The First Identification of Trichinella britovi in the Raccoon Dog (Nyctereutes procyonoides) in Romania
Abstract
1. Introduction
2. Materials and Methods
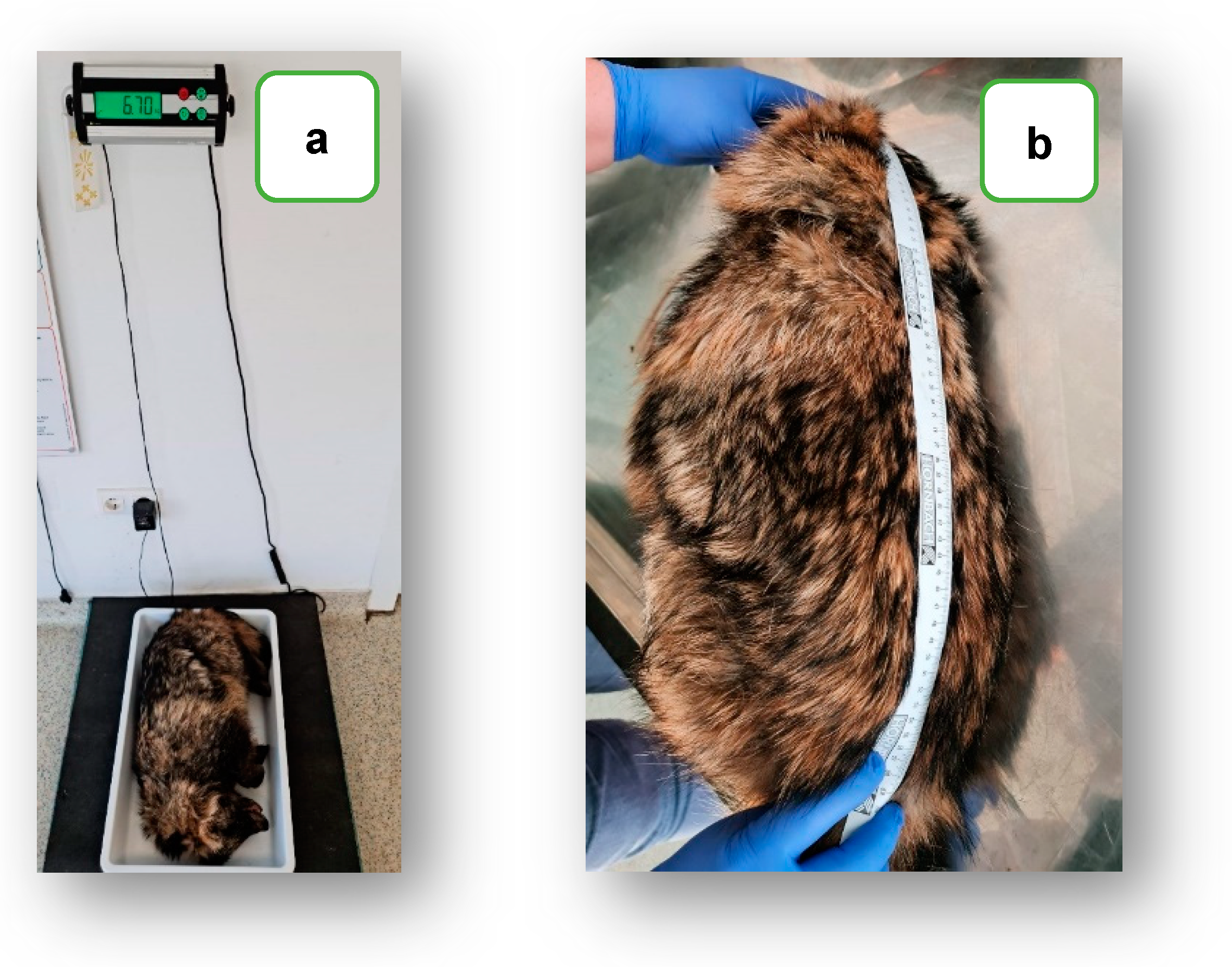
2.1. Case Report
2.1.1. The Study Area
2.1.2. Artificial Digestion
2.1.3. DNA Extraction and Molecular Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kauhala, K.; Kowalczyk, R. Invasion of the raccoon dog Nyctereutes procyonoides in Europe: History of colonization, features behind its success, and threats to native fauna. Curr. Zool. 2011, 57, 584–598. [Google Scholar] [CrossRef]
- Cotta, V.; Bodea, M.; Micu, I. Vânatul și vânătoarea în România. Ed. Ceres. Rom. 2001, 786. [Google Scholar]
- Al-Sabi, M.N.S.; Chriél, M.; Jensen, T.H.; Enemark, H.L. Endoparasites of the raccoon dog (Nyctereutes procyonoides) and the red fox (Vulpes vulpes) in Denmark 2009–2012–A comparative study. Int. J. Parasitol. Parasites Wildl. 2013, 2, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Bružinskaitė-Schmidhalter, R.; Šarkūnas, M.; Malakauskas, A.; Mathis, A.; Torgerson, P.R.; Deplazes, P. Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology 2012, 139, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, L.J.; Jensen, L.M.; Chriel, M.; Bodker, R.; Petersen, H.H. The raccoon dog (Nyctereutes procyonoides) as a reservoir of zoonotic diseases in Denmark. Int. J. Parasitol. Parasites Wildl. 2021, 16, 175–182. [Google Scholar] [CrossRef]
- Maas, M.; van den End, S.; van Roon, A.; Mulder, J.; Franssen, F.; Dam-Deisz, C.; Montizaan, M.; van der Giessen, J. First findings of Trichinella spiralis and DNA of Echinococcus multilocularis in wild raccoon dogs in the Netherlands. Int J Parasitol Parasites Wildl. 2016, 5, 277–279. [Google Scholar] [CrossRef]
- Pozio, E.; Murrell, K.D. Systematics and epidemiology of Trichinella. Adv. Parasitol. 2006, 63, 367–439. [Google Scholar]
- Pozio, E.; Rinaldi, L.; Marucci, G.; Musella, V.; Galati, F.; Cringoli, G.; Boireau, P.; La Rosa, G. Hosts and habitats of Trichinella spiralis and Trichinella britovi in Europe. Int. J. Parasitol. 2009, 39, 71–79. [Google Scholar] [CrossRef]
- Pozio, E. Trichinellosis in the European union: Epidemiology, ecology and economic impact. Parasitol. Today 1998, 14, 35–38. [Google Scholar] [CrossRef]
