McHDV VP60 Virus-like Particles Elicit Protective Immunity Against Moschus chrysogaster Hemorrhagic Disease in Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid, Cells, and Virus
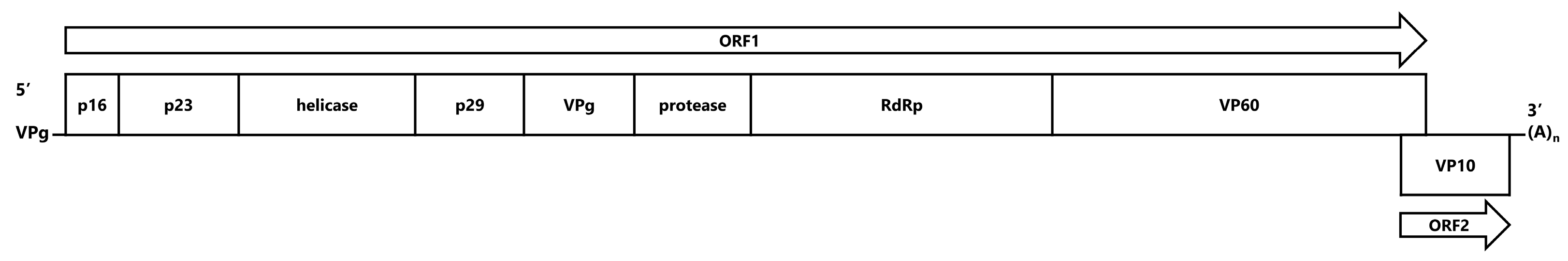
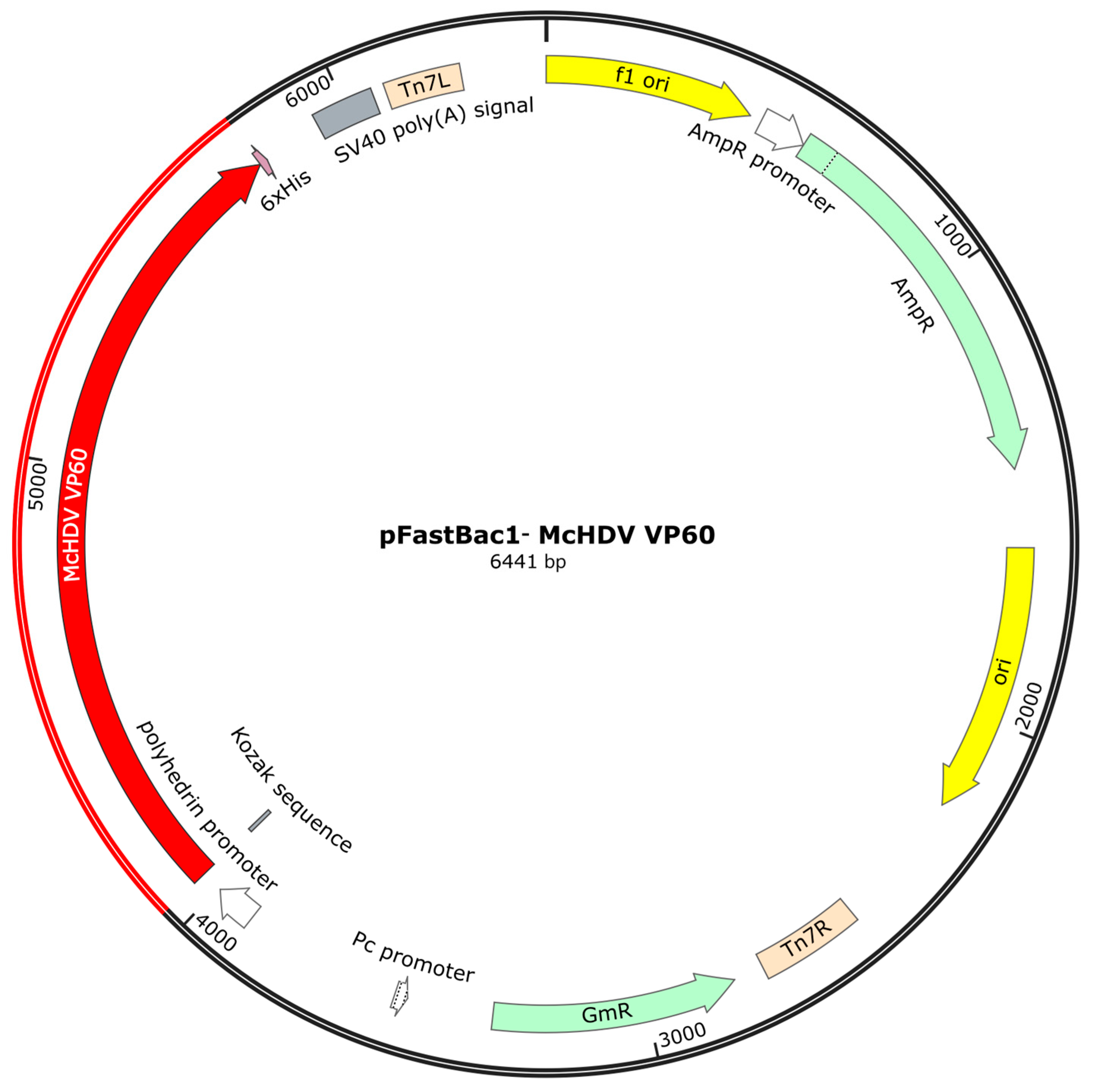
2.2. Construction of Recombinant Baculovirus
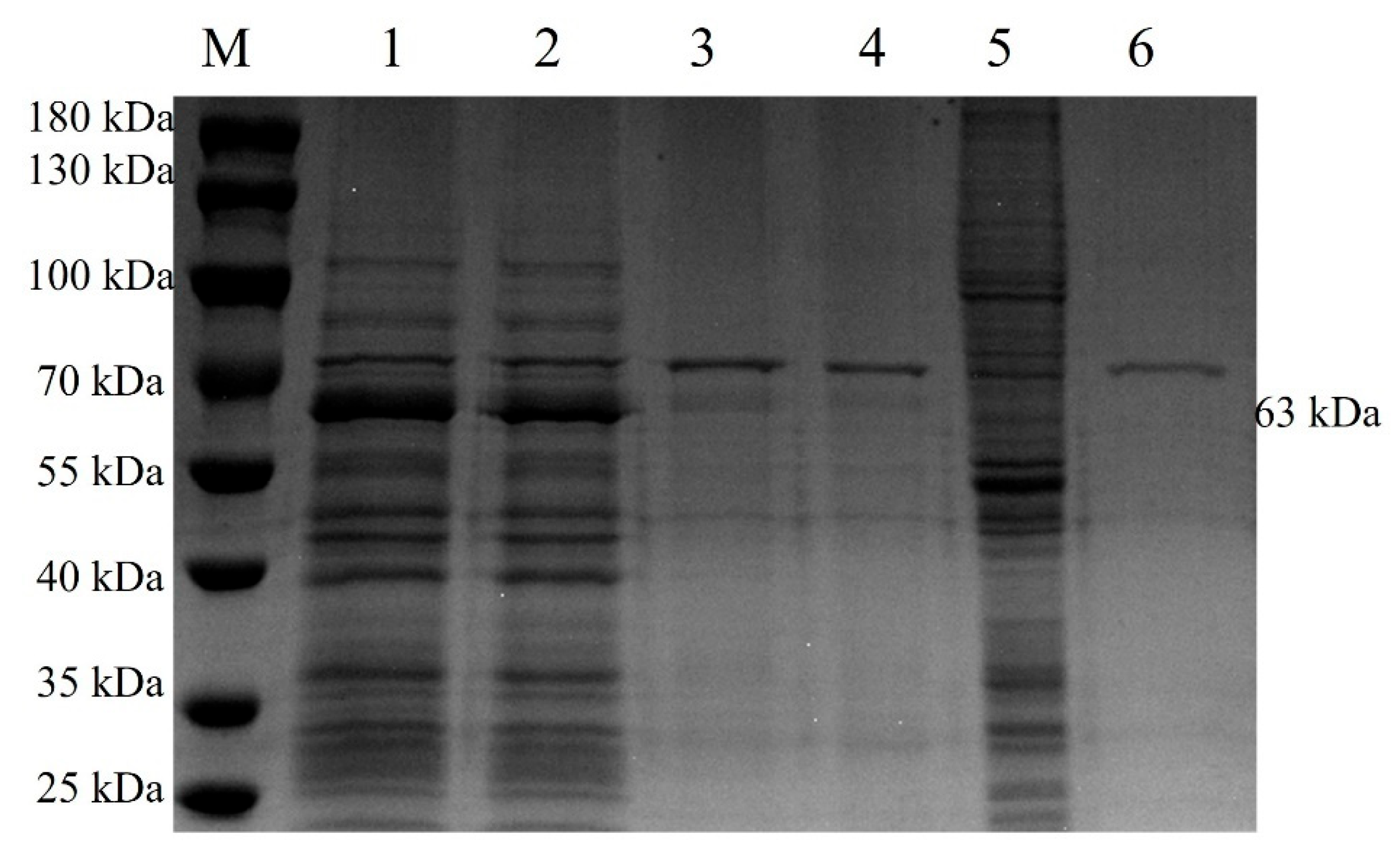
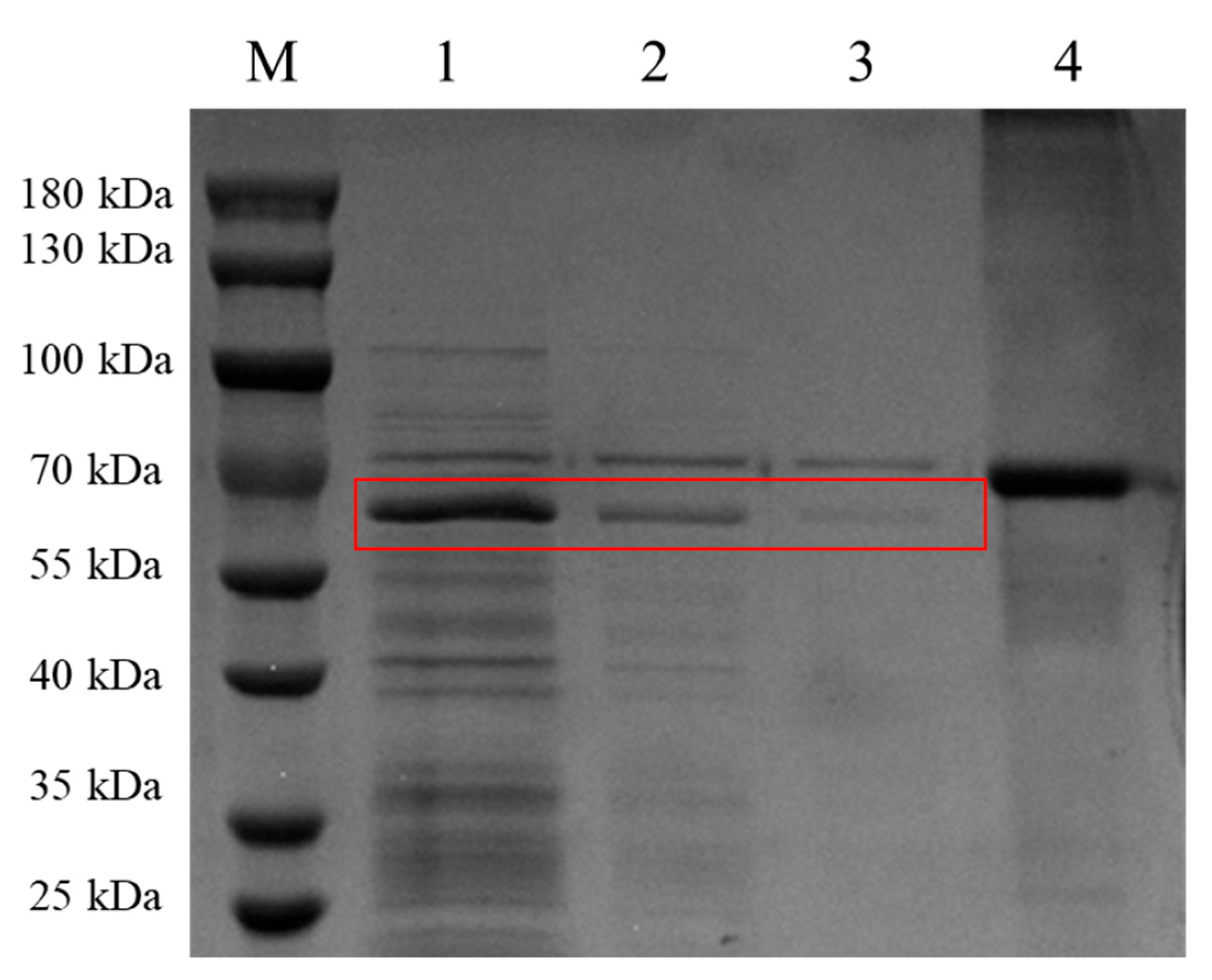
2.3. Expression of McHDV VP60 Protein Was Identified by SDS-PAGE
2.4. Expression of McHDV VP60 Protein Was Identified by Western Blotting
2.5. Transmission Electron Microscopy (TEM) Observation McHDV VLPs
2.6. Animal Experiments
2.6.1. Animals
2.6.2. Vaccine Preparation
2.6.3. Immunization
2.6.4. Challenge Infection
3. Results
3.1. Expression and Distribution of McHDV VP60 Protein Expression in Sf9 Cells
3.2. Characterization of McHDV VLPs by Electron Microscopy
3.3. Antigen Content Test of Vaccine
3.4. Immunogenicity of McHDV VLPs in Rabbits
3.5. McHDV VLPs Vaccine Can Protect Rabbits Against McHDV
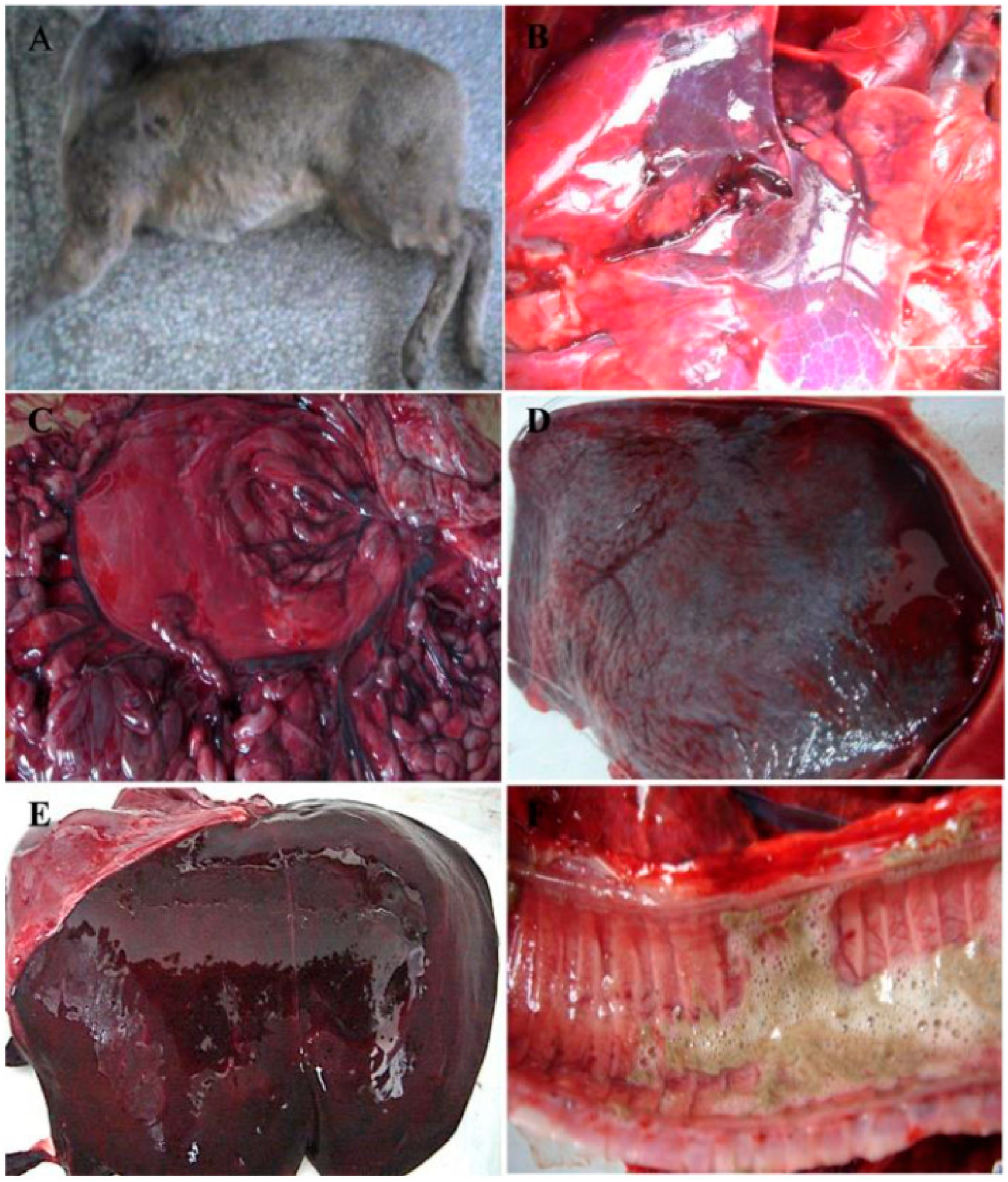
3.6. Macroscopic Examination of Organ Sampling after Challenge
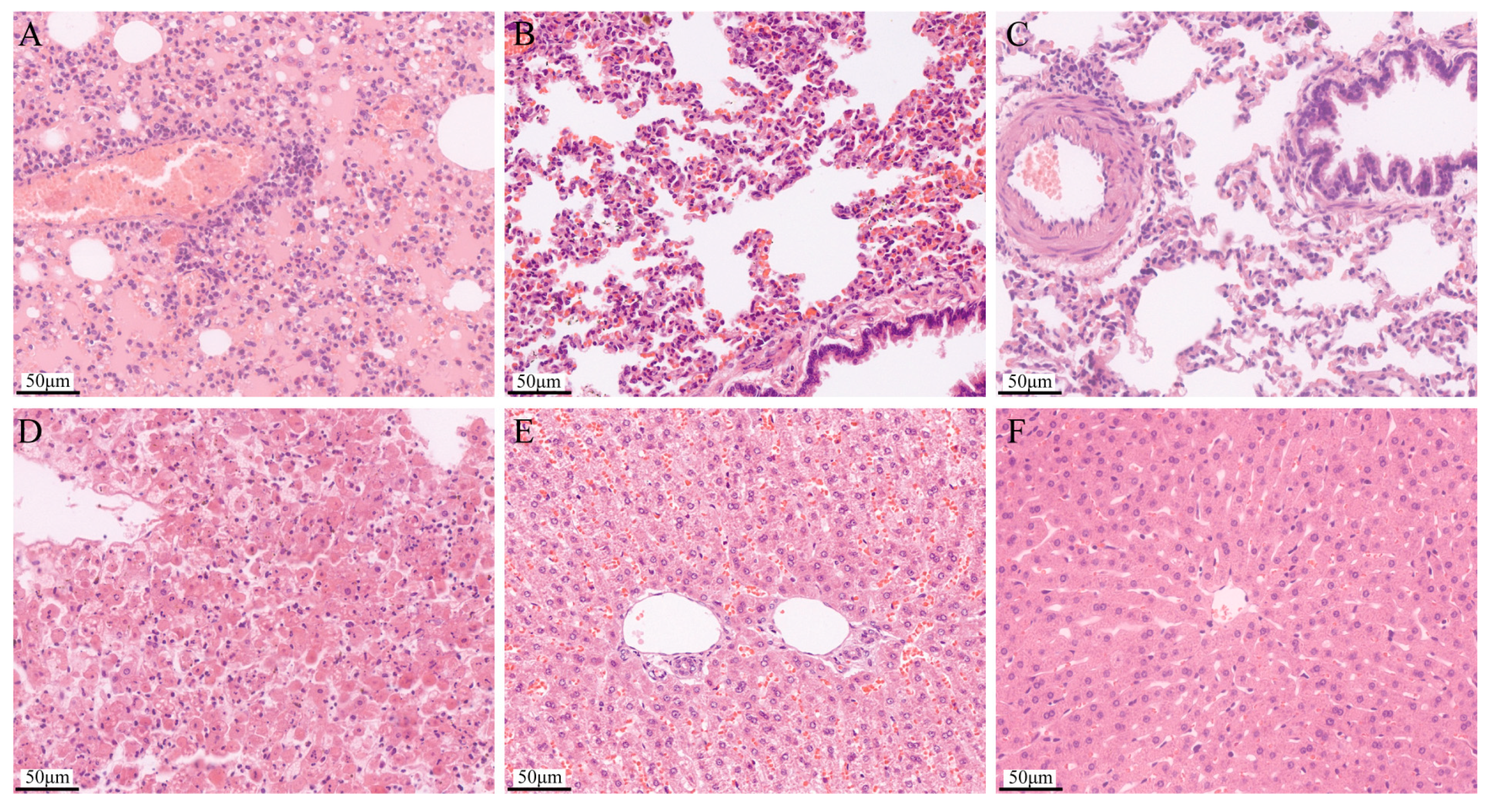
