. 1971, 310, 72–85. [Google Scholar]
- Martín, J. Vaccine-derived poliovirus from long term excretors and the end game of polio eradication. Biologicals 2006, 34, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Weil, M.; Shulman, L.M.; Heiman, S.; Stauber, T.; Alfandari, J.; Weiss, L.; Silberstein, I.; Indenbaum, V.; Mendelson, E.; Sofer, D. Prolonged excretion of type-2 poliovirus from a primary immune deficient patient during the transition to a type-2 poliovirus-free world, Israel, 2016. Eurosurveillance 2016, 21, 30408. [Google Scholar] [CrossRef] [PubMed]
- Odoom, J.K.; Yunus, Z.; Dunn, G.; Minor, P.D.; Martin, J. Changes in population dynamics during long-term evolution of sabin type 1 poliovirus in an immunodeficient patient. J. Virol. 2008, 82, 9179–9190. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Chen, H.Y.; Jorba, J.; Sun, H.C.; Yang, S.J.; Lee, H.C.; Huang, Y.C.; Lin, T.Y.; Chen, P.J.; Shimizu, H.; et al. Intratypic recombination among lineages of type 1 vaccine-derived poliovirus emerging during chronic infection of an immunodeficient patient. J. Virol. 2005, 79, 12623–12634. [Google Scholar] [CrossRef] [PubMed]
- Kew, O.M.; Sutter, R.W.; Nottay, B.K.; McDonough, M.J.; Prevots, D.R.; Quick, L.; Pallansch, M.A. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J. Clin. Microbiol. 1998, 36, 2893–2899. [Google Scholar] [CrossRef]
- Savilahti, E.; Klemola, T.; Carlsson, B.; Mellander, L.; Stenvik, M.; Hovi, T. Inadequacy of mucosal IgM antibodies in selective IgA deficiency: Excretion of attenuated polio viruses is prolonged. J. Clin. Immunol. 1988, 8, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Halsey, N.A.; Pinto, J.; Espinosa-Rosales, F.; Faure-Fontenla, M.A.; da Silva, E.; Khan, A.J.; Webster, A.D.; Minor, P.; Dunn, G.; Asturias, E.; et al. Search for poliovirus carriers among people with primary immune deficiency diseases in the United States, Mexico, Brazil, and the United Kingdom. Bull. World Health Organ. 2004, 82, 3–8. [Google Scholar] [PubMed]
- Asturias, E.J.; Grazioso, C.F.; Luna-Fineman, S.; Torres, O.; Halsey, N.A. Poliovirus excretion in Guatemalan adults and children with HIV infection and children with cancer. Biologicals 2006, 34, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, K.A.; Lago, H.; Diomande, F.; Akoua-Koffi, C.; Caceres, V.M.; Pallansch, M.; Kew, O.; Nolan, M.; Zuber, P. Poliovirus vaccine shedding among persons with HIV in Abidjan, Cote d’Ivoire. J. Infect. Dis. 2005, 192, 2124–2128. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, D.N.; Van Zyl, W.B.; Kruger, M.; Blignaut, L.; Grabow, W.O.; Ehlers, M.M. Poliovirus vaccine strains detected in stool specimens of immunodeficient children in South Africa. Diagn. Microbiol. Infect. Dis. 2006, 54, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ryder, R.W.; Oxtoby, M.J.; Mvula, M.; Batter, V.; Baende, E.; Nsa, W.; Davachi, F.; Hassig, S.; Onorato, I.; Deforest, A.; et al. Safety and immunogenicity of bacille Calmette-Guerin, diphtheria-tetanus-pertussis, and oral polio vaccines in newborn children in Zaire infected with human immunodeficiency virus type 1. J. Pediatr. 1993, 122, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, A.; Abolhassani, H.; Kutukculer, N.; Wassilak, S.G.; Pallansch, M.A.; Kluglein, S.; Quinn, J.; Sutter, R.W.; Wang, X.; Sanal, O.; et al. Patients with Primary Immunodeficiencies Are a Reservoir of Poliovirus and a Risk to Polio Eradication. Front. Immunol. 2017, 8, 685. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, Z.A.; Mach, O.; Hossny, E.; Galal, N.M.; El-Sawy, I.; ElMarsafy, A.; Reda, S.M.; Moussa, I.; Sibak, M.A.; Bassiouni, L.; et al. Poliovirus excretion among persons with Primary Immune Deficiency disorders: Summary of data from enhanced poliovirus surveillance in Egypt, 2011–2014. J. Vaccines Vaccin. 2016, 7, 1000331. [Google Scholar] [CrossRef]
