Susceptibility of Mouse Brain to MCMV Infection and Neuroinflammation During Ontogeny
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Viruses and Cell Lines
2.3. Flow Cytometry
2.4. Statistical Analyses
3. Results
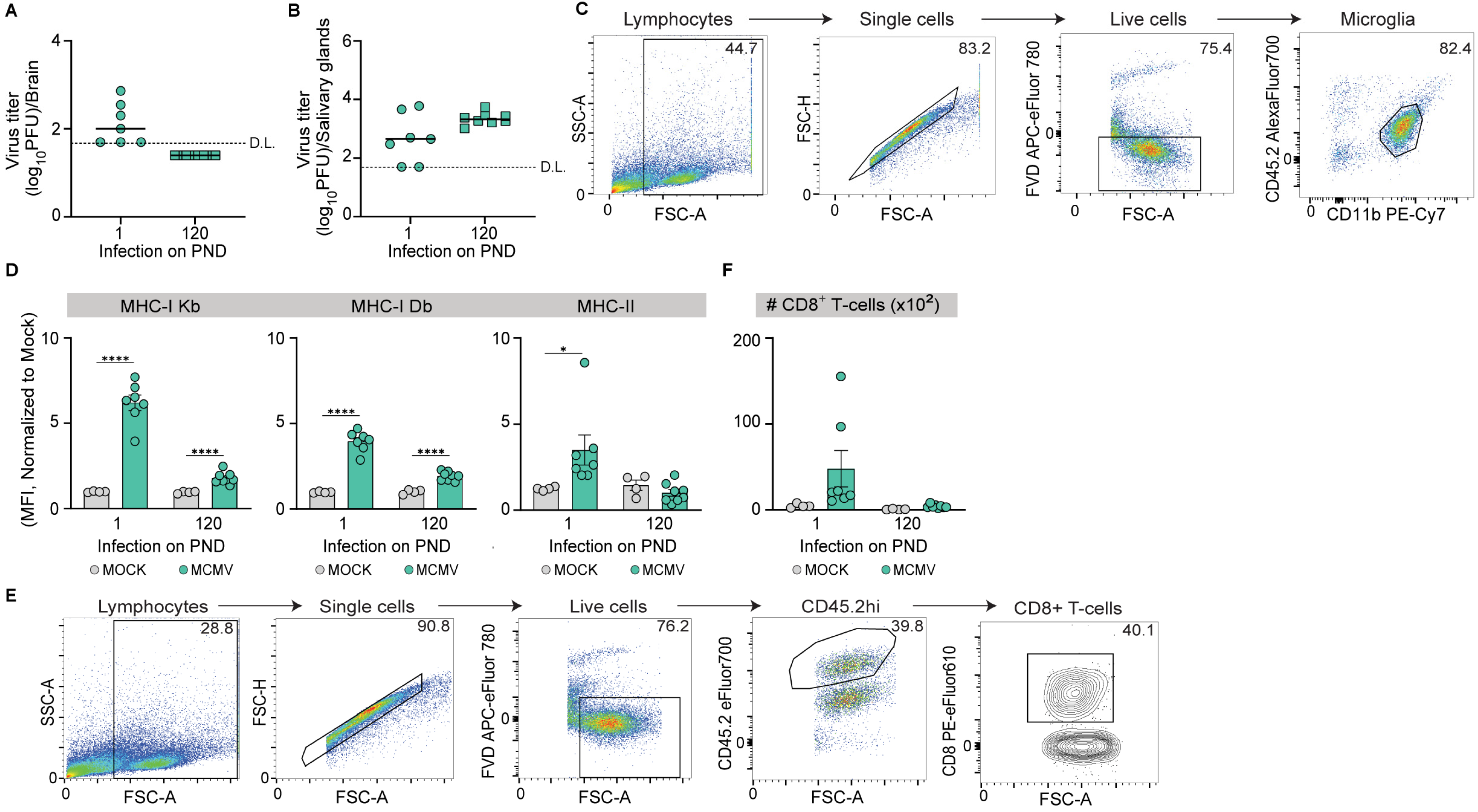
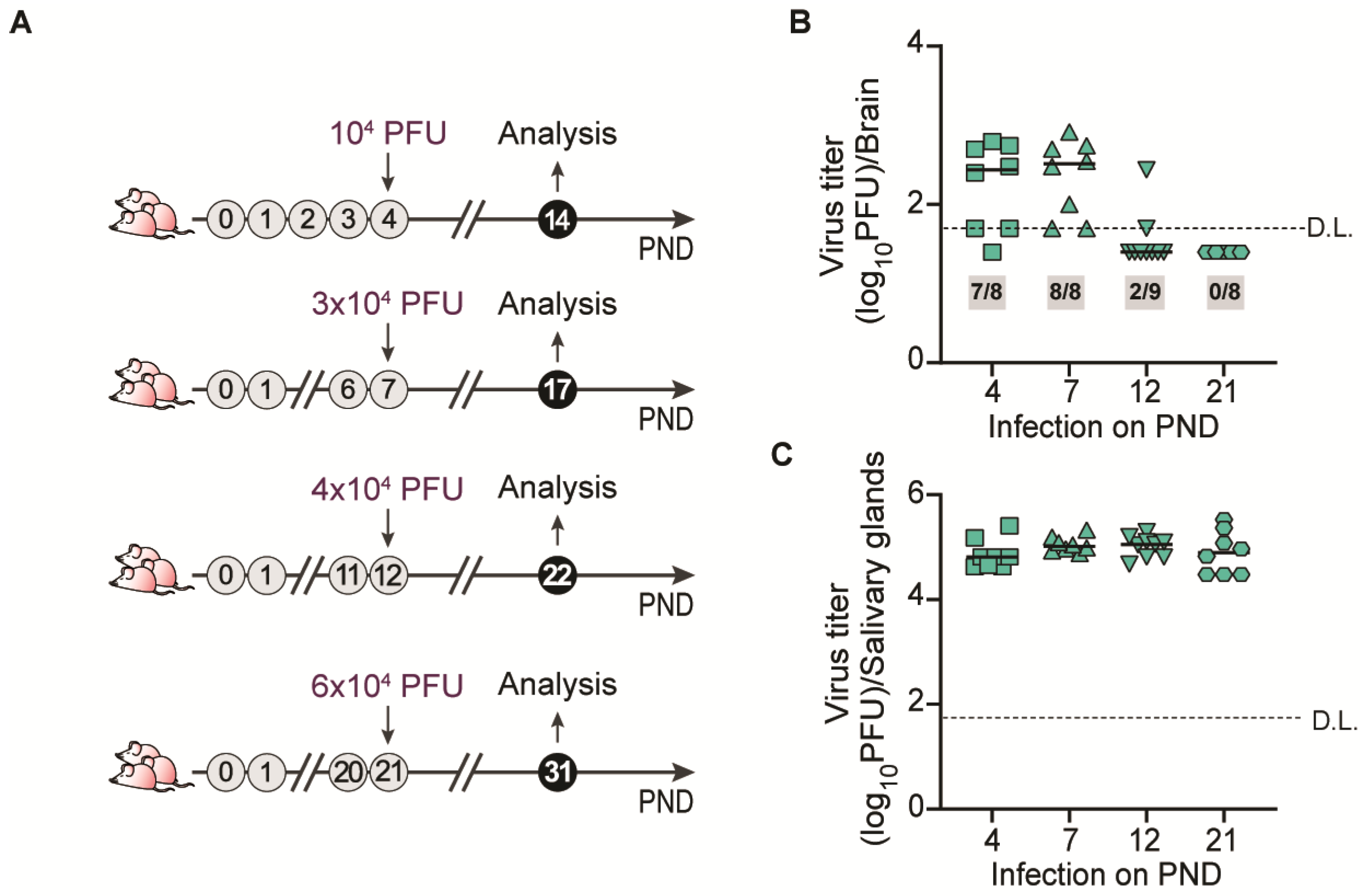
3.1. Age-Dependent Infection of Mouse Brains with MCMV
3.2. MCMV Infection of Mouse Brain During Ontogeny
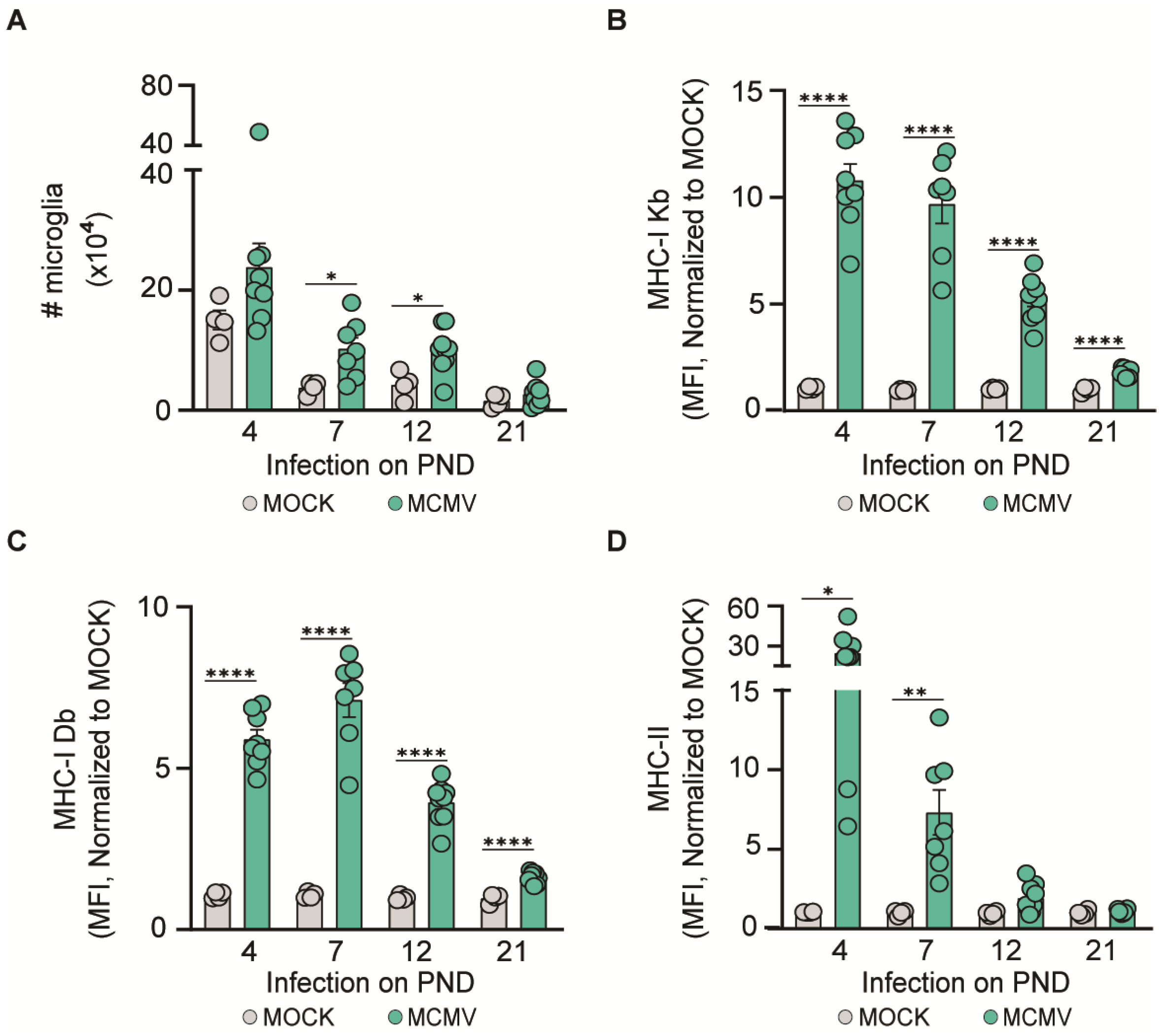
3.3. MCMV Neuroinvasion Results in the Activation of Microglia
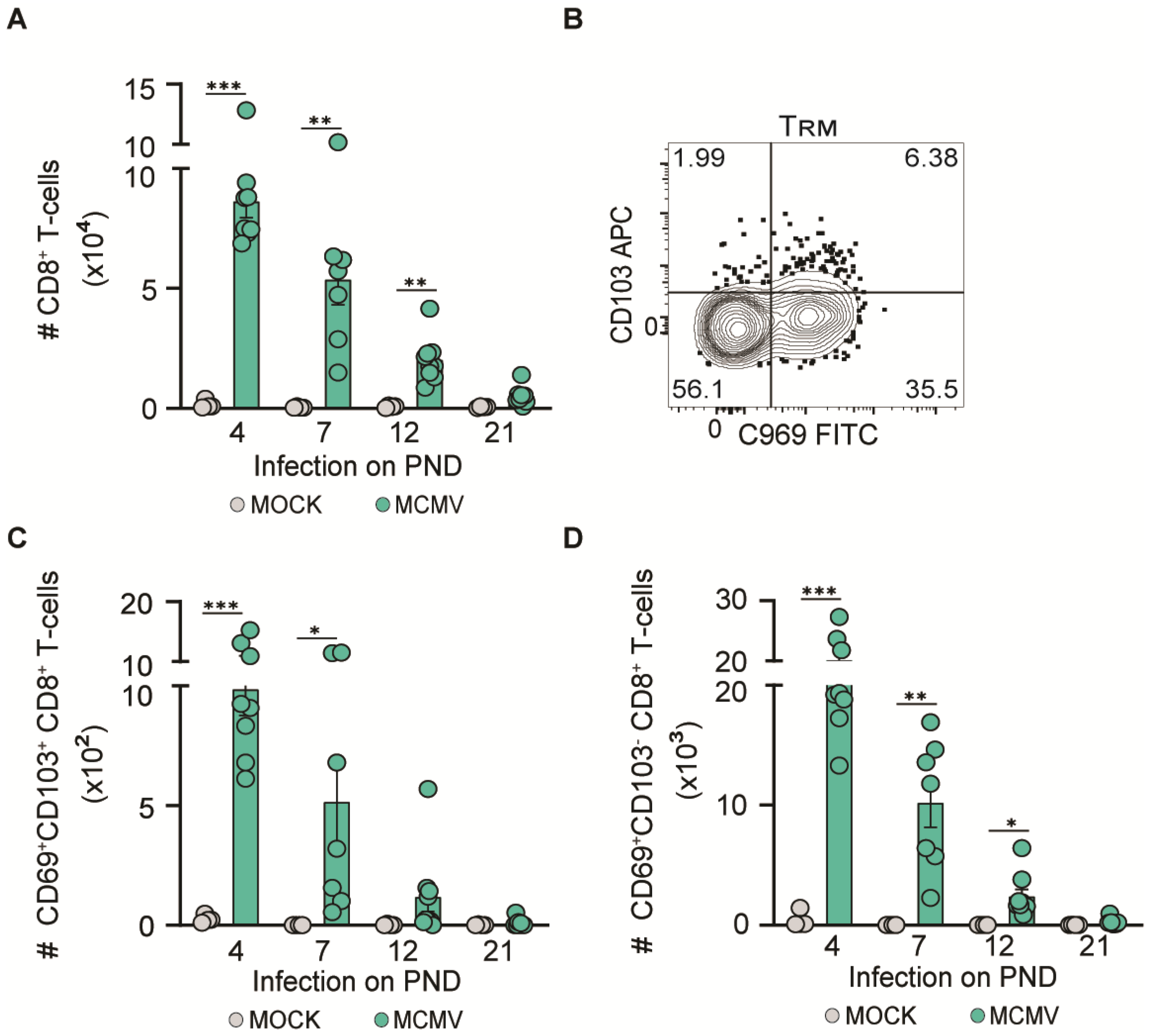
3.4. MCMV Neuroinvasion Promotes CD8+ T-Cell Migration in the CNS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Haddad, B.J.S.; Oler, E.; Armistead, B.; Elsayed, N.A.; Weinberger, D.R.; Bernier, R.; Burd, I.; Kapur, R.; Jacobsson, B.; Wang, C.; et al. The Fetal Origins of Mental Illness. Am. J. Obstet. Gynecol. 2019, 221, 549–562. [Google Scholar] [CrossRef]
- Fowler, K.; Mucha, J.; Neumann, M.; Lewandowski, W.; Kaczanowska, M.; Grys, M.; Schmidt, E.; Natenshon, A.; Talarico, C.; Buck, P.O.; et al. A Systematic Literature Review of the Global Seroprevalence of Cytomegalovirus: Possible Implications for Treatment, Screening, and Vaccine Development. BMC Public Health 2022, 22, 1659. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.K.; Khanna, R. Human Cytomegalovirus: Clinical Aspects, Immune Regulation, and Emerging Treatments. Lancet Infect. Dis. 2004, 4, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Goodrum, F. Human Cytomegalovirus Latency: Approaching the Gordian Knot. Annu. Rev. Virol. 2016, 3, 333–357. [Google Scholar] [CrossRef]
- Griffiths, P.; Baraniak, I.; Reeves, M. The Pathogenesis of Human Cytomegalovirus. J. Pathol. 2015, 235, 288–297. [Google Scholar] [CrossRef]