- Gherman, C.M.; Boros, Z.; Băieș, M.H.; Cozma-Petruț, A.; Cozma, V. A review of Trichinella species infection in wild animals in Romania. Food Waterborne Parasitol. 2022, 27, e00178. [Google Scholar] [CrossRef]
- Marin, A.M.; Oprescu, I.; Dărăbuş, G.; Imre, M.; Hădărugă, N.; Moraru, M.M.F.; Ghilean, B.M.; Mederle, N. Updates on Trichinella species infection in wild boar (Sus scrofa) from Southern Romania. J. Agroaliment. Process. Technol. 2022, 28, 388–393. [Google Scholar]
- Moraru, M.M.F.; Herman, V.; Oprescu, I.; Fodor, J.T.; Morariu, S.; Ilie, M.; Marin, A.M.; Mederle, N. Wild boar-The source of human contamination with Trichinella spp. in Romania. Rev. Rom. Med. Vet. 2022, 32, 38–42. [Google Scholar]
- Commission Implementing Regulation (EU) 2020/1478 of 14 October 2020 Amending Implementing Regulation (EU) 2015/1375 as Regards Sampling, the Reference Method for Detection and Import Conditions Related to Trichinella Control. Available online: http://data.europa.eu/eli/reg_impl/2020/1478/oj (accessed on 11 August 2023).
- Yang, Y.; Cai, Y.N.; Tong, M.W.; Sun, N.; Xuan, Y.H.; Kang, Y.J.; Vallée, I.; Boireau, P.; Cheng, S.P.; Liu, M.Y. Serological tools for detection of Trichinella infection in animals and humans. One Health 2016, 2, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, F.; Gómez-Morales, M.A.; Hill, D.E. International Commission on Trichinellosis: Recommendations on the use of serological tests for the detection of Trichinella infection in animals and humans. Food Waterborne Parasitol. 2019, 14, e00032. [Google Scholar] [CrossRef]
- Pozio, E.; La Rossa, G. PCR-derived methods for the identification of Trichinella parasites from animal and human samples. Methods Mol. Biol. 2003, 216, 299–309. [Google Scholar]
- Pozio, E.; Zarlenga, D. International Commission on Trichinellosis: Recommendations for genotyping Trichinella muscle stage larvae. Food Waterborne Parasitol. 2019, 12, e00033. [Google Scholar] [CrossRef]
- Ordinul Ministrului Mediului, Apelor și Pădurilor nr. 1571/07.06.2022 Privind Aprobarea Cotelor de Recoltă Pentru Unele specii de Faună de Interes Cinegetic. Available online: https://mmediu.ro/articol/ordinul-ministrului-mediului-apelor-si-padurilor-nr-1571 (accessed on 11 August 2023).
