Organ Tropism of Angiostrongylus vasorum Larval Stages in Infected African Giant Snails (Lissachatina fulica)
Abstract
1. Introduction
2. Material and Methods
2.1. Gastropod Maintenance Under Standardized and Parasite-Free Conditions
2.2. Isolation of Vital Angiostronglyus Vasorum L1 and Gastropod Oral Infection
2.3. Gastropod Euthanasia and Organ Isolation
2.4. Artificial Digestion and Identification of Larvae
2.5. Histology
3. Results
3.1. Angiostrongylus Vasorum Larval Development and Organ Tropism in Experimentally Infected L. fulica Snails
3.2. Histological Findings Indicate Hemocyte Responses to Angiostrongylus Vasorum Larval Stages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hermosilla, C.; Kleinertz, S.; Silva, L.M.R.; Hirzmann, J.; Huber, D.; Kusak, J.; Taubert, A. Protozoan and Helminth Parasite Fauna of Free-Living Croatian Wild Wolves (Canis lupus) Analyzed by Scat Collection. Vet. Parasitol. 2017, 233, 14–19. [Google Scholar] [CrossRef] [PubMed]
- De Liberato, C.; Grifoni, G.; Lorenzetti, R.; Meoli, R.; Cocumelli, C.; Mastromattei, A.; Scholl, F.; Rombolà, P.; Calderini, P.; Bruni, G.; et al. Angiostrongylus vasorum in Wolves in Italy: Prevalence and Pathological Findings. Parasites Vectors 2017, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Szabo, L.; Juhász, L.; Takács, A.; Lanszki, J.; Takács, P.; Heltai, M. Data on the Parasitological Status of Golden Jackal (Canis aureus L., 1758) in Hungary. Acta Vet. Hung. 2013, 62, 1–9. [Google Scholar] [CrossRef]
- Gavrilović, P.; Marinković, D.; Vidanović, D.; Dobrosavljević, I.; Gavrilović, A. Are Golden Jackals (Canis aureus) Definitive Hosts for Angiostrongylus vasorum? Transbound. Emerg. Dis. 2019, 66, 2305–2310. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; D’Alessio, N.; Di Prisco, F.; Neola, B.; Restucci, B.; Pagano, T.B.; Veneziano, V. Angiostrongylus vasorum Infection in Red Foxes (Vulpes vulpes) in Southern Italy. Acta Parasitol. 2015, 60, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.R.; Tomlinson, A.; Hunter, S.; Nichols, T.; Roberts, E.; Fox, M.T.; Taylor, M.A. Angiostrongylus vasorum and Eucoleus aerophilus in Foxes (Vulpes vulpes) in Great Britain. Vet. Parasitol. 2008, 154, 48–57. [Google Scholar] [CrossRef]
- Simpson, V.R. Angiostrongylus vasorum Infection in Foxes (Vulpes vulpes) in Cornwall. Vet. Rec. 1996, 139, 443–445. [Google Scholar] [CrossRef]
- Otranto, D.; Cantacessi, C.; Dantas-Torres, F.; Brianti, E.; Pfeffer, M.; Genchi, C.; Guberti, V.; Capelli, G.; Deplazes, P. The Role of Wild Canids and Felids in Spreading Parasites to Dogs and Cats in Europe. Part II: Helminths and Arthropods. Vet. Parasitol. 2015, 213, 24–37. [Google Scholar] [CrossRef]
- Germitsch, N.; KAPEL, C.; THAMSBORG, S.; Deplazes, P.; Schnyder, M. Host-Specific Serological Response to Angiostrongylus vasorum Infection in Red Foxes (Vulpes vulpes): Implications for Parasite Epidemiology. Parasitology 2017, 144, 1–10. [Google Scholar] [CrossRef]