- Cannon, M.J.; Davis, K.F. Washing Our Hands of the Congenital Cytomegalovirus Disease Epidemic. BMC Public Health 2005, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.B.; Pass, R.F.; Britt, W.J.; Stagno, S.; Alford, C.A. Symptomatic Congenital Cytomegalovirus Infection: Neonatal Morbidity and Mortality. Pediatr. Infect. Dis. J. 1992, 11, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.R.; Bialek, S.R.; Boppana, S.B.; Griffiths, P.D.; Laughlin, C.A.; Ljungman, P.; Mocarski, E.S.; Pass, R.F.; Read, J.S.; Schleiss, M.R.; et al. Priorities for CMV Vaccine Development. Vaccine 2013, 32, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.B.; Britt, W.J. Synopsis of Clinical Aspects of Human Cytomegalovirus Disease. In Cytomegaloviruses from Molecular Pathogenesis to Intervention; Caister Academic Press: Norfolk, VA, USA, 2013; pp. 1–26. [Google Scholar]
- Reddehase, M.; Lemmermann, N. Mouse Model of Cytomegalovirus Disease and Immunotherapy in the Immunocompromised Host: Predictions for Medical Translation That Survived the “Test of Time”. Viruses 2018, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Krstanović, F.; Britt, W.J.; Jonjić, S.; Brizić, I. Cytomegalovirus Infection and Inflammation in Developing Brain. Viruses 2021, 13, 1078. [Google Scholar] [CrossRef] [PubMed]
- Brizić, I.; Lisnić, B.; Brune, W.; Hengel, H.; Jonjić, S. Cytomegalovirus Infection: Mouse Model. Curr. Protoc. Immunol. 2018, 122, e51. [Google Scholar] [CrossRef] [PubMed]
- Koontz, T.; Bralic, M.; Tomac, J.; Pernjak-Pugel, E.; Bantug, G.; Jonjic, S.; Britt, W.J. Altered Development of the Brain after Focal Herpesvirus Infection of the Central Nervous System. J. Exp. Med. 2008, 205, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Brizić, I.; Šušak, B.; Arapović, M.; Huszthy, P.C.; Hiršl, L.; Kveštak, D.; Juranić