3.7. Histopathology Examination of Organ Sampling after Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Ethics Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, F.; Gao, H.; Qin, W.; Song, P.; Wang, H.; Zhang, J.; Liu, D.; Wang, D.; Zhang, T. Marked Seasonal Variation in Structure and Function of Gut Microbiota in Forest and Alpine Musk Deer. Front. Microbiol. 2021, 12, 699797. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yang, J.; Wang, J.; Yang, Y.; Fu, W.; Zheng, C.; Cheng, J.; Zeng, Y.; Zhang, Y.; Xu, L.; et al. Changes in the Population Genetic Structure of Captive Forest Musk Deer (Moschus Berezovskii) with the Increasing Number of Generation under Closed Breeding Conditions. Animals 2020, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zan, L.; Zhou, X.; Zhang, R.; Wang, G.; Qi, J.; Meng, X. Impacts of anthropogenic activities on the habitat of musk deer in the Xinglongshan National Nature Reserve on the eastern edge of the Tibet Plateau. Chin. J. Ecol. 2024, 43, 1920. [Google Scholar]
- Zhang, L.; Sun, Z.; An, B.; Zhang, D.; Chen, L. Alpine Musk Deer (Moschus chrysogaster) Adjusts to a Human-Dominated Semi-Arid Mountain Ecosystem. Animals 2022, 12, 3061. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Xu, T.; Qi, J.; Zhang, Y.; Zhang, X.; Meng, X. Population distribution, quantitative characteristics and influencing factors of the wild alpine musk deer in Xinglongshan National Nature Reserve, Gansu Province. Acta Ecol. Sin. 2020, 40, 7997–8004. [Google Scholar]
