Prevalence and Sociodemographic Risk Factors of Soil-Transmitted Helminths in Rural Communities Living in Endemic Foci of Onchocerciasis in Southern Gabon
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Population
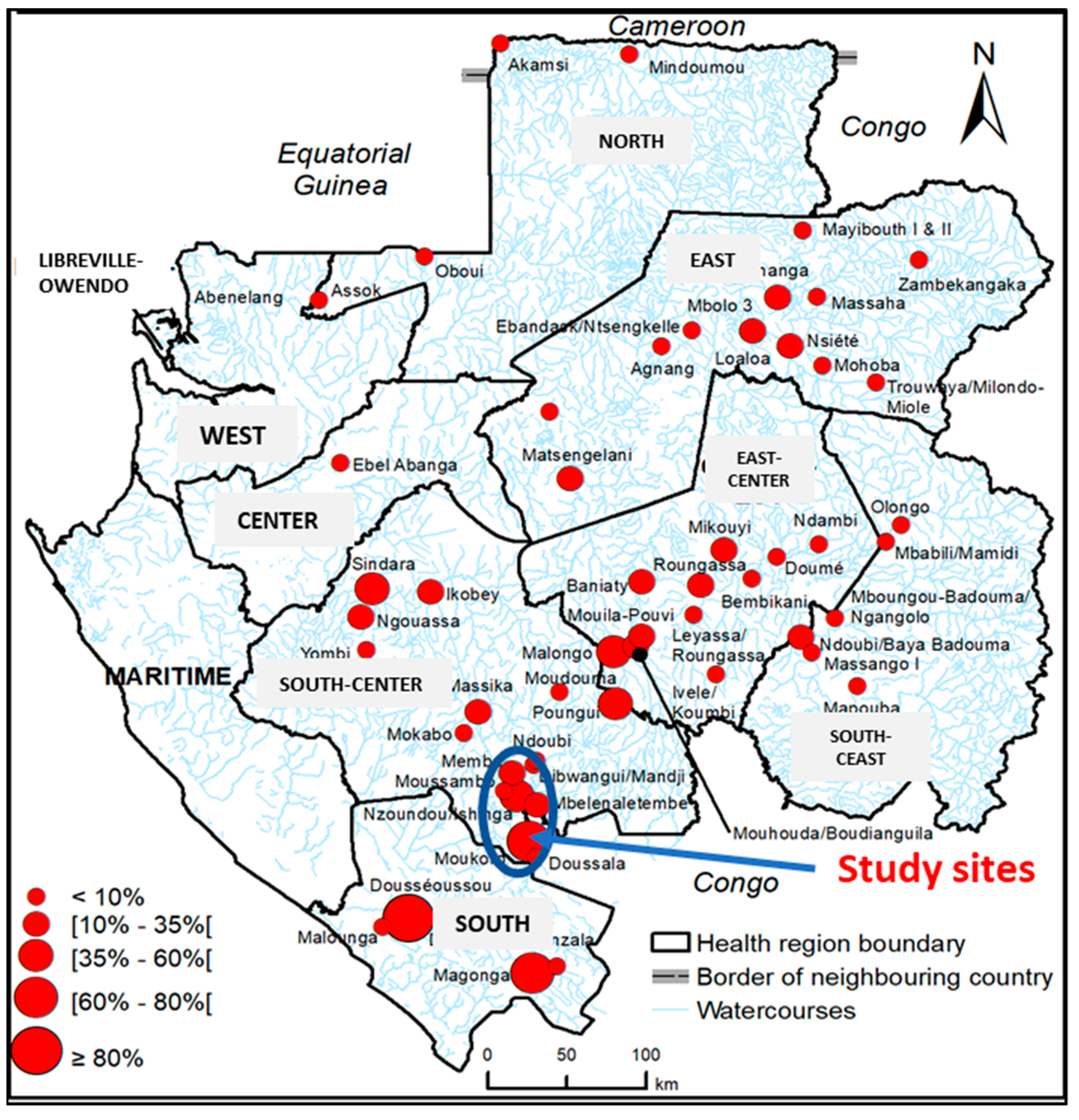
2.1.1. Study Sites
2.1.2. Study Populations
2.1.3. Sample Size Calculation
2.2. Biological Testing
2.2.1. Sample Collection
2.2.2. Kato–Katz Technique
2.2.3. Helminth Culture
2.2.4. Onchocerciasis Seropositivity
2.3. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Population
3.2. Prevalence of Ov16 IgG Seropositivity and Onchocerciasis Endemicity
3.3. STH Prevalence
3.4. Prevalence and Intensity of Intestinal Nematode Species
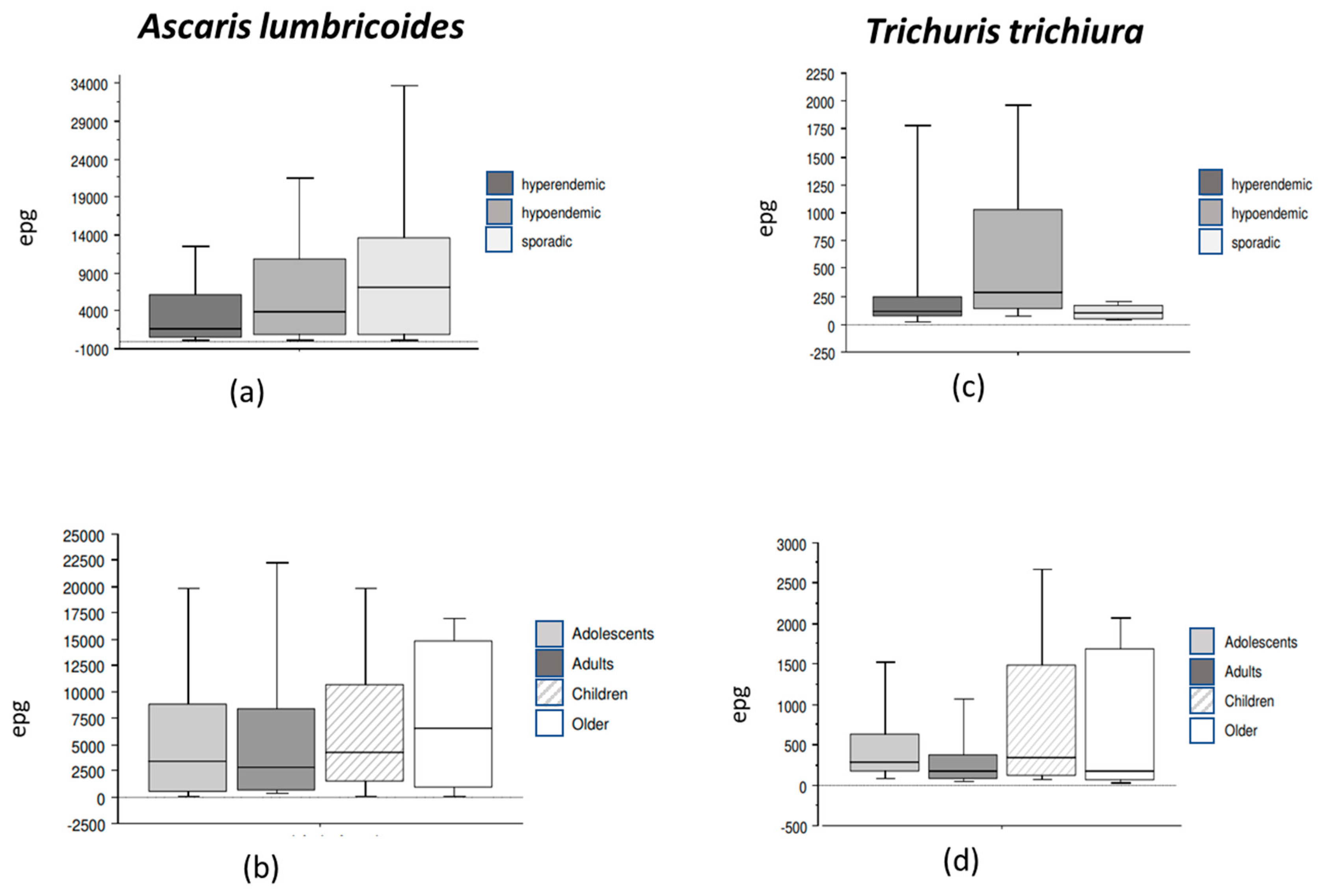
3.5. STH Species Prevalence and Parasite Density According to Onchocerciasis Endemicity
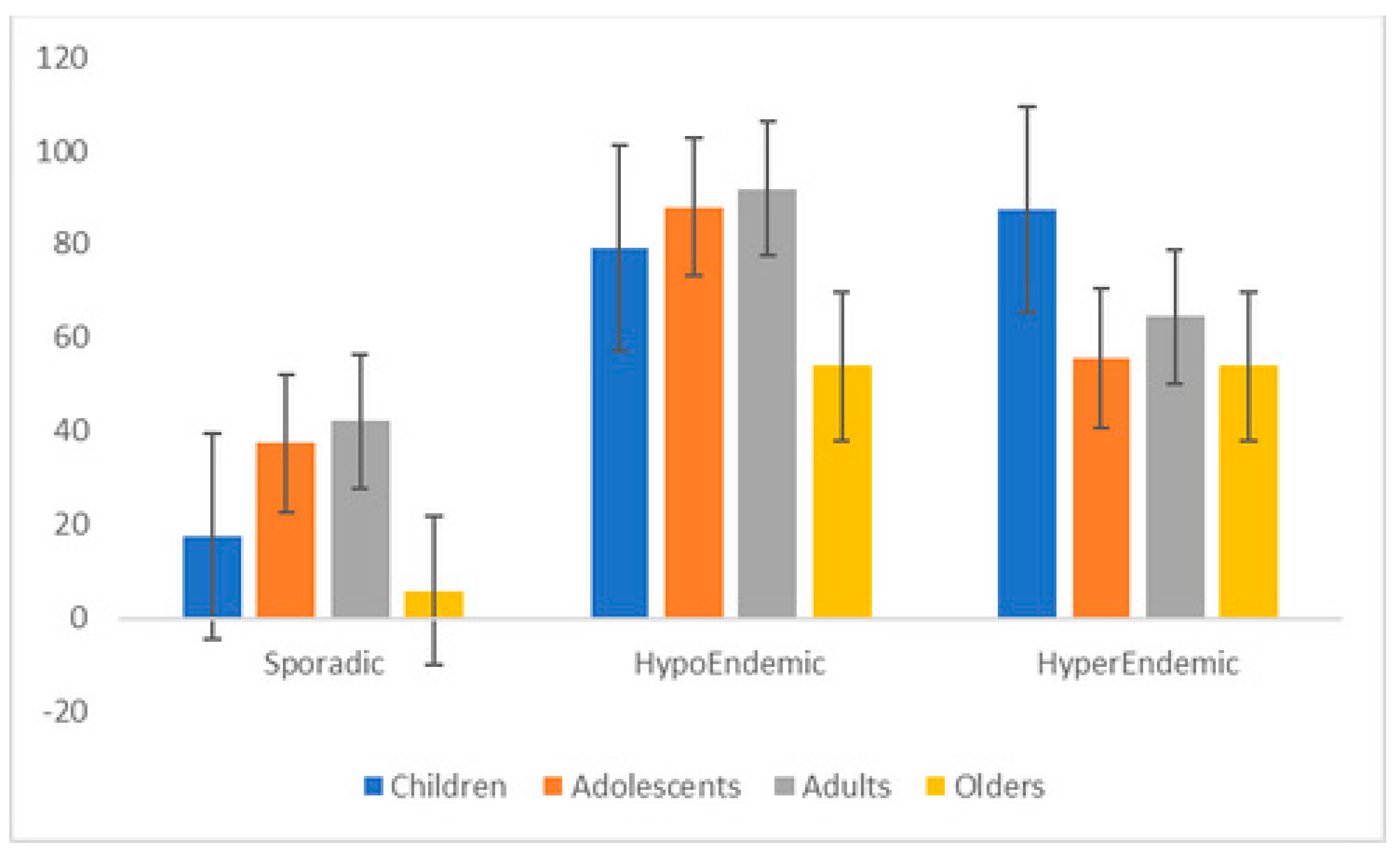
3.6. Intestinal Nematode Prevalence and Intensity According to Age and Gender
3.7. STH Species Prevalence and Intensity According to Previous Antihelminthic Treatment
3.8. STH Species Prevalence and Intensity According to Level of School Attendance
3.9. Factors Associated with STH Carriage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Report on NTD 2024. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/global-report-on-neglected-tropical-diseases-2024 (accessed on 24 July 2024).