- Fiore, L.; Novello, F.; Simeoni, P.; Amato, C.; Vellucci, L.; De Stefano, D.; Grandolfo, M.E.; Luzzi, I. Surveillance of acute flaccid paralysis in Italy: 1996–1997. AFP Study Group. Acute flaccid paralysis. Eur. J. Epidemiol. 1999, 15, 757–763. [Google Scholar] [CrossRef]
- Galal, N.M.; Meshaal, S.; ElHawary, R.; Nasr, E.; Bassiouni, L.; Ashghar, H.; Farag, N.H.; Mach, O.; Burns, C.; Iber, J.; et al. Poliovirus excretion following vaccination with live poliovirus vaccine in patients with primary immunodeficiency disorders: Clinicians’ perspectives in the endgame plan for polio eradication. BMC Res. Notes 2018, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ivanova, O.; Driss, N.; Tiongco-Recto, M.; da Silva, R.; Shahmahmoodi, S.; Sazzad, H.M.; Mach, O.; Kahn, A.L.; Sutter, R.W. Poliovirus excretion among persons with primary immune deficiency disorders: Summary of a seven-country study series. J. Infect. Dis. 2014, 210 (Suppl. S1), S368–S372. [Google Scholar] [CrossRef]
- Mohanty, M.C.; Madkaikar, M.R.; Desai, M.; Taur, P.; Nalavade, U.P.; Sharma, D.K.; Gupta, M.; Dalvi, A.; Shabrish, S.; Kulkarni, M.; et al. Poliovirus Excretion in Children with Primary Immunodeficiency Disorders, India. Emerg. Infect. Dis. 2017, 23, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, M.C.; Desai, M.; Mohammad, A.; Aggarwal, A.; Govindaraj, G.; Bhattad, S.; Lashkari, H.P.; Rajasekhar, L.; Verma, H.; Kumar, A.; et al. Assessment of Enterovirus Excretion and Identification of VDPVs in Patients with Primary Immunodeficiency in India: Outcome of ICMR-WHO Collaborative Study Phase-I. Vaccines 2023, 11, 1211. [Google Scholar] [CrossRef]
- Triki, H.; Barbouche, M.B.; Bahri, O.; Bejaoui, M.; Dellagi, K. Community-acquired poliovirus infection in children with primary immunodeficiencies in Tunisia. J. Clin. Microbiol. 2003, 41, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Driss, N.; Ben-Mustapha, I.; Mellouli, F.; Ben Yahia, A.; Touzi, H.; Bejaoui, M.; Ben Ghorbel, M.; Triki, H.; Barbouche, M.R. High susceptibility for enterovirus infection and virus excretion features in Tunisian patients with primary immunodeficiencies. Clin. Vaccine Immunol. 2012, 19, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Galal, N.M.; Bassiouny, L.; Nasr, E.; Abdelmeguid, N. Isolation of poliovirus shedding following vaccination in children with antibody deficiency disorders. J. Infect. Dev. Ctries. 2012, 6, 881–885. [Google Scholar] [CrossRef] [PubMed]
- de Silva, R.; Gunasena, S.; Ratnayake, D.; Wickremesinghe, G.D.; Kumarasiri, C.D.; Pushpakumara, B.A.; Deshpande, J.; Kahn, A.L.; Sutter, R.W. Prevalence of prolonged and chronic poliovirus excretion among persons with primary immune deficiency disorders in Sri Lanka. Vaccine 2012, 30, 7561–7565. [Google Scholar] [CrossRef]
- Khetsuriani, N.; Prevots, D.R.; Quick, L.; Elder, M.E.; Pallansch, M.; Kew, O.; Sutter, R.W. Persistence of vaccine-derived polioviruses among immunodeficient persons with vaccine-associated paralytic poliomyelitis. J. Infect. Dis. 2003, 188, 1845–1852. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Prolonged poliovirus excretion in an immunodeficient person with vaccine-associated paralytic poliomyelitis. MMWR Morb. Mortal. Wkly. Rep. 1997, 46, 641–643. [Google Scholar]
- Centers for Disease Control and Prevention. Update on vaccine-derived polioviruses. MMWR Morb. Mortal. Wkly. Rep. 2006, 55, 1093–1097. [Google Scholar]