Lisnić, V.; Golemac, M.; Pernjak Pugel, E.; Tomac, J.; et al. Brain-Resident Memory CD8+ T Cells Induced by Congenital CMV Infection Prevent Brain Pathology and Virus Reactivation. Eur. J. Immunol. 2018, 48, 950–964. [Google Scholar] [CrossRef] [PubMed]
- Reuter, J.D.; Gomez, D.L.; Wilson, J.H.; van den Pol, A.N. Systemic Immune Deficiency Necessary for Cytomegalovirus Invasion of the Mature Brain. J. Virol. 2004, 78, 1473–1487. [Google Scholar] [CrossRef]
- Cheeran, M.C.-J.; Gekker, G.; Hu, S.; Min, X.; Cox, D.; Lokensgard, J.R. Intracerebral Infection with Murine Cytomegalovirus Induces CXCL10 and Is Restricted by Adoptive Transfer of Splenocytes. J. Neurovirol. 2004, 10, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Ribalta, T.; Martinez, J.A.; Jares, P.; Muntané, J.; Miquel, R.; Claramonte, X.; Cardesa, A. Presence of Occult Cytomegalovirus Infection in the Brain after Orthotopic Liver Transplantation. Virchows Arch. 2002, 440, 166–171. [Google Scholar] [CrossRef]
- Becroft, D.M. Prenatal Cytomegalovirus Infection: Epidemiology, Pathology and Pathogenesis. Perspect. Pediatr. Pathol. 1981, 6, 203–241. [Google Scholar] [PubMed]
- Brizić, I.; Lisnić, B.; Krstanović, F.; Brune, W.; Hengel, H.; Jonjić, S. Mouse Models for Cytomegalovirus Infections in Newborns and Adults. Curr. Protoc. 2022, 2, e537. [Google Scholar] [CrossRef] [PubMed]
- Bantug, G.R.B.; Cekinovic, D.; Bradford, R.; Koontz, T.; Jonjic, S.; Britt, W.J. CD8+ T Lymphocytes Control Murine Cytomegalovirus Replication in the Central Nervous System of Newborn Animals. J. Immunol. 2008, 181, 2111–2123. [Google Scholar] [CrossRef]