- World Health Organization. Soil-Transmitted Helminthiases: Eliminating as Public Health Problem Soil-Transmitted Helminthiases in Children: Progress Report 2001–2010 and Strategic Plan 2011–2020; World Health Organization: Geneva, Switzerland, 2012.
- Moser, W.; Schindler, C.; Keiser, J. Efficacy of recommended drugs against soil transmitted helminths: Systematic review and network meta-analysis. BMJ 2017, 358, j4307. [Google Scholar] [CrossRef]
- Sumbele, I.U.N.; Otia, O.V.; Bopda, O.S.M.; Ebai, C.B.; Kimbi, H.K.; Nkuo-Akenji, T. Polyparasitism with Schistosoma haematobium, Plasmodium and soil-transmitted helminths in school-aged children in Muyuka-Cameroon following implementation of control measures: A cross sectional study. Infect. Dis. Poverty 2021, 10, 14. [Google Scholar] [CrossRef]
- World Health Organization. Rapport de mission de cartographie de l’onchocercose et de la loase dans 69 village au Gabon. In African Programme for Onchocerciasis Control (APOC); World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- M’Bondoukwe, N.P.; Kendjo, E.; Mawili-Mboumba, D.P.; Koumba Lengongo, J.V.; Offouga Mbouoronde, C.; Nkoghe, D.; Toure, F.; Bouyou-Akotet, M.K. Prevalence of and risk factors for malaria, filariasis, and intestinal parasites as single infections or co-infections in different settlements of Gabon, Central Africa. Infect. Dis. Poverty 2018, 7, 6. [Google Scholar] [CrossRef]
- Dejon-Agobe, J.C.; Honkpehedji, Y.J.; Zinsou, J.F.; Edoa, J.R.; Adegbite, B.R.; Mangaboula, A.; Agnandji, S.T.; Mombo-Ngoma, G.; Ramharter, M.; Kremsner, P.G.; et al. Epidemiology of Schistosomiasis and Soil-Transmitted Helminth Coinfections among Schoolchildren Living in Lambarene, Gabon. Am. J. Trop. Med. Hyg. 2020, 103, 325–333. [Google Scholar] [CrossRef]
- Zouré, H.G.; Noma, M.; Tekle, A.H.; Amazigo, U.V.; Diggle, P.J.; Giorgi, E.; Remme, J.H. The geographic distribution of onchocerciasis in the 20 participating countries of the African Programme for Onchocerciasis Control: (2) pre-control endemicity levels and estimated number infected. Parasites Vectors 2014, 7, 326. [Google Scholar] [CrossRef]
- World Health Organization. Expanded special Project for Elimination of NTD. In Gabon 2020: Status of Onchocerciasis Elimination; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020; p. 196. ISBN 978-92-4-001035-2.