- International Trichinella Reference Center (ITRC) at the ”Istituto Superiore di Sanità” in Rome, Italy. Available online: http://www.iss.it/site/trichinella/scripts/sear.asp (accessed on 27 August 2023).
- Blaga, R.; Gherman, C.; Cozma, V.; Zocevic, A.; Pozio, E.; Boireau, P. Trichinella species circulating among wild and domestic animals in Romania. Vet. Parasitol. 2009, 159, 218–221. [Google Scholar] [CrossRef]
- Blaga, R.; Gherman, C.; Seucom, D.; Cozma, V.; Boireau, P. First identification of Trichinella spp. in golden jackal (Canis aureus) in Romania. J. Wildl. Dis. 2008, 44, 457–459. [Google Scholar] [CrossRef]
- Blaga, R.; Durand, B.; Stoichici, A.; Gherman, C.; Ștefan, N.; Cozma, V.; Boireau, P. Animal Trichinella infection in Romania: Geographical heterogeneity for the last 8 years. Vet. Parasitol. 2009, 159, 290–294. [Google Scholar] [CrossRef]
- Boros, Z.; Ionica, A.M.; Deak, G.; Mihalca, A.D.; Chisamera, G.B.; Gyorke, A.; Gherman, C.M.; Cozma, V. The European badger, Meles meles, as a new host for Trichinella britovi in Romania. Vet. Parasitol. 2021, 297, 109545. [Google Scholar] [CrossRef]
- Boros, Z.; Ionica, A.M.; Deak, G.; Mihalca, A.D.; Chișamera, G.B.; Constantinescu, I.C.; Adam, C.; Gherman, C.; Cozma, V. Trichinella spp. infection in European polecats (Linnaeus, 1758) from Romania. Helminthologia 2021, 58, 323–327. [Google Scholar] [CrossRef]
- Iacob, O. Epidemiological surveillance of Trichinella spp. infection in animals in the eastern part of Romania (Moldova) and the potential risk of human infection. In Actual Problems of Zoology and Parasitology: Achievements and Prospects; Institutul de Zoologie: Chișinău, Moldova, 2017. [Google Scholar]
- Imre, K.; Pozio, E.; Tonanzi, D.; Sala, C.; Ilie, M.S.; Imre, M.; Morar, A. The red fox (Vulpes vulpes) plays a minor role in the epidemiology of the domestic cycle of Trichinella in Romania. Vet. Parasitol. 2015, 212, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Marian, I.; Mihalca, A.D.; Gherman, C.M. Prevalence of Trichinella spp. infection in large wild carnivore species from Romania between Jan 2014 and July 2015. BUASVM CN 2015, 72, 438–440. [Google Scholar] [CrossRef][Green Version]
- Nesterov, V.; Colofan, I.; Nițulescu, A.; Costiov, F.; Dumitrescu, C.; Milla, C.; Popescu, S. Implications of human activity in the epizootiology of silvical trichinosis. Rev. Rom. Paraz. 1991, 1, 71–72. [Google Scholar]
- Nicorescu, I.M.D.; Ionița, M.; Ciupescu, L.; Buzatu, C.V.; Tanasuica, R.; Mitrea, I.L. New insights into the molecular epidemiology of Trichinella infection in domestic pigs, wild boars, and bears in Romania. Vet. Parasitol. 2015, 212, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Oltean, M.; Kalmar, Z.; Kiss, B.J.; Marinov, M.; Vasile, A.; Sandor, A.D.; Rosenthal, B.M. European mustelids occupying pristine wetlands in the Danube delta are infected with Trichinella likely derived from domesticated swine. J. Wildl. Dis. 2014, 50, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Oprescu, I.; Dărăbus, G.; Morariu, S.; Mederle, N.; Ilie, M.; Imre, K.; Imre, M.; Mako, A.; Nincov, I. The prevalence of trichinellosis in Covasna county between 1997–2007. Sci. Work. Vet. Med. 2007, 42, 64–69. [Google Scholar]
- Cabaj, W. Wild and domestic animals as permanent Trichinella reservoir in Poland. Wiad Parazytol. 2006, 52, 175–179. [Google Scholar]
- Osten-Sacken, N.; Solarczyk, P. Trichinella spiralis in road-killed raccoon dogs (Nyctereutes procyonoides) in western Poland. Ann. Parasitol. 2016, 62, 77–79. [Google Scholar]