- Bao, S.; Xing, X.; Ding, X.; Gao, X.; An, K.; Fu, X.; Xue, H. A New Animal Infectious Disease, Moschus chrysogaster Viral Hemorrhagic Disease. Sci. Agric. Sin. 2018, 51, 1012. [Google Scholar]
- Bao, S.; An, K.; Liu, C.; Xing, X.; Fu, X.; Xue, H.; Wen, F.; He, X.; Wang, J. Rabbit Hemorrhagic Disease Virus Isolated from Diseased Alpine Musk Deer (Moschus sifanicus). Viruses 2020, 12, 897. [Google Scholar] [CrossRef]
- Abrantes, J.; van der Loo, W.; Le Pendu, J.; Esteves, P.J. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): A review. Vet. Res. 2012, 43, 12. [Google Scholar] [CrossRef]
- Lopes, A.M.; Dalton, K.P.; Magalhães, M.J.; Parra, F.; Esteves, P.J.; Holmes, E.C.; Abrantes, J. Full genomic analysis of new variant rabbit hemorrhagic disease virus revealed multiple recombination events. J. Gen. Virol. 2015, 96, 1309–1319. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, Y.; Guo, L.; ShiJun, B. Preparation of Tissue Inactivated Vaccine against Hemorrhagic Disease of Musk Deer and Analysis of Its Immune Protection. Prog. Vet. Med. 2024, 45, 78–81. [Google Scholar] [CrossRef]
- Bao, S.; Zhang, J.; He, J.; Zhang, Y.; Xing, X.; Wen, F.; Fu, X.; Wu, X. Tandem expression of the major epitope domains of the Moschus chrysogaster hemorrhagic disease virus VP60 and its protective efficacy to rabbits. Chin. J. Biotechnol. 2020, 36, 1536–1545. [Google Scholar] [CrossRef]