- Steinmann, P.; Utzinger, J.; Du, Z.-W.; Zhou, X.-N. Multiparasitism a neglected reality on global, regional and local scale. Adv. Parasitol. 2010, 73, 21–50. [Google Scholar]
- Kombila, M.; Richard-Lenoble, D. The Mectizan donation program in Gabon: Progress and perspectives of distribution in the focus of onchocerciasis (1991–1997). Sante 1998, 8, 53–57. [Google Scholar]
- Kamgno, J.; Pion, S.D.; Chesnais, C.B.; Bakalar, M.H.; D’Ambrosio, M.V.; Mackenzie, C.D.; Nana-Djeunga, H.C.; Gounoue-Kamkumo, R.; Njitchouang, G.-R.; Nwane, P.; et al. A Test-and-Not-Treat Strategy for Onchocerciasis in Loa loa–Endemic Areas. N. Engl. J. Med. 2017, 377, 2044–2052. [Google Scholar] [CrossRef]
- Blok, D.J.; Kamgno, J.; Pion, S.D.; Nana-Djeunga, H.C.; Niamsi-Emalio, Y.; Chesnais, C.B.; Mackenzie, C.D.; Klion, A.D.; Fletcher, D.A.; Nutman, T.B.; et al. Feasibility of Onchocerciasis Elimination Using a “Test-and-not-treat” Strategy in Loa loa Co-endemic Areas. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 72, e1047–e1055. [Google Scholar] [CrossRef]
- Bah, Y.M.; Bah, M.S.; Paye, J.; Conteh, A.; Saffa, S.; Tia, A.; Sonnie, M.; Veinoglou, A.; Amon, J.J.; Hodges, M.H.; et al. Soil-transmitted helminth infection in school age children in Sierra Leone after a decade of preventive chemotherapy interventions. Infect. Dis. Poverty 2019, 8, 41. [Google Scholar] [CrossRef]
- Tabi, E.S.B.; Eyong, E.M.; Akum, E.A.; Love, J.; Cumber, S.N. Soil-transmitted Helminth infection in the Tiko Health District, South West Region of Cameroon: A post-intervention survey on prevalence and intensity of infection among primary school children. Pan Afr. Med. J. 2018, 30, 74. [Google Scholar]
- Palmeirim, M.S.; Hürlimann, E.; Knopp, S.; Speich, B.; Belizario, V., Jr.; Joseph, S.A.; Vaillant, M.; Olliaro, P.; Keiser, J. Efficacy and safety of co-administered ivermectin plus albendazole for treating soil-transmitted helminths: A systematic review, meta-analysis and individual patient data analysis. PLoS Neglected Trop. Dis. 2018, 12, e0006458. [Google Scholar] [CrossRef]
- Gebrezgabiher, G.; Yewhalaw, D.; Ayana, M.; Hailu, A.; Mekonnen, Z. Impact of ivermectin mass drug administration on burden of soil-transmitted helminths in onchocerciasis control and elimination programs, Yeki district, southwest Ethiopia. PLoS ONE 2022, 17, e0263625. [Google Scholar] [CrossRef]
- de Vos, A.S.; Stolk, W.A.; Coffeng, L.E.; de Vlas, S.J. The impact of mass drug administration expansion to low onchocerciasis prevalence settings in case of connected villages. PLoS Neglected Trop. Dis. 2021, 15, e0009011. [Google Scholar] [CrossRef]
- Metcalfe, C. Biostatistics: A Foundation for Analysis in the Health Sciences, 7th ed.; Wiley: Hoboken, NJ, USA, 1999; ISBN 0-471-16386-4. [Google Scholar]
- Mbondoukwe, P.; Mboumba, P.; Mondouo, F.; Kombila, M.; Akotet, M. Prevalence of Soil-transmitted Helminths and Intestinal Protozoa in Shanty Towns of Libreville, Gabon. Int. J. Trop. Dis. Health 2016, 20, 1–9. [Google Scholar] [CrossRef]
- Kato, K.M.M. Comparative examinations. Jpn. J. Parasitol. 1954, 3, 35. [Google Scholar]
- Harada, Y.; Mori, O.J.Y.A.M. A New Method for culturing Hook Worm. Yonago Acta Med. 1955, 1, 177–179. [Google Scholar]
- Ash, L.R.; Orihe, T.C.; LSavioli, L. Bench Aids for the Diagnosis of Intestinal Parasites, 2nd ed.; WHO: Geneva, Switzerland, 2019; pp. 10–12. [Google Scholar]
- Vlaminck, J.; Fischer, P.U.; Weil, G.J. Diagnostic Tools for Onchocerciasis Elimination Programs. Trends Parasitol. 2015, 31, 571–582. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Perneel, J.; Mandro, M.; Abhafule, G.; Siewe Fodjo, J.N.; Dusabimana, A.; Abrams, S.; Kumar-Singh, S.; Colebunders, R. Comparison of Diagnostic Tests for Onchocerca volvulus in the Democratic Republic of Congo. Pathogens 2020, 9, 435. [Google Scholar] [CrossRef]