- Magi, M.; Guardone, L.; Dell’Omodarme, M.; Prati, M.C.; Mignone, W.; Torracca, B.; Monni, G.; Macchioni, F. Angiostrongylus vasorum in Red Foxes (Vulpes vulpes) and Badgers (Meles meles) from Central and Northern Italy. Hystrix Ital. J. Mamm. 2009, 20, 121–126. [Google Scholar]
- Uribe, M.; Payán, E.; Brabec, J.; Vélez, J.; Taubert, A.; Chaparro-Gutiérrez, J.J.; Hermosilla, C. Intestinal Parasites of Neotropical Wild Jaguars, Pumas, Ocelots, and Jaguarundis in Colombia: Old Friends Brought Back from Oblivion and New Insights. Pathogens 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Bružinskaitė-Schmidhalter, R.; Šarkūnas, M.; Malakauskas, A.; Mathis, A.; Torgerson, P.R.; Deplazes, P. Helminths of Red Foxes (Vulpes vulpes) and Raccoon Dogs (Nyctereutes procyonoides) in Lithuania. Parasitology 2012, 139, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Patterson-Kane, J.C.; Gibbons, L.M.; Jefferies, R.; Morgan, E.R.; Wenzlow, N.; Redrobe, S.P. Pneumonia from Angiostrongylus vasorum Infection in a Red Panda (Ailurus fulgens fulgens). J. Vet. Diagn. Investig. 2009, 21, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Willesen, J.L. Canine Pulmonary Angiostrongylosis: An Update. Vet. J. 2009, 179, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Segeritz, L.; Westhoff, K.M.; Schaper, R.; Hermosilla, C.; Taubert, A. Angiostrongylus vasorum, Aelurostrongylus abstrusus, Crenosoma vulpis and Troglostrongylus brevior Infections in Native Slug Populations of Bavaria and Baden-Wuerttemberg in Germany. Pathogens 2022, 11, 747. [Google Scholar] [CrossRef]
- Maksimov, P.; Hermosilla, C.; Taubert, A.; Staubach, C.; Sauter-Louis, C.; Conraths, F.J.; Vrhovec, M.G.; Pantchev, N. GIS-Supported Epidemiological Analysis on Canine Angiostrongylus vasorum and Crenosoma vulpis Infections in Germany. Parasites Vectors 2017, 10, 108. [Google Scholar] [CrossRef]
- Aziz, N.A.A.; Daly, E.; Allen, S.; Rowson, B.; Greig, C.; Forman, D.; Morgan, E.R. Distribution of Angiostrongylus vasorum and Its Gastropod Intermediate Hosts along the Rural–Urban Gradient in Two Cities in the United Kingdom, Using Real Time PCR. Parasites Vectors 2016, 9, 56. [Google Scholar] [CrossRef]
- Colombo, M.; Traversa, D.; Grillotti, E.; Pezzuto, C.; De Tommaso, C.; Pampurini, F.; Schaper, R.; Drake, J.; Crisi, P.E.; Russi, I.; et al. Highly Variable Clinical Pictures in Dogs Naturally Infected with Angiostrongylus vasorum. Pathogens 2021, 10, 1372. [Google Scholar] [CrossRef]
- Morgan, E.; Shaw, S. Angiostrongylus vasorum Infection in Dogs: Continuing Spread and Developments in Diagnosis and Treatment. J. Small Anim. Pract. 2010, 51, 616–621. [Google Scholar] [CrossRef]
- Traversa, D.; Di Cesare, A.; Meloni, S.; Frangipane di Regalbono, A.; Milillo, P.; Pampurini, F.; Venco, L. Canine Angiostrongylosis in Italy: Occurrence of Angiostrongylus vasorum in Dogs with Compatible Clinical Pictures. Parasitol. Res. 2013, 112, 2473–2480. [Google Scholar] [CrossRef]