- Krmpotic, A.; Bubic, I.; Polic, B.; Lucin, P.; Jonjic, S. Pathogenesis of Murine Cytomegalovirus Infection. Microbes Infect. 2003, 5, 1263–1277. [Google Scholar] [CrossRef]
- Pavlou, A.; Mulenge, F.; Gern, O.L.; Busker, L.M.; Greimel, E.; Waltl, I.; Kalinke, U. Orchestration of Antiviral Responses within the Infected Central Nervous System. Cell. Mol. Immunol. 2024, 21, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and Non-Immune Functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef] [PubMed]
- Kveštak, D.; Juranić Lisnić, V.; Lisnić, B.; Tomac, J.; Golemac, M.; Brizić, I.; Indenbirken, D.; Cokarić Brdovčak, M.; Bernardini, G.; Krstanović, F.; et al. NK/ILC1 Cells Mediate Neuroinflammation and Brain Pathology Following Congenital CMV Infection. J. Exp. Med. 2021, 218, e20201503. [Google Scholar] [CrossRef] [PubMed]
- Teissier, N.; Fallet-Bianco, C.; Delezoide, A.L.; Laquerrière, A.; Marcorelles, P.; Khung-Savatovsky, S.; Nardelli, J.; Cipriani, S.; Csaba, Z.; Picone, O.; et al. Cytomegalovirus-Induced Brain Malformations in Fetuses. J. Neuropathol. Exp. Neurol. 2014, 73, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Bowen, L.N.; Smith, B.; Reich, D.; Quezado, M.; Nath, A. HIV-Associated Opportunistic CNS Infections: Pathophysiology, Diagnosis and Treatment. Nat. Rev. Neurol. 2016, 12, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.J.; Hyde, T.B.; Schmid, D.S. Review of Cytomegalovirus Shedding in Bodily Fluids and Relevance to Congenital Cytomegalovirus Infection. Rev. Med. Virol. 2011, 21, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Chen, S.; Sharp, M.; Dekker, C.; Manganello, A.M.; Tongson, E.C.; Maecker, H.T.; Holmes, T.H.; Wang, Z.; Kemble, G.; et al. Persistent and Selective Deficiency of CD4+ T Cell Immunity to Cytomegalovirus in Immunocompetent Young Children. J. Immunol. 