- Engels, D.; Zhou, X.N. Neglected tropical diseases: An effective global response to local poverty-related disease priorities. Infect Dis. Poverty 2020, 9, 10. [Google Scholar] [CrossRef]
- Duerr, H.P.; Eichner, M. Epidemiology and control of onchocerciasis: The threshold biting rate of savannah onchocerciasis in Africa. Int. J. Parasitol. 2010, 40, 641–650. [Google Scholar] [CrossRef]
- Eyang-Assengone, E.R.; Makouloutou-Nzassi, P.; Mbou-Boutambe, C.; Bangueboussa, F.; Atsame, J.; Boundenga, L. Status of Onchocerciasis Elimination in Gabon and Challenges: A Systematic Review. Microorganisms 2023, 11, 1946. [Google Scholar] [CrossRef]
- Mintsa Nguema, R.; Mavoungou, J.F.; Mengue Me Ngou-Milama, K.; Mabicka Mamfoumbi, M.; Koumba, A.A.; Sani Lamine, M.; Diarra, A.; Nkone Asseko, G.; Mourou, J.R.; Bouyou Akotet, M.K.; et al. Baseline Mapping of Schistosomiasis and Soil Transmitted Helminthiasis in the Northern and Eastern Health Regions of Gabon, Central Africa: Recommendations for Preventive Chemotherapy. Trop. Med. Infect. Dis. 2018, 3, 119. [Google Scholar] [CrossRef]
- Hassen, M.; Mohammed, A.; Endeshaw, T.; Seid, T.; Samuel, F.; Asmare, T.; Birhanu, H.; Bekele, F.; Yayeh, A.; Seife, F.; et al. Integrated Prevalence Assessment of Wuchereria bancrofti and Onchocerca volvulus in Three Co-Endemic Districts of Gambella Region, Ethiopia. Am. J. Trop. Med. Hyg. 2023, 109, 844–849. [Google Scholar] [CrossRef]
- Moutongo Mouandza, R.; Mourou, J.R.; Moutombi Ditombi, B.; Roger Sibi Matotou, H.; Ekomi, B.; Bouyou-Akotet, M.K.; Mawili-Mboumba, D.P. Sociodemographics, Clinical Factors, and Biological Factors Associated with Loiasis in Endemic Onchocerciasis Areas in Southern Gabon. Am. J. Trop. Med. Hyg. 2023, 109, 850–857. [Google Scholar] [CrossRef]
- Tekalign, E.; Bajiro, M.; Ayana, M.; Tiruneh, A.; Belay, T. Prevalence and Intensity of Soil-Transmitted Helminth Infection among Rural Community of Southwest Ethiopia: A Community-Based Study. Biomed. Res. Int. 2019, 2019, 3687873. [Google Scholar] [CrossRef] [PubMed]
- Dieye, Y.; Storey, H.L.; Barrett, K.L.; Gerth-Guyette, E.; Di Giorgio, L.; Golden, A.; Faulx, D.; Kalnoky, M.; Ndiaye, M.K.N.; Sy, N.; et al. Feasibility of utilizing the SD BIOLINE Onchocerciasis IgG4 rapid test in onchocerciasis surveillance in Senegal. PLoS Neglected Trop. Dis. 2017, 11, e0005884. [Google Scholar] [CrossRef]
- WHO. Guidelines for Stopping Mass Drug Administration and Verifying Elimination of Human Onchocerciasis: Criteria and Procedures; WHO/HTM/NTD/PCT/2016.1; World Health Organization: Geneva, Switzerland, 2016; p. 44.
- Yang, D.; Yang, Y.; Wang, Y.; Yang, Y.; Dong, S.; Chen, Y.; Zho, Y.U. Prevalence and Risk Factors of Ascaris lumbricoides, Trichuris trichiura and Cryptosporidium Infections in Elementary School Children in Southwestern China: A School-Based Cross-Sectional Study. Int. J. Environ. Res. Public Health 2018, 15, 16. [Google Scholar] [CrossRef]
- Evans, D.S.; Unnasch, T.R.; Richards, F.O. Onchocerciasis and lymphatic filariasis elimination in Africa: It’s about time. Lancet 2015, 385, 2151–2152. [Google Scholar] [CrossRef]
- Dolo, H.; Coulibaly, Y.I.; Dembele, B.; Guindo, B.; Coulibaly, S.Y.; Dicko, I.; Doumbia, S.S.; Dembele, M.; Traore, M.O.; Goita, S.; et al. Integrated seroprevalence-based assessment of Wuchereria bancrofti and Onchocerca volvulus in two lymphatic filariasis evaluation units of Mali with the SD Bioline Onchocerciasis/LF IgG4 Rapid Test. PLoS Neglected Trop. Dis. 2019, 13, e0007064. [Google Scholar] [CrossRef]