- Schnyder, M.; Fahrion, A.; Riond, B.; Ossent, P.; Webster, P.; Kranjc, A.; Glaus, T.; Deplazes, P. Clinical, Laboratory and Pathological Findings in Dogs Experimentally Infected with Angiostrongylus vasorum. Parasitol. Res. 2010, 107, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Elsheikha, H.M.; Holmes, S.A.; Wright, I.; Morgan, E.R.; Lacher, D.W. Recent Advances in the Epidemiology, Clinical and Diagnostic Features, and Control of Canine Cardio-Pulmonary Angiostrongylosis. Vet. Res. 2014, 45, 92. [Google Scholar] [CrossRef] [PubMed]
- Valente, R.; Robles, M.d.R.; Diaz, J.I. Gastropods as Intermediate Hosts of Angiostrongylus spp. in the Americas: Bioecological Characteristics and Geographical Distribution. Mem. Inst. Oswaldo Cruz 2020, 115, e200236. [Google Scholar] [CrossRef] [PubMed]
- Segeritz, L.; Cardona, A.; Taubert, A.; Hermosilla, C.; Ruiz, A. Autochthonous Angiostrongylus cantonensis, Angiostrongylus vasorum and Aelurostrongylus abstrusus Infections in Native Terrestrial Gastropods from the Macaronesian Archipelago of Spain. Parasitol. Res. 2021, 120, 2671–2680. [Google Scholar] [CrossRef]
- Guilhon, J.; Cens, B. Angiostrongylus vasorum (Baillet, 1866). Etude biologique et morphologique. Ann. Parasitol. Hum. Comp. 1973, 48, 567–596. [Google Scholar] [CrossRef]
- Ferdushy, T.; Kapel, C.M.O.; Webster, P.; Al-Sabi, M.N.S.; Grønvold, J.R. The Effect of Temperature and Host Age on the Infectivity and Development of Angiostrongylus vasorum in the Slug Arion lusitanicus. Parasitol. Res. 2010, 107, 147–151. [Google Scholar] [CrossRef]
- Robbins, W.; Conboy, G.; Greenwood, S.; Schaper, R. Infectivity of Gastropod-Shed Third-Stage Larvae of Angiostrongylus vasorum and Crenosoma vulpis to Dogs. Parasites Vectors 2021, 14, 307. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Lange, M.K.; Vélez, J.; Hirzmann, J.; Gutierrez-Arboleda, J.; Taubert, A.; Hermosilla, C.; Chaparro Gutierrez, J.J. The Invasive Giant African Snail Lissachatina fulica as Natural Intermediate Host of Aelurostrongylus abstrusus, Angiostrongylus vasorum, Troglostrongylus brevior, and Crenosoma vulpis in Colombia. PLoS Negl. Trop. Dis. 2019, 13, e0007277. [Google Scholar] [CrossRef]
- Lange, M.K.; Penagos-Tabares, F.; Vélez, J.; Gutiérrez, J.; Hirzmann, J.; Chaparro-Gutiérrez, J.J.; Piedrahita, D.; Taubert, A.; Hermosilla, C. Regional Report on Angiostrongylus vasorum in Colombia: Genetic Similarity to European Lineage. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 21–23. [Google Scholar] [CrossRef]
- Thiengo, S.C.; Faraco, F.A.; Salgado, N.C.; Cowie, R.H.; Fernandez, M.A. Rapid Spread of an Invasive Snail in South America: The Giant African Snail, Achatina fulica, in Brasil. Biol. Invasions 2007, 9, 693–702. [Google Scholar] [CrossRef]