- Cybulska, A.; Kornacka, A.; Moskwa, B. The occurrence and muscle distribution of Trichinella britovi in raccoon dogs (Nyctereutes procyonoides) in wildlife in the Głęboki Bród Forest District, Poland. Int. J. Parasitol. Parasites Wildl. 2019, 9, 149–153. [Google Scholar] [CrossRef]
- Cybulska, A. Immunoproteomic Analysis of Trichinella britovi Proteins Recognized by IgG Antibodies from Meat Juice of Carnivores Naturally Infected with T. britovi. Pathogens 2022, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Duscher, T.; Hodžić, A.; Glawischnig, W.; Duscher, G.G. The raccoon dog (Nyctereutes procyonoides) and the raccoon (Procyon lotor)-their role and impact of maintaining and transmitting zoonotic diseases in Austria, Central Europe. Parasitol. Res. 2017, 116, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E.; Miller, I.; Järvis, T.; Kapel, C.M.; La Rosa, G. Distribution of sylvatic species of Trichinella in Estonia according to climate zones. J. Parasitol. 1998, 84, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Järvis, T.; Pozio, E. Epidemiological investigations on Trichinella infections in farmed fur animals of Estonia. Vet. Parasitol. 2006, 139, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Kärssin, A.; Häkkinen, L.; Niin, E.; Peik, K.; Vilem, A.; Jokelainen, P.; Lassen, B. Trichinella spp. biomass has increased in raccoon dogs (Nyctereutes procyonoides) and red foxes (Vulpes vulpes) in Estonia. Parasites Vectors 2017, 10, 609. [Google Scholar] [CrossRef] [PubMed]
- Järvis, T.; Miller, I.; Pozio, E. Epidemiological studies on animal and human trichinellosis in Estonia. Parasite 2001, 2, 86–87. [Google Scholar] [CrossRef]
- Mayer-Scholl, A.; Reckinger, S.; Schulze, C.; Nöckler, K. Study on the occurrence of Trichinella spp. in raccoon dogs in Brandenburg, Germany. Vet. Parasitol. 2016, 231, 102–105. [Google Scholar] [CrossRef]
- Thiess, A.; Schuster, R.; Nöckler, K.; Mix, H. Helminth findings in indigenous raccoon dogs Nyctereutes procyonoides (Gray, 1843). Berl. Munch. Tierarztl. Wochenschr. 2001, 114, 273–276. [Google Scholar]
- Nockler, K. Prevalence and importance of Trichinella in Germany. Wien. Tierarztl. Monatsschrif. 2005, 92, 301–307. [Google Scholar]
- Langner, T.; Hamedy, A.; Wellner, H.; Johne, A.; Mayer-Scholl, A.; Birka, S. First detection of Trichinella spiralis in raccoon (Procyon lotor) in Germany. Vet. Parasitol. Reg. Stud. Rep. 2022, 36, 100800. [Google Scholar] [CrossRef]
- Pannwitz, G.; Mayer-Scholl, A.; Balicka-Ramisz, A.; Nöckler, K. Increased prevalence of Trichinella spp., northeastern Germany, 2008. Emerg. Infect. Dis. 2010, 16, 936–942. [Google Scholar] [CrossRef]
- Akkoc, N.; Kuruuzum, Z.; Akar, S.; Yuce, A.; Onen, F.; Yapar, N.; Ozgenc, O.; Turk, M.; Ozdemir, D.; Avci, M.; et al. A large scale outbreak of trichinellosis due to Trichinella britovi in Turkey. Zoonoses Public Health 2009, 56, 65–70. [Google Scholar] [CrossRef]
- Deksne, G.; Segliņa, Z.; Jahundoviča, I.; Esīte, Z.; Bakasejevs, E.; Bagrade, G.; Keidāne, D.; Interisano, M.; Marucci, G.; Tonanzi, D.; et al. High prevalence of Trichinella spp. in sylvatic carnivore mammals of Latvia. Vet. Parasitol. 2016, 231, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Malakauskas, A.; Paulauskas, V.; Järvis, T.; Keidans, P.; Eddi, C.; Kapel, C.M.O. Molecular epidemiology of Trichinella spp. in three Baltic countries: Lithuania, Latvia, and Estonia. Parasitol. Res. 2007, 100, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Boros, Z.; Vallee, I.; Panait, L.C.; Gherman, C.M.; Chevillot, A.; Boireau, P.; Cozma, V. Seroprevalance of Trichinella spp. in wild boars (Sus scrofa) from Bihor county, western Romania. Helmintologia 2020, 57, 235–240. [Google Scholar] [CrossRef]