- Asfaw, M.A.; Gezmu, T.; Wegayehu, T.; Bekele, A.; Hailemariam, Z.; Masresha, N.; Gebre, T. Soil-transmitted helminth infections among pre-school aged children in Gamo Gofa zone, Southern Ethiopia: Prevalence, intensity and intervention status. PLoS ONE 2020, 15, e0243946. [Google Scholar] [CrossRef] [PubMed]
- Fleming, F.M.; Brooker, S.; Geiger, S.M.; Caldas, I.R.; Correa-Oliveira, R.; Hotez, P.J.; Bethony, J.M. Synergistic associations between hookworm and other helminth species in a rural community in Brazil. Trop. Med. Int. Health 2006, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, A.D.; Martin, M.; Kaplan, H.; Gurven, M. Antagonism between two intestinal parasites in humans: The importance of co-infection for infection risk and recovery dynamics. Proc. Biol. Sci. 2013, 280, 20131671. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.C.; Turner, H.C.; Tun, A.; Anderson, R.M. Epidemiological surveys of, and research on, soil-transmitted helminths in Southeast Asia: A systematic review. Parasites Vectors 2016, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Halwindi, H.; Magnussen, P.; Olsen, A.; Lisulo, M. Potential Contribution of Adult Populations to the Maintenance of Schistosomiasis and Soil-Transmitted Helminth Infections in the Siavonga and Mazabuka Districts of Zambia. J. Biosoc. Sci. 2017, 49, 265–275. [Google Scholar] [CrossRef]
- Faulkner, H.; Turner, J.; Behnke, J.; Kamgno, J.; Rowlinson, M.C.; Bradley, J.E.; Boussinesq, M. Associations between filarial and gastrointestinal nematodes. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 301–312. [Google Scholar] [CrossRef]
- Gebrezgabiher, G.; Mekonnen, Z.; Yewhalaw, D.; Hailu, A. Reaching the last mile: Main challenges relating to and recommendations to accelerate onchocerciasis elimination in Africa. Infect. Dis. Poverty 2019, 8, 60. [Google Scholar] [CrossRef]
- Speich, B.; Ali, S.M.; Ame, S.M.; Bogoch, I.I.; Alles, R.; Huwyler, J.; Albonico, M.; Hattendorf, J.; Utzinger, J.; Keiser, J. Efficacy and safety of albendazole plus ivermectin, albendazole plus mebendazole, albendazole plus oxantel pamoate, and mebendazole alone against Trichuris trichiura and concomitant soil-transmitted helminth infections: A four-arm, randomised controlled trial. Lancet Infect. Dis. 2015, 15, 277–284. [Google Scholar]
- Le, B.; Clarke, N.E.; Hii, S.F.; Byrne, A.; Khattak, A.; Lake, S.; Lazu, E.; Wickham, S.; Wand, H.; Olsen, N.; et al. Effectiveness of one and two doses of ivermectin mass drug administration in reducing the prevalence and intensity of soil-transmitted helminth (STH) infections in Western Province, Solomon Islands: A cluster-randomised, before-after analysis. Lancet Reg. Health West. Pac. 2024, 42, 100942. [Google Scholar] [CrossRef]
- Clarke, N.E.; Doi, S.A.R.; Wangdi, K.; Chen, Y.; Clements, A.C.A.; Nery, S.V. Efficacy of Anthelminthic Drugs and Drug Combinations Against Soil-transmitted Helminths: A Systematic Review and Network Meta-analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 68, 96–105. [Google Scholar] [CrossRef]
- Belizario, V.Y.; Amarillo, M.E.; de Leon, W.U.; de los Reyes, A.E.; Bugayong, M.G.; Macatangay, B.J. A comparison of the efficacy of single doses of albendazole, ivermectin, and diethylcarbamazine alone or in combinations against Ascaris and Trichuris spp. Bull. World Health Organ. 2003, 81, 35–42. [Google Scholar] [PubMed]
| N | % | |
|---|---|---|
| Communities | ||
| Memba | 82 | 22.8 |
| Mayanga | 32 | 8.9 |