- Odaibo, A.B.; Olayinka, S.O. Shell Morphology, Radula and Genital Structures of New Invasive Giant African Land Snail Species, Achatina fulica (Bowdich, 1822), Achatina albopicta (E.A. Smith, 1878) and Achatina reticulata (Pfeiffer, 1845), (Gastropoda:Achatinidae) in Southwest Nigeria. bioRxiv 2019. [Google Scholar] [CrossRef]
- Sobrepeña, J.M.M.; Demayo, C.G. Banding Pattern and Shape Morphology Variations on Shells of the Invasive Giant African Land Snail Achatina fulica (Bowdic,h 1822) from the Philippines. Ann. Biol. Res. 2014, 5, 64–79. [Google Scholar]
- van Bruggen, A.C. Achatina fulica on the Island of Timor, Lesser Sunda Islands (Indonesia). Basteria 1981, 45, 90. [Google Scholar]
- Rekha Sarma, R.; Munsi, M.; Neelavara Ananthram, A. Effect of Climate Change on Invasion Risk of Giant African Snail (Achatina fulica, Férussac, 1821: Achatinidae) in India. PLoS ONE 2015, 10, e0143724. [Google Scholar] [CrossRef]
- Traversa, D.; Di Cesare, A.; Conboy, G. Canine and Feline Cardiopulmonary Parasitic Nematodes in Europe: Emerging and Underestimated. Parasit. Vectors 2010, 3, 62. [Google Scholar] [CrossRef]
- Sauerländer, R.; Eckert, J. Die Achatschnecke (Achatina fulica) als experimenteller Zwischenwirt für Angiostrongylus vasorum (Nematoda). Z. Parasitenk. 1974, 44, 59–72. [Google Scholar] [CrossRef]
- Sauerländer, R. Histologische Veränderungen bei experimentell mit Angiostrongylus vasorum oder Angiostrongylus cantonensis (Nematoda) infizierten Achatschnecken (Achatina fulica). Z. Parasitenk. 1976, 49, 263–280. [Google Scholar] [CrossRef]
- Giannelli, A.; Colella, V.; Abramo, F.; Ramos, R.A.d.N.; Falsone, L.; Brianti, E.; Varcasia, A.; Dantas-Torres, F.; Knaus, M.; Fox, M.T.; et al. Release of Lungworm Larvae from Snails in the Environment: Potential for Alternative Transmission Pathways. PLOS Negl. Trop.-Dis. 2015, 9, e0003722. [Google Scholar] [CrossRef]
- Pila, E.A.; Sullivan, J.T.; Wu, X.Z.; Fang, J.; Rudko, S.P.; Gordy, M.A.; Hanington, P.C. Haematopoiesis in Molluscs: A Review of Haemocyte Development and Function in Gastropods, Cephalopods and Bivalves. Dev. Comp. Immunol. 2016, 58, 119–128. [Google Scholar] [CrossRef]
- Lange, M.K.; Penagos-Tabares, F.; Muñoz-Caro, T.; Gärtner, U.; Mejer, H.; Schaper, R.; Hermosilla, C.; Taubert, A. Gastropod-Derived Haemocyte Extracellular Traps Entrap Metastrongyloid Larval Stages of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Troglostrongylus brevior. Parasit. Vectors 2017, 10, 50. [Google Scholar] [CrossRef]
- Nakayama, K.; Nomoto, A.M.; Nishijima, M.; Maruyama, T. Morphological and Functional Characterization of Hemocytes in the Giant Clam Tridacna crocea. J. Invertebr. Pathol. 1997, 69, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Loker, E.S. Gastropod Immunobiology. In Invertebrate Immunity; Söderhäll, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–43. ISBN 978-1-4419-8059-5. [Google Scholar]