- Bellmunt, A.; May, G.; Zell, R.; Pring-Akerblom, P.; Verhagen, W.; Heim, A. Evolution of poliovirus type I during 5.5 years of prolonged enteral replication in an immunodeficient patient. Virology 1999, 265, 178–184. [Google Scholar] [CrossRef]
- MacLennan, C.A.; Huissoon, A.P.; Kumararatne, D.S. Vaccine-derived poliomyelitis 12 years after infection. N. Engl. J. Med. 2011, 365, 1355. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, W.H.; Canning, B.; Wilton, T.; Kidd, M.; Klapsa, D.; Majumdar, M.; Sooriyakumar, K.; Martin, J.; Huissoon, A.P. Case report: Clearance of longstanding, immune-deficiency-associated, vaccine-derived polio virus infection following remdesivir therapy for chronic SARS-CoV-2 infection. Front. Immunol. 2023, 14, 1135834. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Bolivar-Wagers, S.; Srinivas, N.; Holubar, M.; Maldonado, Y. Immunodeficiency-related vaccine-derived poliovirus (iVDPV) cases: A systematic review and implications for polio eradication. Vaccine 2015, 33, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Macklin, G.; Diop, O.M.; Humayun, A.; Shahmahmoodi, S.; El-Sayed, Z.A.; Triki, H.; Rey, G.; Avagyan, T.; Grabovac, V.; Jorba, J.; et al. Update on Immunodeficiency-Associated Vaccine-Derived Polioviruses—Worldwide, July 2018–December 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, M.C.; Madkaikar, M.R.; Desai, M.; Aluri, J.; Varose, S.Y.; Taur, P.; Sharma, D.K.; Nalavade, U.P.; Rane, S.V.; Gupta, M.; et al. Natural Clearance of Prolonged VDPV Infection in a Child With Primary Immunodeficiency Disorder. Front. Immunol. 2019, 10, 1567. [Google Scholar] [CrossRef]
- Alexander, J.P.; Ehresmann, K.; Seward, J.; Wax, G.; Harriman, K.; Fuller, S.; Cebelinski, E.A.; Chen, Q.; Jorba, J.; Kew, O.M.; et al. Transmission of imported vaccine-derived poliovirus in an undervaccinated community in Minnesota. J. Infect. Dis. 2009, 199, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Klapsa, D.; Burton-Fanning, S.; Hand, J.; Wilton, T.; Stephens, L.; Mate, R.; Shillitoe, B.; Celma, C.; Slatter, M.; et al. Asymptomatic immunodeficiency-associated vaccine-derived poliovirus infections in two UK children. Nat. Commun. 2023, 14, 3413. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.S.; Zipursky, S. A novel tool to eradicate an ancient scourge: The novel oral polio vaccine type 2 story. Lancet Infect. Dis. 2023, 23, e67–e71. [Google Scholar] [CrossRef]
- Wyatt, H.V. Poliomyelitis in hypogammaglobulinemics. J. Infect. Dis. 1973, 128, 802–806. [Google Scholar] [CrossRef]
- Jorba, J.; Diop, O.M.; Iber, J.; Sutter, R.W.; Wassilak, S.G.; Burns, C.C. Update on Vaccine-Derived Polioviruses—Worldwide, January 2015–May 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 763–769. [Google Scholar] [CrossRef]
- Shaghaghi, M.; Parvaneh, N.; Ostad-Rahimi, P.; Fathi, S.M.; Shahmahmoodi, S.; Abolhassani, H.; Aghamohammadi, A. Combined immunodeficiency presenting with vaccine-associated paralytic poliomyelitis: A case report and narrative review of literature. Immunol. Investig. 2014, 43, 292–298. [Google Scholar] [CrossRef]
- Shahmahmoodi, S.; Mamishi, S.; Aghamohammadi, A.; Aghazadeh, N.; Tabatabaie, H.; Gooya, M.M.; Zahraei, S.M.; Mousavi, T.; Yousefi, M.; Farrokhi, K.; et al. Vaccine-associated paralytic poliomyelitis in immunodeficient children, Iran, 1995–2008. Emerg. Infect. Dis. 2010, 16, 1133–1136. [Google Scholar] [CrossRef]
- Ion-Nedelcu, N.; Dobrescu, A.; Strebel, P.M.; Sutter, R.W. Vaccine-associated paralytic poliomyelitis and HIV infection. Lancet 1994, 343, 51–52. [Google Scholar] [CrossRef]
- Chitsike, I.; van Furth, R. Paralytic poliomyelitis associated with live oral poliomyelitis vaccine in child with HIV infection in Zimbabwe: Case report. BMJ 1999, 318, 841–843. [Google Scholar] [CrossRef]