2004, 172, 3260–3267. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.; Surendran, N.; Pichichero, M. Immune Responses in Neonates. Expert Rev. Clin. Immunol. 2014, 10, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.F.; Ostermann, E.; Perez, A.; Tödter, S.; Virdi, S.; Indenbirken, D.; Glau, L.; Gieras, A.; Brixel, R.; Arens, R.; et al. Limited Protection against Early-Life Cytomegalovirus Infection Results from Deficiency of Cytotoxic CD8 T Cells. bioRxiv 2024. [Google Scholar] [CrossRef]
- Huygens, A.; Lecomte, S.; Tackoen, M.; Olislagers, V.; Delmarcelle, Y.; Burny, W.; Van Rysselberge, M.; Liesnard, C.; Larsen, M.; Appay, V.; et al. Functional Exhaustion Limits CD4+ and CD8+ T-Cell Responses to Congenital Cytomegalovirus Infection. J. Infect. Dis. 2015, 212, 484–494. [Google Scholar] [CrossRef]
- Liu, J.; Jaijyan, D.K.; Tang, Q.; Zhu, H. Promising Cytomegalovirus-Based Vaccine Vector Induces Robust CD8+ T-Cell Response. Int. J. Mol. Sci. 2019, 20, 4457. [Google Scholar] [CrossRef] [PubMed]
- Karner, D.; Kvestak, D.; Kucan Brlic, P.; Cokaric Brdovcak, M.; Lisnic, B.; Brizic, I.; Juranic Lisnic, V.; Golemac, M.; Tomac, J.; Krmpotic, A.; et al. Prion Protein Alters Viral Control and Enhances Pathology after Perinatal Cytomegalovirus Infection. Nat. Commun. 2024, 15, 7754. [Google Scholar] [CrossRef]
- Rožmanić, C.; Lisnić, B.; Pribanić Matešić, M.; Mihalić, A.; Hiršl, L.; Park, E.; Lesac Brizić, A.; Indenbirken, D.; Viduka, I.; Šantić, M.; et al. Perinatal Murine Cytomegalovirus Infection Reshapes the Transcriptional Profile and Functionality of NK Cells. Nat. Commun. 2023, 14, 6412. [Google Scholar] [CrossRef] [PubMed]