- Schoenberg, D.A.; Cheng, T.C. The Behavior of Biomphalaria glabrata (Gastropoda: Pulmonata) Hemocytes Following Exposure to Lectins. Trans. Am. Microsc. Soc. 1981, 100, 345–354. [Google Scholar] [CrossRef]
- Hanington, P.C.; Forys, M.A.; Dragoo, J.W.; Zhang, S.-M.; Adema, C.M.; Loker, E.S. Role for a Somatically Diversified Lectin in Resistance of an Invertebrate to Parasite Infection. Proc. Natl. Ac. Sci. USA 2010, 107, 21087–21092. [Google Scholar] [CrossRef] [PubMed]
- Loker, E.S.; Bayne, C.J.; Buckley, P.M.; Kruse, K.T. Ultrastructure of Encapsulation of Schistosoma mansoni Mother Sporocysts by Hemocytes of Juveniles of the 10-R2 Strain of Biomphalaria glabrata. J. Parasitol. 1982, 68, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Hahn, U.; Bender, R.; Bayne, C. Production of Reactive Oxygen Species by Hemocytes of Biomphalaria glabrata. Dev. Comp. Immunol. 2000, 24, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Kassai, T. Larvae of protostrongylins in snails. Acta Vet. 1958, 8, 223–236. [Google Scholar]
- Zmoray, I.; Svarc, R.; Lestan, P. Localization of Larvae of Muellerius capillaris in the Tissues of the Intermediate Host Cepaea vindobonensis (Fér.). (Study of the Development of Muellerius capillaris in the Intermediate Host, IV). Biologia 1969, 24, 113–128. [Google Scholar]
- Napoli, E.; Sfacteria, A.; Rifici, C.; Mazzullo, G.; Gaglio, G.; Brianti, E. Reaction of Cornu aspersum Immune System against Different Aelurostrongylus abstrusus Developmental Stages. Pathogens 2023, 12, 542. [Google Scholar] [CrossRef]
- Conboy, G.; Guselle, N.; Schaper, R. Spontaneous Shedding of Metastrongyloid Third-Stage Larvae by Experimentally Infected Limax maximus. Parasitol. Res. 2017, 116, 41–54. [Google Scholar] [CrossRef]
- Lange, M.K.; Penagos-Tabares, F.; Hirzmann, J.; Failing, K.; Schaper, R.; Van Bourgonie, Y.R.; Backeljau, T.; Hermosilla, C.; Taubert, A. Prevalence of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Crenosoma vulpis Larvae in Native Slug Populations in Germany. Vet. Parasitol. 2018, 254, 120–130. [Google Scholar] [CrossRef]
- Ash, L.R. Diagnostic Morphology of the Third-Stage Larvae of Angiostrongylus cantonensis, Angiostrongylus vasorum, Aelurostrongylus abstrusus, and Anafilaroides rostratus (Nematoda: Metastrongyloidea). J. Parasitol. 1970, 56, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Bolt, G.; Monrad, J.; Koch, J.; Jensen, A.L. Canine Angiostrongylosis: A Review. Vet. Rec. 1994, 135, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Tabares, F.; Lange, M.K.; Seipp, A.; Gärtner, U.; Mejer, H.; Taubert, A.; Hermosilla, C. Novel Approach to Study Gastropod-Mediated Innate Immune Reactions against Metastrongyloid Parasites. Parasitol. Res. 2018, 117, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, A.; Cantacessi, C.; Colella, V.; Dantas-Torres, F.; Otranto, D. Gastropod-Borne Helminths: A Look at the Snail–Parasite Interplay. Trends Parasitol. 2016, 32, 255–264. [Google Scholar] [CrossRef]