| Mbelnaletembe | 56 | 15.6 |
| Nzingui/Matamatsengue | 127 | 35.4 |
| Issinga/Nzoundou | 62 | 17.3 |
| Age range * | ||
| Children | 68 | 23.1 |
| Adolescents | 58 | 19.7 |
| Adults | 112 | 38.1 |
| Elders | 56 | 19.1 |
| Gender | ||
| Male | 179 | 49.9 |
| Female | 180 | 50.1 |
| School attendance | ||
| None/pre-school | 70 | 19.5 |
| Primary school | 244 | 68.0 |
| Secondary school | 45 | 12.5 |
| Previous treatment with an antihelmintic | ||
| Yes | 36 | 10.0 |
| No | 323 | 90.0 |
| Wearing shoes when outside | ||
| Sometimes | 179 | 49.9 |
| Regularly | 180 | 50.1 |
| Source of drinking water | ||
| River and rain | 282 | 78.6 |
| River and/or tap | 77 | 21.4 |
| Hand washing after defecation | ||
| Always | 80 | 22.3 |
| Sometimes | 251 | 69.9 |
| Never | 28 | 7.8 |
| Overall Population | Onchocerciasis Endemicity | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sporadic | Hypoendemic | Hyperendemic | |||||||||
| N | % | n | % | n | % | n | % | ||||
| N | 298 | - | 69 | 168 | 61 | - | |||||
| Positive | 193 | 64.8 | 18 | 26.1 | 136 | 80.9 | 39 | 63.9 | <0.01 | ||
| Monoparasitism | 86 | 28.8 | 16 | 23.2 | 47 | 30.0 | 23 | 37.7 | <0.01 | ||
| A. lumbricoides | 44 | 14.8 | 6 | 8.7 | 28 | 16.7 | 10 | 16.4 | |||
| T. trichiura | 34 | 11.4 | 6 | 8.7 | 17 | 10.1 | 11 | 18.0 | |||
| Hookworms | 6 | 2.0 | 3 | 4.3 | 2 | 1.2 | 1 | 1.6 | |||
| S. stercoralis | 2 | 0.6 | 1 | 1.4 | 0 | 0.0 | 1 | 1.6 | |||
| Polyparasitism | 107 | 35.9 | 2 | 2.9 | 89 | 53.0 | 16 | 26.2 | 0.01 | ||
| A. lumbricoides—T. trichiura | 75 | 25.2 | 2 | 2.9 | 57 | 33.9 | 16 | 26.2 | |||
| T. trichiura—hookworms | 7 | 2.3 | 0 | 0.0 | 7 | 4.2 | 0 | 0.0 | |||
| A. lumbricoides—hookworms | 11 | 3.7 | 0 | 0.0 | 11 | 6.5 | 0 | 0.0 | |||
| A. lumbricoides—T. trichiura Hookworms | 11 | 3.7 | 0 | 0.0 | 11 | 6.5 | 0 | 0.0 | |||
| A. lumbricoides—T. trichiura —S. stercoralis | 3 | 1.0 | 0 | 0.0 | 3 | 1.8 | 0 | 0.0 | |||
| Characteristic | A. lumbricoides | T. trichiura | Hookworms | S. stercoralis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | p-Value | n | % | 95% CI | p-Value | n | % | 95% CI | p-Value | n | % | 95% CI | p-Value | |
| Onchoendemicity | <0.01 | <0.01 | <0.01 | 0.835 | ||||||||||||
| Sporadic | 8 | 11.6 | 5.1–21.6 | 8 | 11.6 | 5.1–21.6 | 4 | 5.8 | 1.6–14.2 | 2 | 2.9 | 0.3–10.1 | ||||
| Hypoendemic | 110 | 65.5 | 57.8–72.6 | 96 | 57.1 | 49.3–64.7 | 29 | 17.3 | 11.9–23.8 | 3 | 1.8 | 0.4–5.1 | ||||
| Hyperendemic | 26 | 42.6 | 30.0–55.9 | 27 | 44.3 | 31.5–57.5 | 1 | 1.6 | 0.0–8.8 | 1 | 1.6 | 0.0–8.8 | ||||
| Gender | 0.19 | 0.17 | 0.05 | 0.01 | ||||||||||||
| Female | 79 | 52.0 | 43.7–60.1 | 61 | 40.1 | 32.3–48.4 | 12 | 7.9 | 4.1–13.4 | 0 | 0.0 | 0.0–24.0 | ||||
| Male | 65 | 44.5 | 36.3–53.0 | 70 | 47.9 | 39.6–56.4 | 22 | 15.1 | 9.7–21.9 | 6 | 4.1 | 1.5–8.7 | ||||
| Age groups * | 0.03 | <0.01 | 0.13 | 0.01 | ||||||||||||
| Children | 31 | 45.6 | 33.4–58.1 | 33 | 48.5 | 36.2–61.0 | 5 | 7.3 | 2.4–16.3 | 0 | 0.0 | 0.0–5.2 | ||||
| Adolescents | 32 | 55.2 | 41.5–68.2 | 25 | 43.1 | 30.2–56.8 | 10 | 17.2 | 8.6–29.4 | 2 | 3.4 | 0.4–11.9 | ||||
| Adults | 61 | 54.5 | 44.8–63.9 | 60 | 53.6 | 43.9–63.0 | 15 | 13.4 | 7.7–21.1 | 2 | 1.8 | 0.2–6.3 | ||||
| Elders | 18 | 32.1 | 20.3–46.0 | 12 | 21.4 | 11.6–34.4 | 3 | 5.3 | 1.1–14.9 | 1 | 1.8 | 0.0–9.5 | ||||
| History of anthelminthic treatment in the last 3 months | ||||||||||||||||
| <0.01 | 0.72 | 0.02 | 0.70 | |||||||||||||
| Yes | 9 | 26.5 | 12.9–44.4 | 14 | 41.2 | 24.6–59.3 | 0 | 0.0 | 0.0–10.3 | 1 | 2.9 | 0.0–15.3 | ||||
| No | 135 | 51.1 | 44.5–57.3 | 117 | 44.3 | 38.2–50.5 | 34 | 12.9 | 9.1–17.5 | 5 | 1.9 | 0.6–4.4 | ||||