- Cavadini, P. Rabbit Haemorrhagic Disease. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; WOAH: Paris, France, 2024. [Google Scholar]
- Urakova, N.; Hall, R.; Strive, T.; Frese, M. Restricted Host Specificity of Rabbit Hemorrhagic Disease Virus Is Supported by Challenge Experiments in Immune-compromised Mice (Mus musculus). J. Wildl. Dis. 2019, 55, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Frölich, K.; Klima, F.; Dedek, J. Antibodies against rabbit hemorrhagic disease virus in free-ranging red foxes from Germany. J. Wildl. Dis. 1998, 34, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Merchán, T.; Rocha, G.; Alda, F.; Silva, E.; Thompson, G.; de Trucios, S.H.; Pagés, A. Detection of rabbit haemorrhagic disease virus (RHDV) in nonspecific vertebrate hosts sympatric to the European wild rabbit (Oryctolagus cuniculus). Infect. Genet. Evol. 2011, 11, 1469–1474. [Google Scholar] [CrossRef]
- Chong, R.; Shi, M.; Grueber, C.E.; Holmes, E.C.; Hogg, C.J.; Belov, K.; Barrs, V.R. Fecal Viral Diversity of Captive and Wild Tasmanian Devils Characterized Using Virion-Enriched Metagenomics and Metatranscriptomics. J. Virol. 2019, 93, e00205-19. [Google Scholar] [CrossRef]
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 2012, 31, 58–83. [Google Scholar] [CrossRef]