- Avellon, A.; Cabrerizo, M.; de Miguel, T.; Perez-Brena, P.; Tenorio, A.; Perez, J.L.; de Aragon, M.V.; Trallero, G. Paralysis case and contact spread of recombinant vaccine-derived poliovirus, Spain. Emerg. Infect. Dis. 2008, 14, 1807–1809. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, M.C.; Govindaraj, G.; Ahmad, M.; Varose, S.Y.; Tatkare, M.; Shete, A.; Yadav, S.; Joshi, Y.; Yadav, P.; Sharma, D.; et al. Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Excretion in an Infant with Severe Combined Immune Deficiency with Spillover to a Parent. Vaccines 2024, 12, 759. [Google Scholar] [CrossRef] [PubMed]
- Snider, C.J.; Boualam, L.; Tallis, G.; Takashima, Y.; Abeyasinghe, R.; Lo, Y.R.; Grabovac, V.; Avagyan, T.; Aslam, S.K.; Eltayeb, A.O.; et al. Concurrent outbreaks of circulating vaccine-derived poliovirus types 1 and 2 affecting the Republic of the Philippines and Malaysia, 2019–2021. Vaccine 2023, 41 (Suppl. S1), A58–A69. [Google Scholar] [CrossRef]
- Dowdle, W.R.; De Gourville, E.; Kew, O.M.; Pallansch, M.A.; Wood, D.J. Polio eradication: The OPV paradox. Rev. Med. Virol. 2003, 13, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Kalkowska, D.A.; Pallansch, M.A.; Thompson, K.M. Updated modelling of the prevalence of immunodeficiency-associated long-term vaccine-derived poliovirus (iVDPV) excreters. Epidemiol. Infect. 2019, 147, e295. [Google Scholar] [CrossRef] [PubMed]
- Tebbens, R.J.; Sangrujee, N.; Thompson, K.M. The costs of future polio risk management policies. Risk Anal. 2006, 26, 1507–1531. [Google Scholar] [CrossRef]
- Khetsuriani, N.; Helfand, R.; Pallansch, M.; Kew, O.; Fowlkes, A.; Oberste, M.S.; Tukei, P.; Muli, J.; Makokha, E.; Gary, H. Limited duration of vaccine poliovirus and other enterovirus excretion among human immunodeficiency virus infected children in Kenya. BMC Infect. Dis. 2009, 9, 136. [Google Scholar] [CrossRef][Green Version]
- Duintjer Tebbens, R.J.; Thompson, K.M. Comprehensive screening for immunodeficiency-associated vaccine-derived poliovirus: An essential oral poliovirus vaccine cessation risk management strategy. Epidemiol. Infect. 2017, 145, 217–226. [Google Scholar] [CrossRef]
- Sazzad, H.M.; Rainey, J.J.; Kahn, A.L.; Mach, O.; Liyanage, J.B.; Alam, A.N.; Kawser, C.A.; Hossain, A.; Sutter, R.; Luby, S.P. Screening for long-term poliovirus excretion among children with primary immunodeficiency disorders: Preparation for the polio posteradication era in Bangladesh. J. Infect. Dis. 2014, 210 (Suppl. S1), S373–S379. [Google Scholar] [CrossRef]
- Shaghaghi, M.; Shahmahmoodi, S.; Abolhassani, H.; Soleyman-Jahi, S.; Parvaneh, L.; Mahmoudi, S.; Chavoshzadeh, Z.; Yazdani, R.; Zahraei, S.M.; Ebrahimi, M.; et al. Vaccine-Derived Polioviruses and Children with Primary Immunodeficiency, Iran, 1995–2014. Emerg. Infect. Dis. 2016, 22, 1712–1719. [Google Scholar] [CrossRef]
- Shaghaghi, M.; Shahmahmoodi, S.; Nili, A.; Abolhassani, H.; Madani, S.P.; Nejati, A.; Yousefi, M.; Kandelousi, Y.M.; Irannejad, M.; Shaghaghi, S.; et al. Vaccine-Derived Poliovirus Infection among Patients with Primary Immunodeficiency and Effect of Patient Screening on Disease Outcomes, Iran. Emerg. Infect. Dis. 2019, 25, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Liu, Y.; Xu, J.W.; Wang, Q.; Yin, Z.D.; Wen, N.; Yang, H.; Rodewald, L.E.; Zhang, Z.Y. Detection of a Highly Divergent Type 3 Vaccine-Derived Poliovirus in a Child with a Severe Primary Immunodeficiency Disorder—Chongqing, China, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1148–1150. [Google Scholar] [CrossRef]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, October 2016—Conclusions and recommendation. Wkly. Epidemiol. Rec. 2016, 91, 561–584. [Google Scholar]