- Schnyder, M. Slugs and Angiostrongylus vasorum—How Much Do We Know? Vet. Rec. 2015, 177, 44–45. [Google Scholar] [CrossRef]
- Sullivan, M. Giant African Snail Cooperative Eradication Program in Florida. Final Environmental Assessment; U.S. Department of Agriculture: Washington, DC, USA, 2024. [Google Scholar]
- Dias, S.R.C.; dos Santos Lima, W. Effect of Temperature on Activity of Third-Stage Larvae of Angiostrongylus vasorum. Parasitol. Res. 2012, 110, 1327–1330. [Google Scholar] [CrossRef]
- Morgan, E.R.; Jefferies, R.; Krajewski, M.; Ward, P.; Shaw, S.E. Canine Pulmonary Angiostrongylosis: The Influence of Climate on Parasite Distribution. Parasitol. Int. 2009, 58, 406–410. [Google Scholar] [CrossRef]
- Patz, J.A.; Graczyk, T.K.; Geller, N.; Vittor, A.Y. Effects of Environmental Change on Emerging Parasitic Diseases. Int. J. Parasitol. 2000, 30, 1395–1405. [Google Scholar] [CrossRef]
- Poirier, A.C.; Schmitt, P.; Rosa, R.D.; Vanhove, A.S.; Kieffer-Jaquinod, S.; Rubio, T.P.; Charrière, G.M.; Destoumieux-Garzón, D. Antimicrobial Histones and DNA Traps in Invertebrate Immunity: Evidences in Crassostrea gigas. J. Biol. Chem. 2014, 289, 24821–24831. [Google Scholar] [CrossRef]
- Robb, C.T.; Dyrynda, E.A.; Gray, R.D.; Rossi, A.G.; Smith, V.J. Invertebrate Extracellular Phagocyte Traps Show That Chromatin Is an Ancient Defence Weapon. Nat. Commun. 2014, 5, 4627. [Google Scholar] [CrossRef]
- Alesci, A.; Fumia, A.; Albano, M.; Messina, E.; D’Angelo, R.; Mangano, A.; Miller, A.; Spanò, N.; Savoca, S.; Capillo, G. Investigating the Internal System of Defense of Gastropoda Aplysia depilans (Gmelin, 1791): Focus on Hemocytes. Fish Shellfish Immunol. 2023, 137, 108791. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M. Apoptosis in Molluscan Immune Defense. Invertebr. Surv. J. 2009, 6, 49–58. [Google Scholar]
- Smith, P.J.S. Molluscan Circulation: Haemodynamics and the Heart. In Circulation, Respiration, and Metabolism; Gilles, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 344–355. [Google Scholar]
- Sommerville, B.A. The Circulatory Physiology of Helix pomatia: I. Observations on the Mechanism by Which Helix Emerges from Its Shell and on the Effects of Body Movement on Cardiac Function. J. Exp. Biol. 1973, 59, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Beier, J.C. Malaria Parasite Development in Mosquitoes. Annu. Rev. Entomol. 1998, 43, 519–543. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.L.; Suh, E.; Lynch, P.A.; Thomas, M.B. Exploring the Lower Thermal Limits for Development of the Human Malaria Parasite, Plasmodium falciparum. Biol. Lett. 2019, 15, 20190275. [Google Scholar] [CrossRef]
- Laabs, E.-M.; Schnieder, T.; Strube, C. Transcriptional Differences between Hypobiotic and Non-Hypobiotic Preadult Larvae of the Bovine Lungworm Dictyocaulus viviparus. Parasitol. Res. 2012, 110, 151–159. [Google Scholar] [CrossRef]