- Fakruddin, J.M.; Lempicki, R.A.; Gorelick, R.J.; Yang, J.; Adelsberger, J.W.; Garcia-Pineres, A.J.; Pinto, L.A.; Lane, H.C.; Imamichi, T. Noninfectious papilloma virus-like particles inhibit HIV-1 replication: Implications for immune control of HIV-1 infection by IL-27. Blood 2007, 109, 1841–1849. [Google Scholar] [CrossRef]
- Raghunandan, R. Virus-like particles: Innate immune stimulators. Expert Rev. Vaccines 2011, 10, 409–411. [Google Scholar] [CrossRef]
- Keller, S.A.; Bauer, M.; Manolova, V.; Muntwiler, S.; Saudan, P.; Bachmann, M.F. Cutting edge: Limited specialization of dendritic cell subsets for MHC class II-associated presentation of viral particles. J. Immunol. 2010, 184, 26–29. [Google Scholar] [CrossRef]
- Ponterio, E.; Petrizzo, A.; Di Bartolo, I.; Buonaguro, F.M.; Buonaguro, L.; Ruggeri, F.M. Pattern of activation of human antigen presenting cells by genotype GII.4 norovirus virus-like particles. J. Transl. Med. 2013, 11, 127. [Google Scholar] [CrossRef]
- Laurent, S.; Vautherot, J.F.; Madelaine, M.F.; Le Gall, G.; Rasschaert, D. Recombinant rabbit hemorrhagic disease virus capsid protein expressed in baculovirus self-assembles into viruslike particles and induces protection. J. Virol. 1994, 68, 6794–6798. [Google Scholar] [CrossRef] [PubMed]