- Global Polio Eradication Initiative. Guidelines for Implementing Polio Surveillance Among Patients with Primary Immunodeficiency Disorders (PIDs); Global Polio Eradication Initiative: Geneva, Switzerland, 2022. [Google Scholar]
- Álamo-Junquera, D.; Politi, J.; Simón, P.; Dieli-Crimi, R.; Borrell, R.P.; Colobran, R.; Martínez-Gallo, M.; Campins, M.; Antón, A.; Esperalba, J.; et al. Coordinated Response to Imported Vaccine-Derived Poliovirus Infection, Barcelona, Spain, 2019–2020. Emerg. Infect. Dis. 2021, 27, 1513. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.S.; Harper, J.; Murray, A.; Lexau, C.; Bahta, L.; Christensen, J.; Cebelinski, E.; Fuller, S.; Kline, S.; Wallace, G.S.; et al. Vaccine-derived poliomyelitis 12 years after infection in Minnesota. N. Engl. J. Med. 2011, 364, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Kew, O.M.; Sutter, R.W.; de Gourville, E.M.; Dowdle, W.R.; Pallansch, M.A. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Ann. Rev. Microbiol. 2005, 59, 587–635. [Google Scholar] [CrossRef]
- Minor, P. Vaccine-derived poliovirus (VDPV): Impact on poliomyelitis eradication. Vaccine 2009, 27, 2649–2652. [Google Scholar] [CrossRef]
- Labadie, K.; Pelletier, I.; Saulnier, A.; Martin, J.; Colbere-Garapin, F. Poliovirus mutants excreted by a chronically infected hypogammaglobulinemic patient establish persistent infections in human intestinal cells. Virology 2004, 318, 66–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoneyama, T.; Hagiwara, A.; Hara, M.; Shimojo, H. Alteration in oligonucleotide fingerprint patterns of the viral genome in poliovirus type 2 isolated from paralytic patients. Infect. Immun. 1982, 37, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Saito, Y.; Komatsu, T.; Kodama, H.; Abo, W.; Chiba, S.; Nakao, T. Antigenic analysis of polioviruses isolated from a child with agammaglobulinemia and paralytic poliomyelitis after Sabin vaccine administration. Microbiol. Immunol. 1981, 25, 905–913. [Google Scholar] [CrossRef]
- Abo, W.; Chiba, S.; Yamanaka, T.; Nakao, T.; Hara, M.; Tagaya, I. Paralytic poliomyelitis in a child with agammaglobulinemia. Eur. J. Pediatr. 1979, 132, 11–16. [Google Scholar] [CrossRef]
- Misbah, S.A.; Lawrence, P.A.; Kurtz, J.B.; Chapel, H.M. Prolonged faecal excretion of poliovirus in a nurse with common variable hypogammaglobulinaemia. Postgrad. Med. J. 1991, 67, 301–303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Minor, P. Characteristics of poliovirus strains from long-term excretors with primary immunodeficiencies. Dev. Biol. 2001, 105, 75–80. [Google Scholar]
- MacLennan, C.; Dunn, G.; Huissoon, A.P.; Kumararatne, D.S.; Martin, J.; O’Leary, P.; Thompson, R.A.; Osman, H.; Wood, P.; Minor, P.; et al. Failure to clear persistent vaccine-derived neurovirulent poliovirus infection in an immunodeficient man. Lancet 2004, 363, 1509–1513. [Google Scholar] [CrossRef]
- Dunn, G.; Klapsa, D.; Wilton, T.; Stone, L.; Minor, P.D.; Martin, J. Twenty-Eight Years of Poliovirus Replication in an Immunodeficient Individual: Impact on the Global Polio Eradication Initiative. PLoS Pathog. 2015, 11, e1005114. [Google Scholar] [CrossRef]
- Shahmahmoodi, S.; Parvaneh, N.; Burns, C.; Asghar, H.; Mamishi, S.; Tabatabaie, H.; Chen, Q.; Teimourian, S.; Gooya, M.M.; Esteghamati, A.R.; et al. Isolation of a type 3 vaccine-derived poliovirus (VDPV) from an Iranian child with X-linked agammaglobulinemia. Virus Res. 2008, 137, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, S.; Garcia Erro, M.; Cisterna, D.; Freire, M.C. Paralytic poliomyelitis caused by a vaccine-derived polio virus in an antibody-deficient Argentinean child. Pediatr. Infect. Dis. J. 2003, 22, 570–572. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Laboratory surveillance for wild and vaccine-derived polioviruses—worldwide, January 2007–June 2008. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 967–970. [Google Scholar]