| School attendance | ||||||||||||||||
| 0.53 | 0.64 | 0.23 | 0.54 | |||||||||||||
| None/pre-school | 29 | 46.8 | 44.0–59.9 | 24 | 38.7 | 26.6–51.9 | 5 | 8.1 | 2.7–17.8 | 2 | 3.2 | 0.4–11.2 | ||||
| Primary school | 100 | 51.8 | 44.5–59.0 | 90 | 46.6 | 39.4–53.9 | 27 | 14.0 | 9.4–19.7 | 4 | 2.1 | 0.5–5.2 | ||||
| Secondary school | 15 | 40.5 | 24.7–57.9 | 17 | 45.9 | 29.5–63.1 | 2 | 5.4 | 0.7–18.2 | 0 | 0.0 | 0.0–9.4 | ||||
| All STH | Ascariasis | Trichuriasis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | |
| Onchocerciasis endemicity | <0.01 | <0.01 | <0.01 | ||||||||
| Sporadic | 18 | 26.1 | 1 | 1 | 1 | ||||||
| Hypoendemic | 136 | 80.9 | 14.5 | 6.8–34.6 | 17.4 | 7.9–43.1 | 9.9 | 4.7–23.6 | |||
| Hyperendemic | 39 | 63.9 | 5.6 | 2.4–14.6 | 7.2 | 2.9–19.6 | 6.1 | 2.6–15.6 | |||
| Gender | 0.20 | 0.08 | 0.14 | ||||||||
| Female | 96 | 63.1 | 1 | 1 | 1 | ||||||
| Male | 97 | 66.4 | 0.8 | 0.4–1.4 | 0.61 | 0.4–1.1 | 1.4 | 0.9–2.2 | |||
| Age groups * | 0.03 | 0.06 | <0.01 | ||||||||
| Children | 44 | 64.7 | 1 | 1 | 1 | ||||||
| Adolescent | 40 | 69.0 | 1.1 | 0.7–3.0 | 1.8 | 0.8–4.2 | 0.8 | 0.6–1.5 | |||
| Adults | 85 | 75.9 | 1.4 | 0.8–2.6 | 1.4 | 0.7–2.9 | 1.2 | 0.4–1.6 | |||
| Elders | 22 | 39.3 | 0.6 | 0.3–1.2 | 0.6 | 0.3–1.4 | 0.3 | 0.1–0.6 | |||
| History of anthelminthic treatment in the last 3 months | <0.01 | <0.01 | 0.76 | ||||||||
| No | 177 | 67.0 | 1 | 1 | 1 | ||||||
| Yes | 16 | 47.0 | 0.3 | 0.1–0.7 | 0.3 | 0.1–0.6 | 0.9 | 0.4–1.8 | |||
| Educational level | 0.53 | 0.6 | 0.08 | ||||||||
| None/pre-school | 36 | 58.1 | 1 | 1 | 1 | ||||||
| Primary school | 135 | 70.0 | 1.2 | 0.6–2.0 | 1.1 | 0.5–2.3 | 1.3 | 0.7–2.3 | |||
| Secondary school | 22 | 59.4 | 0.8 | 0.3–1.8 | 1.7 | 0.6–4.8 | 1.3 | 0.6–3.1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moutongo Mouandza, R.; Mourou Mbina, J.R.; Moutombi Ditombi, B.; Mihindou, J.C.; Moussavou Mabicka, D.A.; Mayandza, C.; Mbondoukwe, N.P.; Pongui Ngondza, B.; Ndong Akomezoghe, L.; Mawili Mboumba, D.P.; et al. Prevalence and Sociodemographic Risk Factors of Soil-Transmitted Helminths in Rural Communities Living in Endemic Foci of Onchocerciasis in Southern Gabon. Pathogens 2024, 13, 967. https://doi.org/10.3390/pathogens13110967
Moutongo Mouandza R, Mourou Mbina JR, Moutombi Ditombi B, Mihindou JC, Moussavou Mabicka DA, Mayandza C, Mbondoukwe NP, Pongui Ngondza B, Ndong Akomezoghe L, Mawili Mboumba DP, et al. Prevalence and Sociodemographic Risk Factors of Soil-Transmitted Helminths in Rural Communities Living in Endemic Foci of Onchocerciasis in Southern Gabon. Pathogens. 2024; 13(11):967. https://doi.org/10.3390/pathogens13110967
Chicago/Turabian StyleMoutongo Mouandza, Reinne, Jean Romain Mourou Mbina, Bridy Moutombi Ditombi, Joyce Coella Mihindou, Dimitri Ardrin Moussavou Mabicka, Christian Mayandza, Noe Patrick Mbondoukwe, Bedrich Pongui Ngondza, Luccheri Ndong Akomezoghe, Denise Patricia Mawili Mboumba, and et al. 2024. "Prevalence and Sociodemographic Risk Factors of Soil-Transmitted Helminths in Rural Communities Living in Endemic Foci of Onchocerciasis in Southern Gabon" Pathogens 13, no. 11: 967. https://doi.org/10.3390/pathogens13110967
APA StyleMoutongo Mouandza, R., Mourou Mbina, J. R., Moutombi Ditombi, B., Mihindou, J. C., Moussavou Mabicka, D. A., Mayandza, C., Mbondoukwe, N. P., Pongui Ngondza, B., Ndong Akomezoghe, L., Mawili Mboumba, D. P., & Bouyou Akotet, M. K. (2024). Prevalence and Sociodemographic Risk Factors of Soil-Transmitted Helminths in Rural Communities Living in Endemic Foci of Onchocerciasis in Southern Gabon. Pathogens, 13(11), 967. https://doi.org/10.3390/pathogens13110967