- Blitz, N.M.; Gibbs, H.C. Studies on the Arrested Development of Haemonchus Contortus in Sheep—I. The Induction of Arrested Development. Int. J. Parasitol. 1972, 2, 5–12. [Google Scholar] [CrossRef]
- Sorensen, R.E.; Minchella, D.J. Snail-Trematode Life History Interactions: Past Trends and Future Directions. Parasitology 2001, 123, S3–S18. [Google Scholar] [CrossRef]
- Raut, S.; Barker, G. Achatina fulica Bowdich and Other Achatinidae as Pests in Tropical Agriculture. In Molluscs as Crop Pests; CABI Publishing: Wallingford, UK, 2002; pp. 55–114. ISBN 978-0-85199-320-1. [Google Scholar]
- Rosen, L.; Ash, L.R.; Wallace, G.D. Life History of the Canine Lungworm Angiostrongylus vasorum (Baillet). Am. J. Vet. Res. 1970, 31, 131–143. [Google Scholar]
- Ghose, K.C. Reproductive System of the Snail Achatina fulica. Proc. Zool. Soc. Lond. 1963, 140, 681–695. [Google Scholar] [CrossRef]
- Faro, M.J.; Perazzini, M.; Corrêa, L.d.R.; Mello-Silva, C.C.; Pinheiro, J.; Mota, E.M.; de Souza, S.; de Andrade, Z.; Júnior, A.M. Biological, Biochemical and Histopathological Features Related to Parasitic Castration of Biomphalaria glabrata Infected by Schistosoma mansoni. Exp. Parasitol 2013, 134, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Vázquez Perera, A.; Sánchez Noda, J. First Record of the Invasive Land Snail Achatina (Lissachatina) fulica (Bowdich, 1822) (Gastropoda: Achatinidae), Vector of Angiostrongylus cantonensis (Nematoda: Angiostrongylidae), in Havana, Cuba. Mollusc. Res. 2014, 35, 139–142. [Google Scholar] [CrossRef]
- Gołdyn, B.; Ríos Guayasamín, P.; Sanchez, K.; Hepting, L. Notes on the Distribution and Invasion Potential of Achatina fulica Bowdich, 1822 (Gastropoda: Pulmonata: Achatinidae) in Ecuador. Folia Malacol. 2016, 24, 85–90. [Google Scholar] [CrossRef][Green Version]
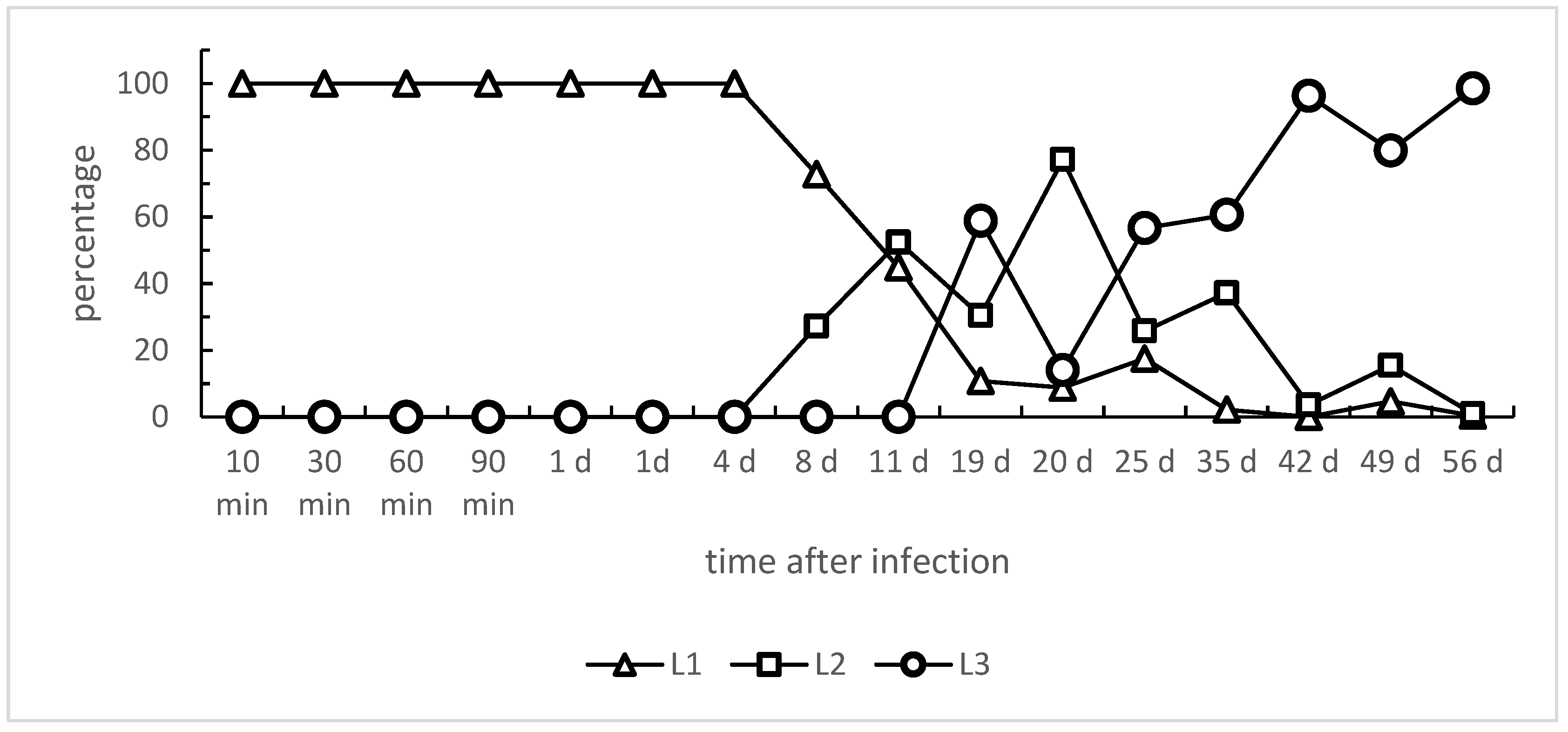
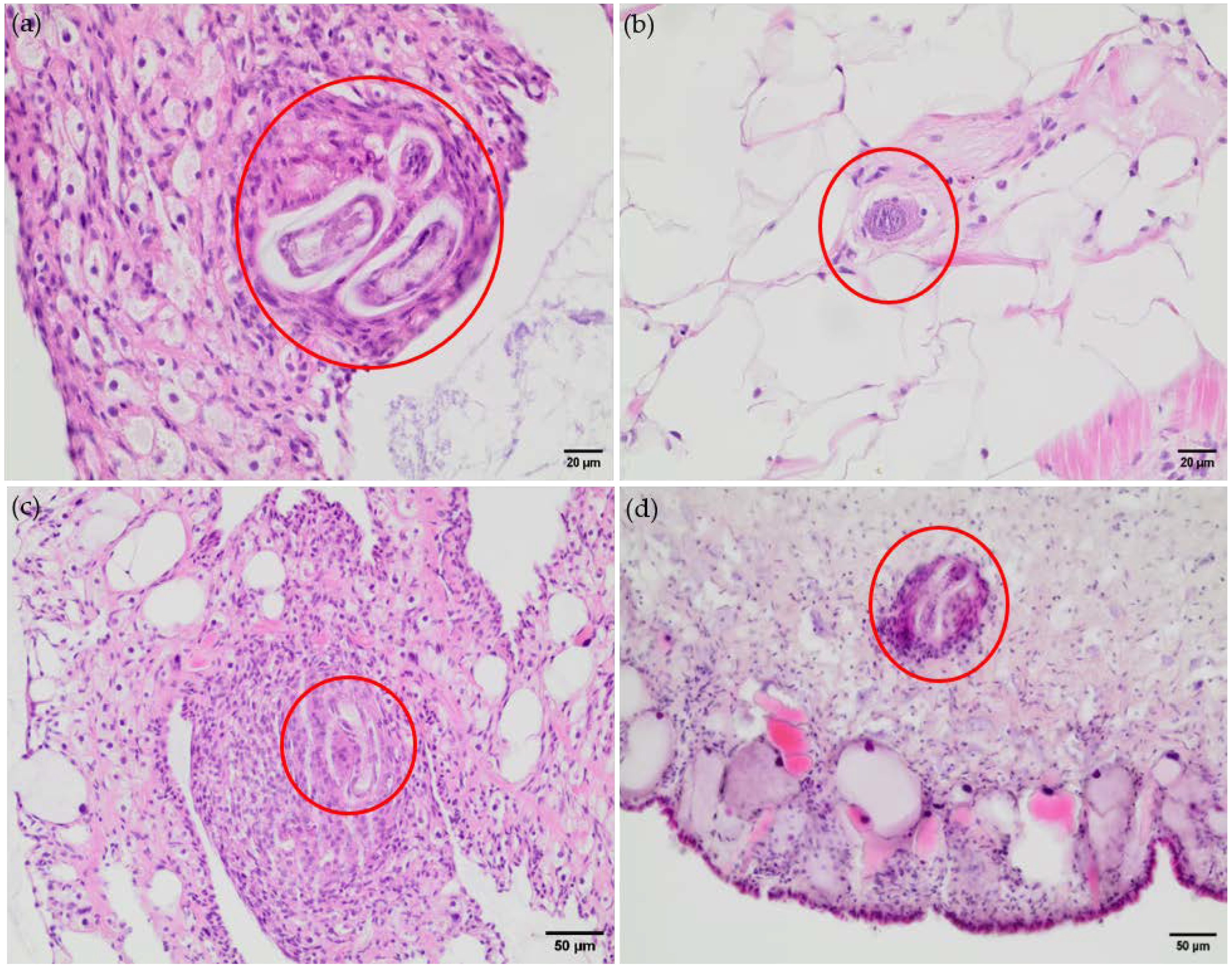
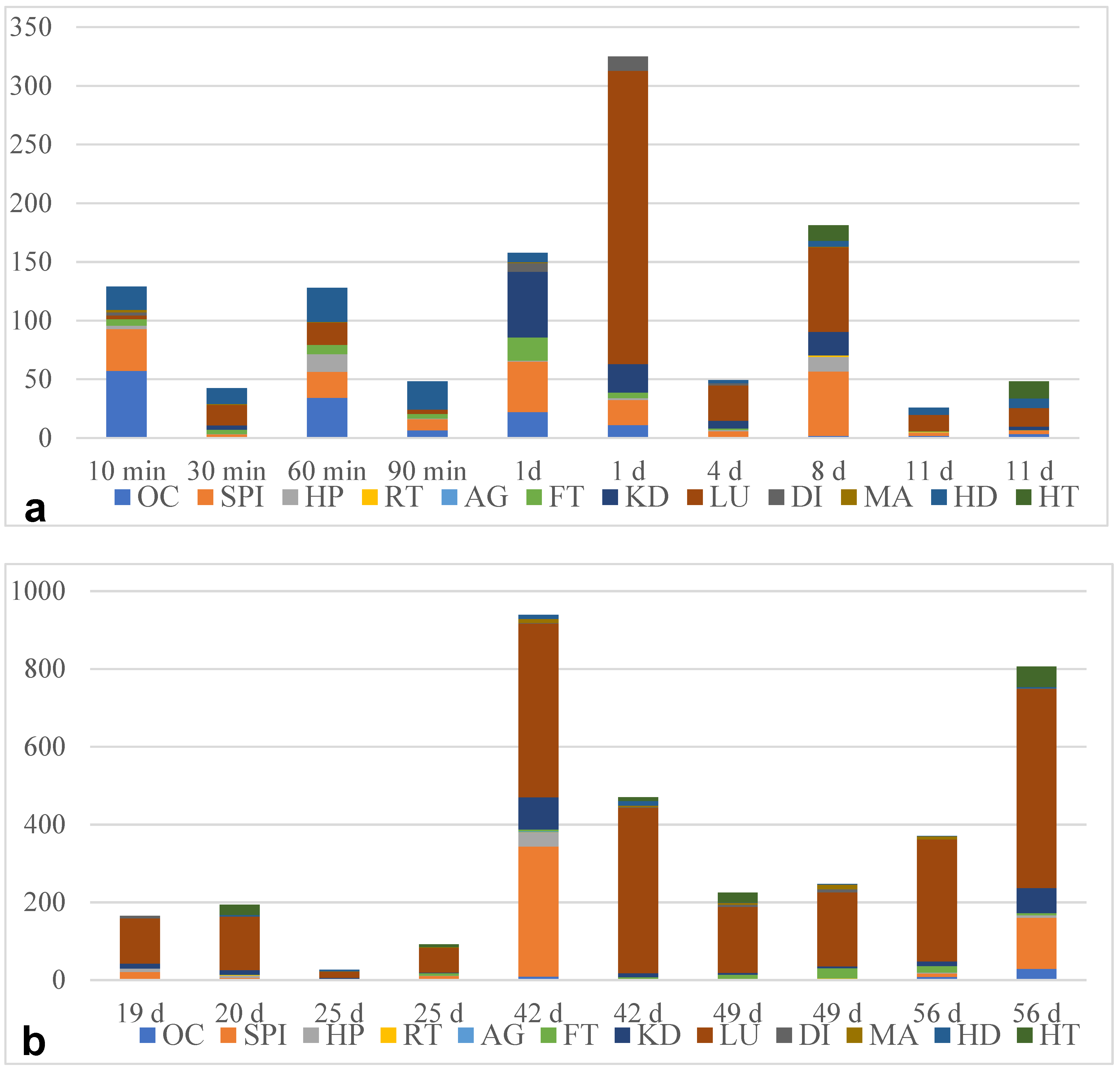
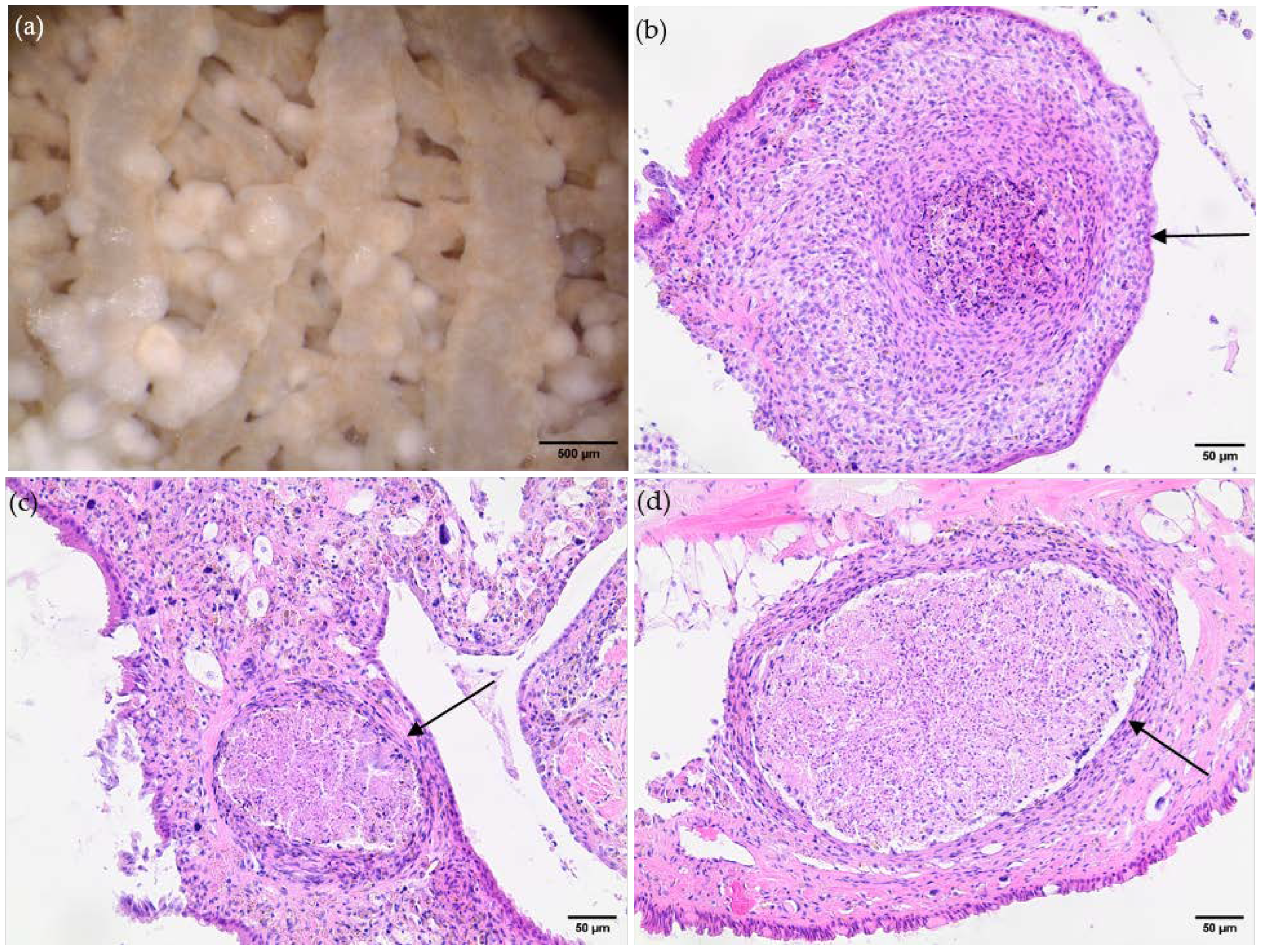
| OC | SPI | HP | RT | AG | FT | KD | LU | DI | MA | HD | HT | RR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 min | L1 | L1 | L1 | n.l.d. | n.l.d. | L1 | n.l.d. | L1 | L1 | L1 | L1 | n.l.d. | 10 |
| 30 min | L1 | L1 | L1 | n.l.d. | n.l.d. | L1 | L1 | L1 | n.l.d. | L1 | L1 | n.l.d. | 4.8 |
| 60 min | L1 | L1 | L1 | n.l.d. | n.l.d. | L1 | n.l.d. | L1 | n.l.d. | L1 | L1 | n.l.d. | 18.3 |
| 90 min | L1 | L1 | L1 | n.l.d. | n.l.d. | L1 | n.l.d. | L1 | n.l.d. | n.l.d. | L1 | n.l.d. | 8.8 |
| 1 day | L1 | L1 | L1 | n.l.d. | n.l.d. | L1 | L1 | n.l.d. | L1 | L1 | L1 | n.l.d. | 9.6 |
| 1 day | L1 | L1 | L1 | n.l.d. | n.l.d. | L1 | L1 | L1 | L1 | n.l.d. | n.l.d. | n.l.d. | 7.1 |
| 4 days | n.l.d. | L1 | L1 | n.l.d. | n.l.d. | L1 | L1 | L1 | L1 | n.l.d. | L1 | n.l.d. | 4.1 |
| 8 days | L1 | L1, L2 | L1, L2 | L2 | n.l.d. | n.l.d. | L1, L2 | L1, L2 | n.l.d. | L1 | L1, L2 | L1 | 15.8 |