- Buttinelli, G.; Donati, V.; Fiore, S.; Marturano, J.; Plebani, A.; Balestri, P.; Soresina, A.R.; Vivarelli, R.; Delpeyroux, F.; Martin, J.; et al. Nucleotide variation in Sabin type 2 poliovirus from an immunodeficient patient with poliomyelitis. J. Gen. Virol. 2003, 84, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Tharmaphornpilas, P. Vaccine-derived poliovirus, Thailand, 2003. Emerg. Infect. Dis. 2005, 11, 777–778. [Google Scholar] [CrossRef]
- Parvaneh, N.; Shahmahmoudi, S.; Tabatabai, H.; Zahraei, M.; Mousavi, T.; Esteghamati, A.R.; Gooya, M.M.; Mamishi, S.; Nategh, R.; Kew, O.M.; et al. Vaccine-associated paralytic poliomyelitis in a patient with MHC class II deficiency. J. Clin. Virol. 2007, 39, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Poliovirus infections in four unvaccinated children—Minnesota, August–October 2005. MMWR Morb. Mortal. Wkly. Rep. 2005, 54, 1053–1055. [Google Scholar]
- Centers for Disease Control and Prevention. Update on vaccine-derived polioviruses—worldwide, January 2006-August 2007. MMWR Morb. Mortal. Wkly. Rep. 2007, 56, 996–1001. [Google Scholar]
- Mamishi, S.; Shahmahmoudi, S.; Tabatabaie, H.; Teimourian, S.; Pourakbari, B.; Gheisari, Y.; Yeganeh, M.; Salavati, A.; Esteghamati, A.-R.; Gooya, M.M.; et al. Novel BTK mutation presenting with vaccine-associated paralytic poliomyelitis. Eur. J. Pediatr. 2008, 167, 1335–1338. [Google Scholar] [CrossRef]
- Driss, N.; Mellouli, F.; Ben Yahia, A.; Touzi, H.; Barbouche, M.R.; Triki, H.; Bejaoui, M. Sequential asymptomatic enterovirus infections in a patient with major histocompatibility complex class II primary immunodeficiency. J. Clin. Microbiol. 2014, 52, 3486–3489. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Update on Vaccine-Derived Polioviruses—Worldwide, January 2008–June 2009. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 1002–1006. [Google Scholar]
- Burgos, M.E.; Elkik, S.; Barbosa, P.; Oleastro, M.; Freire, C.; Parra, A.; Caparelli, M.; Sarkis, C. A vaccine derived poliovirus case in an immunocompromised argentinian child. Int. J. Infect. Dis. 2010, 14, e450–e451. [Google Scholar] [CrossRef][Green Version]
- Centers for Disease Control and Prevention. Update on Vaccine-Derived Polioviruses—Worldwide, July 2009–March 2011. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 846–850. [Google Scholar]
- Centers for Disease Control and Prevention. Update on vaccine-derived polioviruses—worldwide, April 2011–June 2012. MMWR Morb. Mortal. Wkly. Rep. 2012, 61, 741–746. [Google Scholar]
- Diop, O.M.; Burns, C.C.; Wassilak, S.G.; Kew, O.M. Update on vaccine-derived polioviruses—Worldwide, july 2012–december 2013. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 242–248. [Google Scholar] [PubMed]
- Wang, H.-B.; Luo, H.-M.; Li, L.; Fan, C.-X.; Hao, L.-X.; Ma, C.; Su, Q.-R.; Yang, H.; Reilly, K.H.; Wang, H.-Q.; et al. Vaccine-derived poliovirus surveillance in China during 2001–2013: The potential challenge for maintaining polio free status. BMC Infect. Dis. 2017, 17, 742. [Google Scholar] [CrossRef]
- Gumede, N.; Muthambi, V.; Schoub, B.D. Immunodeficiency-associated vaccine-derived poliovirus type 3 in infant, South Africa, 2011. Emerg. Infect. Dis. 2012, 18, 992–994. [Google Scholar] [CrossRef]