- Bárcena, J.; Guerra, B.; Angulo, I.; González, J.; Valcárcel, F.; Mata, C.P.; Castón, J.R.; Blanco, E.; Alejo, A. Comparative analysis of rabbit hemorrhagic disease virus (RHDV) and new RHDV2 virus antigenicity, using specific virus-like particles. Vet. Res. 2015, 46, 106. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Qi, R.; Veldkamp, L.; Ijzer, J.; Kik, M.L.; Zhu, J.; Tang, A.; Dong, D.; Shi, Y.; van Oers, M.M.; et al. Immunogenicity in Rabbits of Virus-Like Particles from a Contemporary Rabbit Haemorrhagic Disease Virus Type 2 (GI.2/RHDV2/b) Isolated in The Netherlands. Viruses 2019, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Plana-Duran, J.; Bastons, M.; Rodriguez, M.J.; Climent, I.; Cortés, E.; Vela, C.; Casal, I. Oral immunization of rabbits with VP60 particles confers protection against rabbit hemorrhagic disease. Arch. Virol. 1996, 141, 1423–1436. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Ulrich, R.; Schinköthe, J.; Müller, M.; Köllner, B. Characterization of protective humoral and cellular immune responses against RHDV2 induced by a new vaccine based on recombinant baculovirus. Vaccine 2019, 37, 4195–4203. [Google Scholar] [CrossRef]
- Bosco-Lauth, A.M.; Cominsky, B.; Porter, S.; Root, J.J.; Schueler, A.; Anderson, G.; VanderWal, S.; Benson, A. A novel vaccine candidate against rabbit hemorrhagic disease virus 2 (RHDV2) confers protection in domestic rabbits. Am. J. Vet. Res. 2022, 83, ajvr.22.05.0095. [Google Scholar] [CrossRef]