- Marcoe, J.P.; Lim, J.R.; Schaubert, K.L.; Fodil-Cornu, N.; Matka, M.; McCubbrey, A.L.; Farr, A.R.; Vidal, S.M.; Laouar, Y. TGF-β Is Responsible for NK Cell Immaturity during Ontogeny and Increased Susceptibility to Infection during Mouse Infancy. Nat. Immunol. 2012, 13, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, M.; Daneman, R. Formation and Maintenance of the BBB. Mech. Dev. 2015, 138, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Crivellato, E.; Artico, M. Development of the Blood-brain Barrier: A Historical Point of View. Anat. Rec. Part B New Anat. 2006, 289B, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Saili, K.S.; Zurlinden, T.J.; Schwab, A.J.; Silvin, A.; Baker, N.C.; Hunter, E.S.; Ginhoux, F.; Knudsen, T.B. Blood-Brain Barrier Development: Systems Modeling and Predictive Toxicology. Birth Defects Res. 2017, 109, 1680–1710. [Google Scholar] [CrossRef] [PubMed]
- Galea, I. The Blood-Brain Barrier in Systemic Infection and Inflammation. Cell. Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef]
- Koyuncu, O.O.; Hogue, I.B.; Enquist, L.W. Virus Infections in the Nervous System. Cell Host Microbe 2013, 13, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Nunez, N.V.; Gaudin, R. A Viral Journey to the Brain: Current Considerations and Future Developments. PLoS Pathog. 2020, 16, e1008434. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.A.A.; Morris, S.L.; Rudman, G.A.; Rittenhouse, D.J.; Monk, C.H.; Sakamuri, S.S.V.P.; Mehedi Hasan, M.; Shamima Khatun, M.; Wang, H.; Garfinkel, L.P.; et al. Intermittent Cytomegalovirus Infection Alters Neurobiological Metabolism and Induces Cognitive Deficits in Mice. Brain. Behav. Immun. 2024, 117, 36–50. [Google Scholar] [CrossRef] [PubMed]
- van den Pol, A.N. Brain Trauma Enhances Transient Cytomegalovirus Invasion of the Brain Only in Mice That Are Immunodeficient. J. Virol. 2009, 83, 420–427. [Google Scholar] [CrossRef]