- Diop, O.M.; Burns, C.C.; Sutter, R.W.; Wassilak, S.G.; Kew, O.M. Update on Vaccine-Derived Polioviruses—Worldwide, January 2014–March 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 640–646. [Google Scholar] [PubMed]
- Schubert, A.; Bottcher, S.; Eis-Hubinger, A.M. Two Cases of Vaccine-Derived Poliovirus Infection in an Oncology Ward. N. Engl. J. Med. 2016, 374, 1296–1298. [Google Scholar] [CrossRef] [PubMed]
- Trimble, R.; Atkins, J.; Quigg, T.C.; Burns, C.C.; Wallace, G.S.; Thomas, M.; Mangla, A.T.; Infante, A.J. Vaccine-Associated Paralytic Poliomyelitis and BCG-osis in an Immigrant Child with Severe Combined Immunodeficiency Syndrome—Texas, 2013. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 724–741. [Google Scholar]
- Jorba, J.; Diop, O.M.; Iber, J.; Henderson, E.; Sutter, R.W.; Wassilak, S.G.F.; Burns, C.C. Update on Vaccine-Derived Polioviruses—Worldwide, January 2016-June 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1185–1191. [Google Scholar] [CrossRef]
- Jorba, J.; Diop, O.M.; Iber, J.; Henderson, E.; Zhao, K.; Sutter, R.W.; Wassilak, S.G.F.; Burns, C.C. Update on Vaccine-Derived Polioviruses—Worldwide, January 2017–June 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1189–1194. [Google Scholar] [CrossRef]
- Howard, W.; Moonsamy, S.; Seakamela, L.; Jallow, S.; Modiko, F.; du Plessis, H.; Sibiya, R.; Kamupira, M.; Maseti, E.; Suchard, M. Sensitivity of the acute flaccid paralysis surveillance system for poliovirus in South Africa, 2016–2019. J. Med. Microbiol. 2021, 70, 001441. [Google Scholar] [CrossRef]

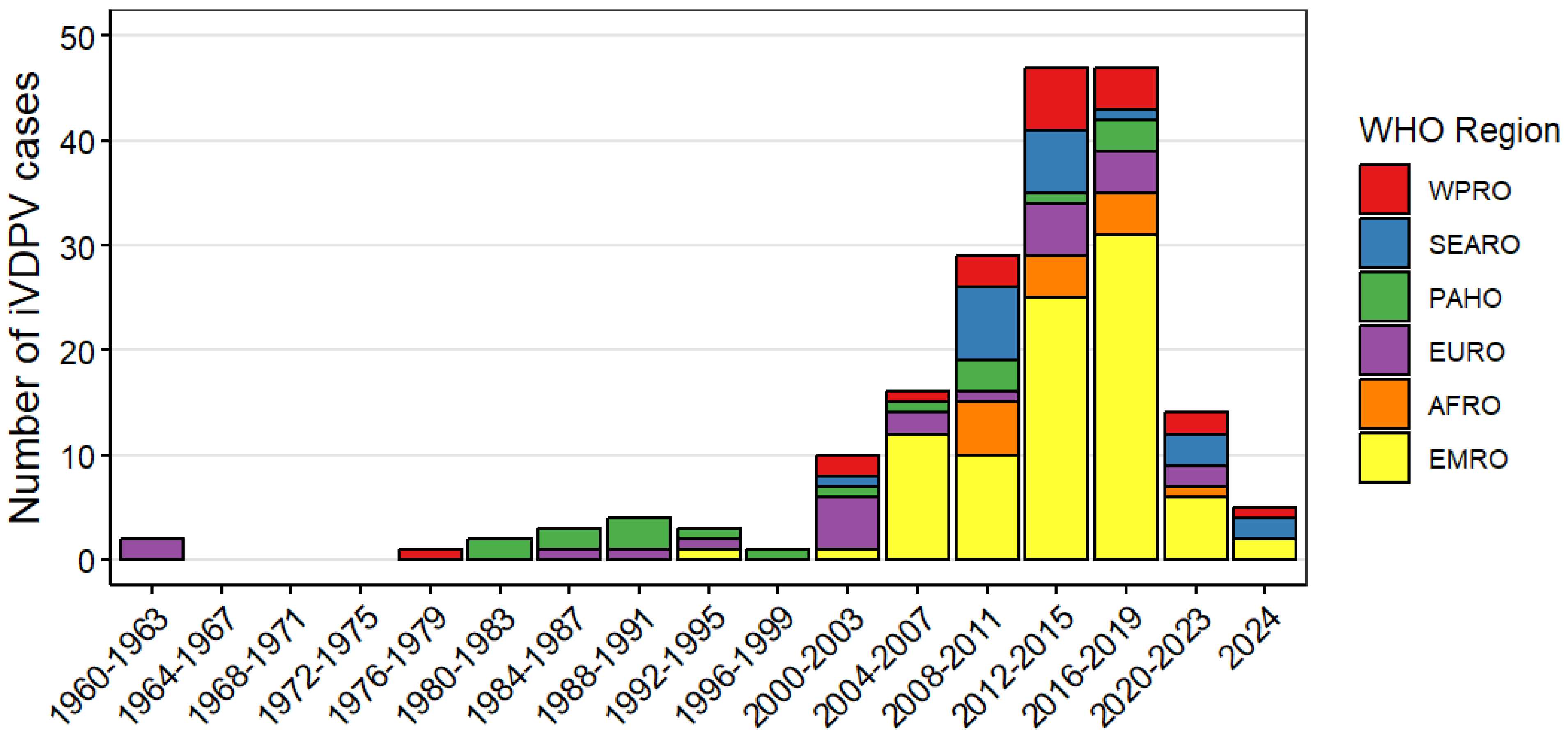
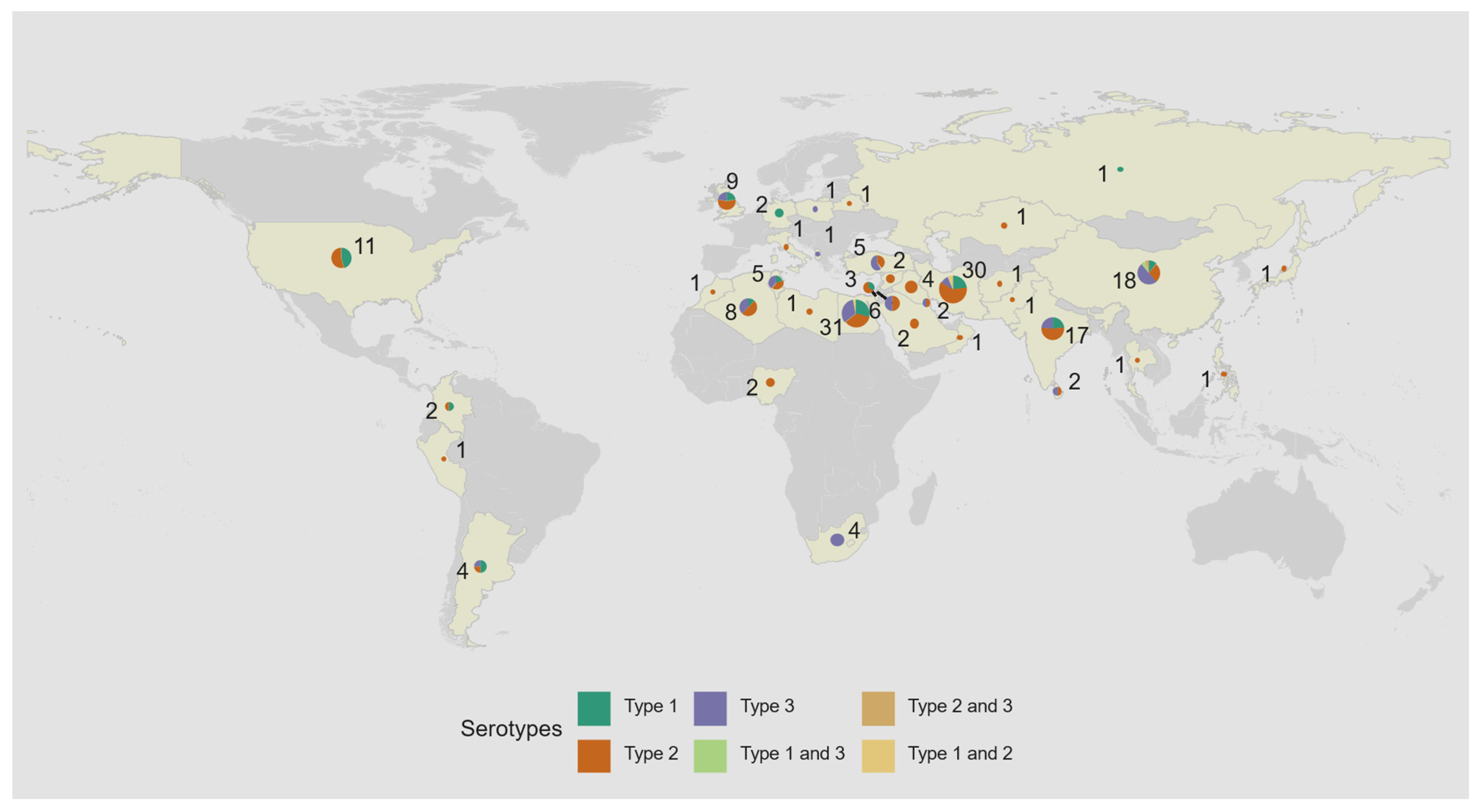
| Country | Patients with PIDs Studied | Poliovirus Excretors | iVDPV Excretors | Years of Study | Reference | ||
|---|---|---|---|---|---|---|---|
| Bangladesh | 13 | 1 | 8% | 0 | 0% | 2008–2013 | [54] |
| Brazil | 95 | 3 | 3% | 0 | 0% | 2001–2002 | [45] |
| China | 167 | 3 | 2% | 0 | 0% | 2008–2013 | [54] |
| China | 63 | 0 | 0% | 0 | 0% | 2014–2015 | [50] |
| Colombia | 25 | 0 | 0% | 0 | 0% | 2014–2015 | [50] |
| Egypt | 15 | 2 | 13% | 0 | 0% | 2006–2009 | [59] |
| Egypt | 104 | 12 | 12% | 6 | 6% | 2011–2014 | [51] |
| India | 23 | 0 | 0% | 0 | 0% | 2014–2015 | [50] |
| India | 42 | 3 | 7% | 1 | 2% | 2014–2017 | [55] |
| India | 154 | 4 | 3% | 1 | 1% | 2019 | [56] |
| Iran | 43 | 1 | 2% | 0 | 0% | 2008–2013 | [54] |
| Iran | 102 | 4 | 4% | 3 | 3% | 2014–2015 | [50] |
| Israel | 24 | 0 | 0% | 0 | 0% | 2014–2015 | [50] |
| Italy | 38 | 0 | 0% | 0 | 0% | 2001–2002 | [52] |
| Japan | 9 | 0 | 0% | 0 | 0% | 2014–2015 | [50] |
| Mexico | 33 | 1 | 3% | 0 | 0% | 2001–2002 | [45] |
| Mexico | 20 | 0 | 0% | 0 | 0% | 2014–2015 | [50] |
| Poland | 0 | 0 | 0% | 0 | 0% | 2014–2015 | [50] |
| Philippines | 70 | 1 | 1% | 0 | 0% | 2008–2013 | [54] |
| Sri Lanka | 51 | 5 | 10% | 2 | 4% | 2008–2013 | [54,60] |
| Russia | 136 | 0 | 0% | 0 | 0% | 2008–2013 | [54] |
| Russia | 83 | 2 | 2% | 0 | 0% | 2014–2015 | [50] |
| Tunisia | 16 | 4 | 25% | 0 | 0% | 1996–1997 | [57] |
| Tunisia | 82 | 6 | 7% | 0 | 0% | 2008–2013 | [54,58] |
| Tunisia | 40 | 3 | 8% | 1 | 3% | 2014–2015 | [50] |
| Turkey | 172 | 4 | 2% | 1 | 1% | 2014–2015 | [50] |
| United Kingdom | 125 | 0 | 0% | 0 | 0% | 2001–2002 | [45] |
| United States | 94 | 0 | 0% | 0 | 0% | 2001–2002 | [45] |
| Total | 1717 | 59 | 3% | 15 | 1% | ||
| Variable | Categories | iVDPV Cases, n (%) |
|---|---|---|
| Gender | Male | 103 (56) |
| Female | 67 (36) | |
| Missing | 14 (8) | |
| Age at onset or first positive specimen | <1 year | 83 (45) |
| 1 to <5 years | 64 (34) | |
| 5 to <10 years | 11 (6) | |
| 10 to <20 years | 7 (4) | |
| 20 to <30 years | 3 (2) | |
| ≥30 years | 2 (1) | |
| Missing | 14 (8) | |
| Paralysis | Yes | 106 (58) |
| No | 69 (38) | |
| Unknown | 9 (5) | |