- Bosco-Lauth, A.M.; Schueler, A.; Midthun, E.; Tyra, H.; Held, A.; Hood, C.; Quilici, M.; Erickson, S.; Glover, S.; Gustafson, B.; et al. Vaccination against Rabbit Hemorrhagic Disease Virus 2 (RHDV2) Using a Baculovirus Recombinant Vaccine Provides Durable Immunity in Rabbits. Viruses 2024, 16, 538. [Google Scholar] [CrossRef]
- Marín, M.S.; Martín Alonso, J.M.; Pérez Ordoyo García, L.I.; Boga, J.A.; Argüello-Villares, J.L.; Casais, R.; Venugopal, K.; Jiang, W.; Gould, E.A.; Parra, F. Immunogenic properties of rabbit haemorrhagic disease virus structural protein VP60 expressed by a recombinant baculovirus: An efficient vaccine. Virus Res. 1995, 39, 119–128. [Google Scholar] [CrossRef]



| Groups | Number | Antigen | Dosage | Method | Time-Point for Sera Collection |
|---|---|---|---|---|---|
| 1 | 4 | 6log2 HAU VLPs vaccine | 0.5 mL | Subcutaneous | 0 days before immunization; 1, 2, 3 and 4 weeks after immunization |
| 2 | 4 | 8log2 HAU VLPs vaccine | 0.5 mL | Subcutaneous | |
| 3 | 4 | 10log2 HAU VLPs vaccine | 0.5 mL | Subcutaneous | |
| 4 | 4 | PBS | 0.5 mL | Subcutaneous |
| Groups | Number | Challenge Experiment | ||
|---|---|---|---|---|
| Death | Clinical Signs Rate * | Protection Rate | ||
| 1 | 4 | 0 | 0% | 4/4 (100%) |
| 2 | 4 | 0 | 0% | 4/4 (100%) |