- Kawasaki, H.; Kosugi, I.; Sakao-Suzuki, M.; Meguro, S.; Arai, Y.; Tsutsui, Y.; Iwashita, T. Cytomegalovirus Initiates Infection Selectively from High-Level Β1 Integrin-Expressing Cells in the Brain. Am. J. Pathol. 2015, 185, 1304–1323. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Kosugi, I.; Arai, Y.; Tsutsui, Y. The Amount of Immature Glial Cells in Organotypic Brain Slices Determines the Susceptibility to Murine Cytomegalovirus Infection. Lab. Investig. 2002, 82, 1347–1358. [Google Scholar] [CrossRef]
- Johnson, R.T.; McFarland, H.F.; Levy, S.E. Age-Dependent Resistance to Viral Encephalitis: Studies of Infections Due to Sindbis Virus in Mice. J. Infect. Dis. 1972, 125, 257–262. [Google Scholar] [CrossRef]
- Oliver, K.R.; Scallan, M.F.; Dyson, H.; Fazakerley, J.K. Susceptibility to a Neurotropic Virus and Its Changing Distribution in the Developing Brain Is a Function of CNS Maturity. J. Neurovirol. 1997, 3, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Sellier, Y.; Marliot, F.; Bessières, B.; Stirnemann, J.; Encha-Razavi, F.; Guilleminot, T.; Haicheur, N.; Pages, F.; Ville, Y.; Leruez-Ville, M. Adaptive and Innate Immune Cells in Fetal Human Cytomegalovirus-Infected Brains. Microorganisms 2020, 8, 176. [Google Scholar] [CrossRef]
- Brizić, I.; Mihalić, A.; Kveštak, D.; Lisnić, B.; Krstanović, F.; Hosseini, S.; Sitnik, K.; Golemac, M.; Lisnić, V.J.; Rashidi, A.; et al. Persistently Primed Microglia Restrict the Reactivation of Latent Cytomegalovirus at the Expense of Neuronal Synaptic Connectivity. Available online: https://www.researchsquare.com/article/rs-5144336/v1 (accessed on 5 October 2024).