| 11 days | L1 | L2 | L1 | L1 | n.l.d. | L1, L2 | n.l.d. | L2 | n.l.d. | n.l.d. | L1 | n.l.d. | 2.1 |
| 11 days | L2 | L2 | n.l.d. | n.l.d. | n.l.d. | L1 | L2 | L2 | n.l.d. | n.l.d. | L1, L2 | L3 | 1.8 |
| 19 days | L3 | L1, L2, L3 | L1, L2, L3 | n.l.d. | n.l.d. | L1, L2, L3 | L1, L2, L3 | L2, L3 | L2 | n.l.d. | n.l.d. | n.l.d. | 10.2 |
| 20 days | n.l.d. | L2, L3 | L1, L2, L3 | L2 | n.l.d. | L1, L2 | L2 | L2, L3 | n.l.d. | L2 | L1, L2 | L2, L3 | 11.4 |
| 25 days | n.l.d. | L2, L3 | n.l.d. | n.l.d. | n.l.d. | L1, L2, L3 | L2, L3 | L3 | n.l.d. | L3 | L1, L2, L3 | n.l.d. | 2.7 |
| 25 days | n.l.d. | L2, L3 | L1, L2 | n.l.d. | n.l.d. | L1, L2, L3 | L3 | L3 | n.l.d. | L3 | n.l.d. | L3 | 10.9 |
| 35 days | n.l.d. | L2, L3 | L2, L3 | L2, L3 | n.l.d. | L2, L3 | n.l.d. | L2, L3 | n.l.d. | L2, L3 | L2 | L2, L3 | 11.6 |
| 35 days | n.l.d. | n.l.d. | L2, L3 | L2 | n.l.d. | L1, L2, L3 | L1, L2, L3 | L2, L3 | L3 | L3 | L2, L3 | L2, L3 | 13.9 |
| 42 days | L3 | L3 | L2, L3 | L3 | L3 | L2, L3 | L3 | L3 | L3 | L3 | L3 | n.l.d. | 40 |
| 42 days | n.l.d. | n.l.d. | L2, L3 | L3 | n.l.d. | L2, L3 | L3 | L3 | L3 | L3 | L2, L3 | L3 | 10 |
| 49 days | L3 | L3 | L3 | L1 | n.l.d. | L1, L2, L3 | L1, L3 | L1, L3 | L3 | L2, L3 | L1 | L3 | 14.2 |
| 49 days | n.l.d. | n.l.d. | L1, L2, L3 | L3 | n.l.d. | L1, L2, L3 | L3 | L2, L3 | L3 | L2, L3 | L2, L3 | n.l.d. | 29.6 |
| 56 days | L3 | L3 | L3 | n.l.d. | n.l.d. | L1, L2, L3 | L3 | L3 | n.l.d. | L3 | L3 | 26.9 | |
| 56 days | L2, L3 | L3 | L3 | n.l.d. | n.l.d. | L3 | L3 | L3 | L3 | L3 | L3 | L3 | 49.7 |
| MA, HT | RT | AG | HD | KD | FT | OC | HP | ST | LU | IR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 y | L3 | L3 | L3 | 0.8 | |||||||
| 2 y | L3 | L3 | 0.2 | ||||||||
| 2 y | L3 | L3 | L3 | L3 | L3 | L3 | 4.4 | ||||
| 2 y | L3 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dusch, A.; Segeritz, L.; Henrich, M.; Taubert, A.; Hermosilla, C. Organ Tropism of Angiostrongylus vasorum Larval Stages in Infected African Giant Snails (Lissachatina fulica). Pathogens 2024, 13, 946. https://doi.org/10.3390/pathogens13110946
Dusch A, Segeritz L, Henrich M, Taubert A, Hermosilla C. Organ Tropism of Angiostrongylus vasorum Larval Stages in Infected African Giant Snails (Lissachatina fulica). Pathogens. 2024; 13(11):946. https://doi.org/10.3390/pathogens13110946
Chicago/Turabian StyleDusch, Alena, Lisa Segeritz, Manfred Henrich, Anja Taubert, and Carlos Hermosilla. 2024. "Organ Tropism of Angiostrongylus vasorum Larval Stages in Infected African Giant Snails (Lissachatina fulica)" Pathogens 13, no. 11: 946. https://doi.org/10.3390/pathogens13110946
APA StyleDusch, A., Segeritz, L., Henrich, M., Taubert, A., & Hermosilla, C. (2024). Organ Tropism of Angiostrongylus vasorum Larval Stages in Infected African Giant Snails (Lissachatina fulica). Pathogens, 13(11), 946. https://doi.org/10.3390/pathogens13110946