- Różycki, M.; Korpysa-Dzirba, W.; Bełcik, A.; Pelec, T.; Mazurek, J.; Cencek, T. Analysis of a Trichinellosis Outbreak in Poland after Consumption of Sausage Made of Wild Boar Meat. J. Clin. Med. 2022, 11, 485. [Google Scholar] [CrossRef]
- Pavic, S.; Andric, A.; Sofronic-Milosavljevic, L.J.; Gnjatovic, M.; Mitic, I.; Vasilev, S.; Sparic, R.; Pavic, A. Trichinella britovi outbreak: Epidemiological, clinical, and biological features. Med. Mal. Infect. 2020, 50, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Pinto, M.; Fernandes, A.R.G.; Santos, M.H.; Marucci, G. Trichinella britovi infection in wild boar in Portugal. Zoonoses Public Health 2021, 68, 103–109. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Cui, J.; Shen, L.J. The epidemiology of animal trichinellosis in China. Vet. J. 2007, 173, 391–398. [Google Scholar] [CrossRef]
- Marin, A.M.; Dărăbuș, G.; Herman, V.; Morariu, S.; Olariu, T.R.; Dărăbuș, R.G.; Sîrbu, B.; Mederle, N. Study on the prevalence and larval burden of the nematode Trichinella spp. in red foxes from hunting grounds in Timis County. Rev. Rom. Med. Vet. 2021, 31, 23–26. [Google Scholar]
- Pozio, E. New patterns of Trichinella infections. Vet. Parasitol. 2001, 98, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carrasco, C.; Moroni, B.; García-Garrigós, A.; Robetto, S.; Carella, E.; Zoppi, S.; Tizzani, P.; Gonzálvez, M.; Orusa, R.; Rossi, L. Wolf Is Back: A Novel Sensitive Sentinel Rejoins the Trichinella Cycle in the Western Alps. Vet. Sci. 2023, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Oivanen, L.; Kapel, C.M.O.; Pozio, E.; La Rosa, G.; Mikkonen, T.; Sukura, A. Associations between Trichinella species and host species in Finland. J. Parasitol. 2002, 88, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Airas, N.; Saari, S.; Mikkonen, T.; Virtala, A.M.; Pellikka, J.; Oksanen, A.; Isomursu, M.; Kilpelä, S.S.; Lim, C.W.; Sukura, A. Sylvatic Trichinella spp. infection in Finland. J. Parasitol. 2010, 96, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Interisano, M.; Deksne, G.; Pozio, E. The subnivium, a haven for Trichinella larvae in host carcasses. Int. J. Parasitol. Parasites Wildl. 2019, 8, 229–233. [Google Scholar] [CrossRef]
- Pozio, E.; La Rosa, G.; Yamaguchi, T.; Saito, S. Trichinella britovi from Japan. J. Parasitol. 1996, 82, 847–849. [Google Scholar] [CrossRef]
- Kanai, Y.; Inoue, T.; Mano, T.; Nonaka, N.; Katakura, K.; Oku, Y. Epizootiological survey of Trichinella spp. infection in carnivores, rodents and insectivores in Hokkaido, Japan. Jpn. J. Vet. Res. 2007, 54, 175–182. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, A.-M.; Popovici, D.-C.; Dărăbuș, G.; Marian, C.; Nițușcă, D.; Mederle, N. The First Identification of Trichinella britovi in the Raccoon Dog (Nyctereutes procyonoides) in Romania. Pathogens 2023, 12, 1132. https://doi.org/10.3390/pathogens12091132
Marin A-M, Popovici D-C, Dărăbuș G, Marian C, Nițușcă D, Mederle N. The First Identification of Trichinella britovi in the Raccoon Dog (Nyctereutes procyonoides) in Romania. Pathogens. 2023; 12(9):1132. https://doi.org/10.3390/pathogens12091132
Chicago/Turabian StyleMarin, Ana-Maria, Dan-Cornel Popovici, Gheorghe Dărăbuș, Cătălin Marian, Diana Nițușcă, and Narcisa Mederle. 2023. "The First Identification of Trichinella britovi in the Raccoon Dog (Nyctereutes procyonoides) in Romania" Pathogens 12, no. 9: 1132. https://doi.org/10.3390/pathogens12091132
APA StyleMarin, A.-M., Popovici, D.-C., Dărăbuș, G., Marian, C., Nițușcă, D., & Mederle, N. (2023). The First Identification of Trichinella britovi in the Raccoon Dog (Nyctereutes procyonoides) in Romania. Pathogens, 12(9), 1132. https://doi.org/10.3390/pathogens12091132