- Zheng, H.; Webster, M.J.; Weickert, C.S.; Beasley, C.L.; Paulus, M.P.; Yolken, R.H.; Savitz, J. Cytomegalovirus Antibodies Are Associated with Mood Disorders, Suicide, Markers of Neuroinflammation, and Microglia Activation in Postmortem Brain Samples. Mol. Psychiatry 2023, 28, 5282–5292. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The Semantics of Microglia Activation: Neuroinflammation, Homeostasis, and Stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- Düsedau, H.P.; Steffen, J.; Figueiredo, C.A.; Boehme, J.D.; Schultz, K.; Erck, C.; Korte, M.; Faber-Zuschratter, H.; Smalla, K.-H.; Dieterich, D.; et al. Influenza A Virus (H1N1) Infection Induces Microglial Activation and Temporal Dysbalance in Glutamatergic Synaptic Transmission. MBio 2021, 12, e0177621. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstanović, F.; Mihalić, A.; Šakota, L.; Lisnić, B.; Jonjić, S.; Brizić, I. Susceptibility of Mouse Brain to MCMV Infection and Neuroinflammation During Ontogeny. Pathogens 2024, 13, 1108. https://doi.org/10.3390/pathogens13121108
Krstanović F, Mihalić A, Šakota L, Lisnić B, Jonjić S, Brizić I. Susceptibility of Mouse Brain to MCMV Infection and Neuroinflammation During Ontogeny. Pathogens. 2024; 13(12):1108. https://doi.org/10.3390/pathogens13121108
Chicago/Turabian StyleKrstanović, Fran, Andrea Mihalić, Lucija Šakota, Berislav Lisnić, Stipan Jonjić, and Ilija Brizić. 2024. "Susceptibility of Mouse Brain to MCMV Infection and Neuroinflammation During Ontogeny" Pathogens 13, no. 12: 1108. https://doi.org/10.3390/pathogens13121108
APA StyleKrstanović, F., Mihalić, A., Šakota, L., Lisnić, B., Jonjić, S., & Brizić, I. (2024). Susceptibility of Mouse Brain to MCMV Infection and Neuroinflammation During Ontogeny. Pathogens, 13(12), 1108. https://doi.org/10.3390/pathogens13121108