| 3 | 4 | 0 | 0% | 4/4 (100%) |
| 4 | 4 | 4 | 100% | 0/4 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Liu, Y.; Huang, D.; Wang, Q.; He, X.; Zhao, W.; Zhao, Y.; Ma, H.; Xing, X.; Zhang, Z.; et al. McHDV VP60 Virus-like Particles Elicit Protective Immunity Against Moschus chrysogaster Hemorrhagic Disease in Rabbits. Pathogens 2024, 13, 925. https://doi.org/10.3390/pathogens13110925
Shao Y, Liu Y, Huang D, Wang Q, He X, Zhao W, Zhao Y, Ma H, Xing X, Zhang Z, et al. McHDV VP60 Virus-like Particles Elicit Protective Immunity Against Moschus chrysogaster Hemorrhagic Disease in Rabbits. Pathogens. 2024; 13(11):925. https://doi.org/10.3390/pathogens13110925
Chicago/Turabian StyleShao, Yu, Yudong Liu, Dong Huang, Qing Wang, Xiaoxiao He, Wenjing Zhao, Yunhai Zhao, Haiyun Ma, Xiaoyong Xing, Zhixiong Zhang, and et al. 2024. "McHDV VP60 Virus-like Particles Elicit Protective Immunity Against Moschus chrysogaster Hemorrhagic Disease in Rabbits" Pathogens 13, no. 11: 925. https://doi.org/10.3390/pathogens13110925
APA StyleShao, Y., Liu, Y., Huang, D., Wang, Q., He, X., Zhao, W., Zhao, Y., Ma, H., Xing, X., Zhang, Z., & Bao, S. (2024). McHDV VP60 Virus-like Particles Elicit Protective Immunity Against Moschus chrysogaster Hemorrhagic Disease in Rabbits. Pathogens, 13(11), 925. https://doi.org/10.3390/pathogens13110925