| Country of residence World Bank income classification | High income | 38 (21) |
| Upper-middle income | 77 (42) | |
| Lower-middle income | 68 (37) | |
| Low income | 1 (1) | |
| WHO region of residence | African Region | 14 (8) |
| Eastern Mediterranean Region | 88 (48) | |
| European Region | 24 (13) | |
| Region of the Americas | 18 (10) | |
| South-East Asian Region | 20 (11) | |
| Western Pacific Region | 20 (11) | |
| B-cell immunodeficiencies | CVID | 23 (12) |
| AGG | 23 (12) | |
| HGG | 13 (7) | |
| Other B-cell immunodeficiencies | 2 (1) | |
| Combined B- and T-cell immunodeficiencies | SCID | 45 (24) |
| MHC class II deficiency | 19 (10) | |
| Other combined immunodeficiencies | 9 (5) | |
| Other immune disorders | Other disorders | 4 (2) |
| Unknown | 46 (25) | |
| Poliovirus serotype a | 1 | 42 (22) |
| 2 | 97 (53) | |
| 3 | 50 (27) | |
| Outcome status | Dead | 74 (40) |
| Alive (stopped excreting) | 62 (34) | |
| Alive (excreting at last specimen) | 27 (15) | |
| Unknown | 21 (11) |
| Immunodeficiency Disorder | Patients n (%) | Paralysis n (%) | Dead n (%) | Alive (Excreting at Last Specimen) n (%) | Number of Nucleotides at Time of Detection Median (Range) | Length of Excretion in Years Median (Range) | Type 1 n (%) | Type 2 n (%) | Type 3 n (%) |
|---|---|---|---|---|---|---|---|---|---|
| B-cell immunodeficiencies | |||||||||
| AGG | 23 (13.2) | 22 (96%) | 4 (17%) | 1 (4%) | 1.5 (0.5–3.5) | 0.3 (0.05–4.92) | 5 (22%) | 14 (61%) | 4 (17%) |
| CVID | 22 (12.6) | 16 (73%) | 8 (36%) | 1 (5%) | 2.2 (0.7–12.3) | 0.625 (0.04–26.42) | 6 (27%) | 13 (59%) | 4 (18%) |
| HGG | 13 (7.5) | 10 (77%) | 8 (62%) | 0 (0%) | 1.55 (0.6–2.2) | 0.485 (0.01–1.83) | 2 (15%) | 9 (69%) | 2 (15%) |
| Other antibody disorders | 2 (1.1) | 2 (100%) | 0 (0%) | 1 (50%) | 1.1 (0.6–1.6) | 0.45 (0.45–0.45) | 1 (50%) | 1 (50%) | 0 (0%) |
| Combined B- and T-cell immunodeficiencies | |||||||||
| SCID | 45 (25.9) | 16 (36%) | 30 (67%) | 3 (7%) | 1.4 (0.66–4.5) | 0.2 (0.02–2.13) | 11 (24%) | 28 (62%) | 9 (20%) |
| MHC class II deficiency | 19 (10.9) | 7 (37%) | 8 (42%) | 3 (16%) | 1.6 (0.67–4) | 0.405 (0.09–1.74) | 3 (16%) | 9 (47%) | 7 (37%) |
| Other combined immunodeficiencies | 9 (5.2) | 3 (33%) | 3 (33%) | 0 (0%) | 1.7 (1–2.2) | 0.205 (0.05–0.46) | 3 (33%) | 3 (33%) | 3 (33%) |
| Other immunodeficiency disorders | |||||||||
| Other disorders | 4 (2.3) | 2 (50%) | 1 (25%) | 0 (0%) | 1 (0.9–1.6) | 0.68 (0.07–1.29) | 1 (25%) | 3 (75%) | 0 (0%) |
| Unknown | 47 (27) | 28 (60%) | 11 (23%) | 21 (45%) | 1.35 (0.67–7) | 0.505 (0.01–3.37) | 10 (21%) | 17 (36%) | 21 (45%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estivariz, C.F.; Krow-Lucal, E.R.; Mach, O. Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Infections: A Review of Epidemiology and Progress in Detection and Management. Pathogens 2024, 13, 1128. https://doi.org/10.3390/pathogens13121128
Estivariz CF, Krow-Lucal ER, Mach O. Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Infections: A Review of Epidemiology and Progress in Detection and Management. Pathogens. 2024; 13(12):1128. https://doi.org/10.3390/pathogens13121128
Chicago/Turabian StyleEstivariz, Concepcion F., Elisabeth R. Krow-Lucal, and Ondrej Mach. 2024. "Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Infections: A Review of Epidemiology and Progress in Detection and Management" Pathogens 13, no. 12: 1128. https://doi.org/10.3390/pathogens13121128
APA StyleEstivariz, C. F., Krow-Lucal, E. R., & Mach, O. (2024). Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Infections: A Review of Epidemiology and Progress in Detection and Management. Pathogens, 13(12), 1128. https://doi.org/10.3390/pathogens13121128







