In Vitro Antibody-Dependent Enhancement of SARS-CoV-2 Infection Could Be Abolished by Adding Human IgG
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Serum Samples
2.3. Monoclonal Antibodies
2.4. Construction and Production of Variant Pseudoviruses
2.5. Pseudovirus Neutralization Assays
2.6. Antibody-Dependent Enhancement (ADE) Assay
2.7. Generation of Mutated mAbs
2.8. Quantitative and Statistical Analysis
3. Results
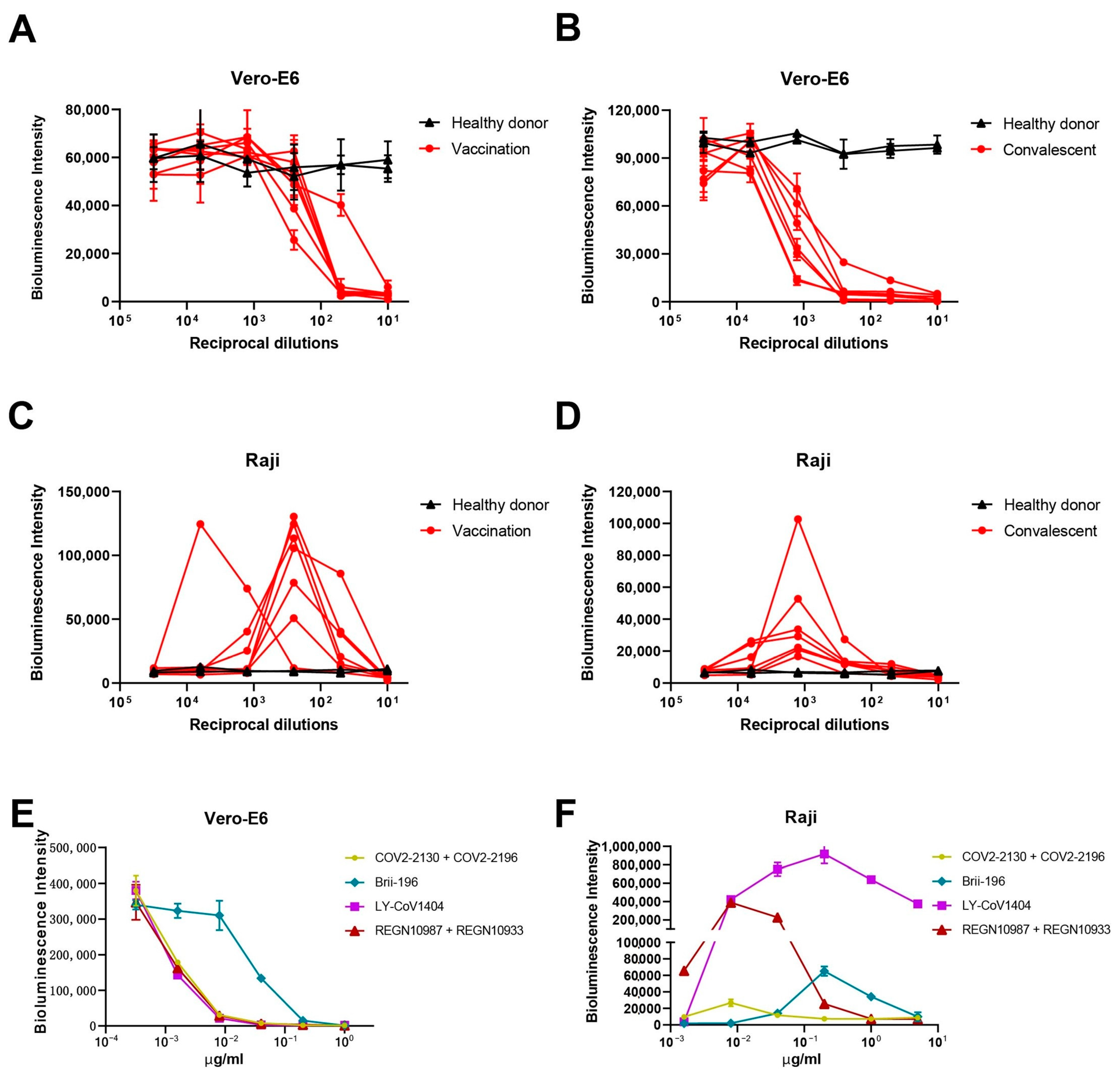
3.1. Vaccination and Convalescent Sera from COVID-19 Patients Induced ADE of SARS-CoV-2 Pseudoviral Infection on Raji Cells In Vitro
3.2. Approved mAbs Induced ADE of SARS-CoV-2 Pseudoviral Infection on Raji Cells
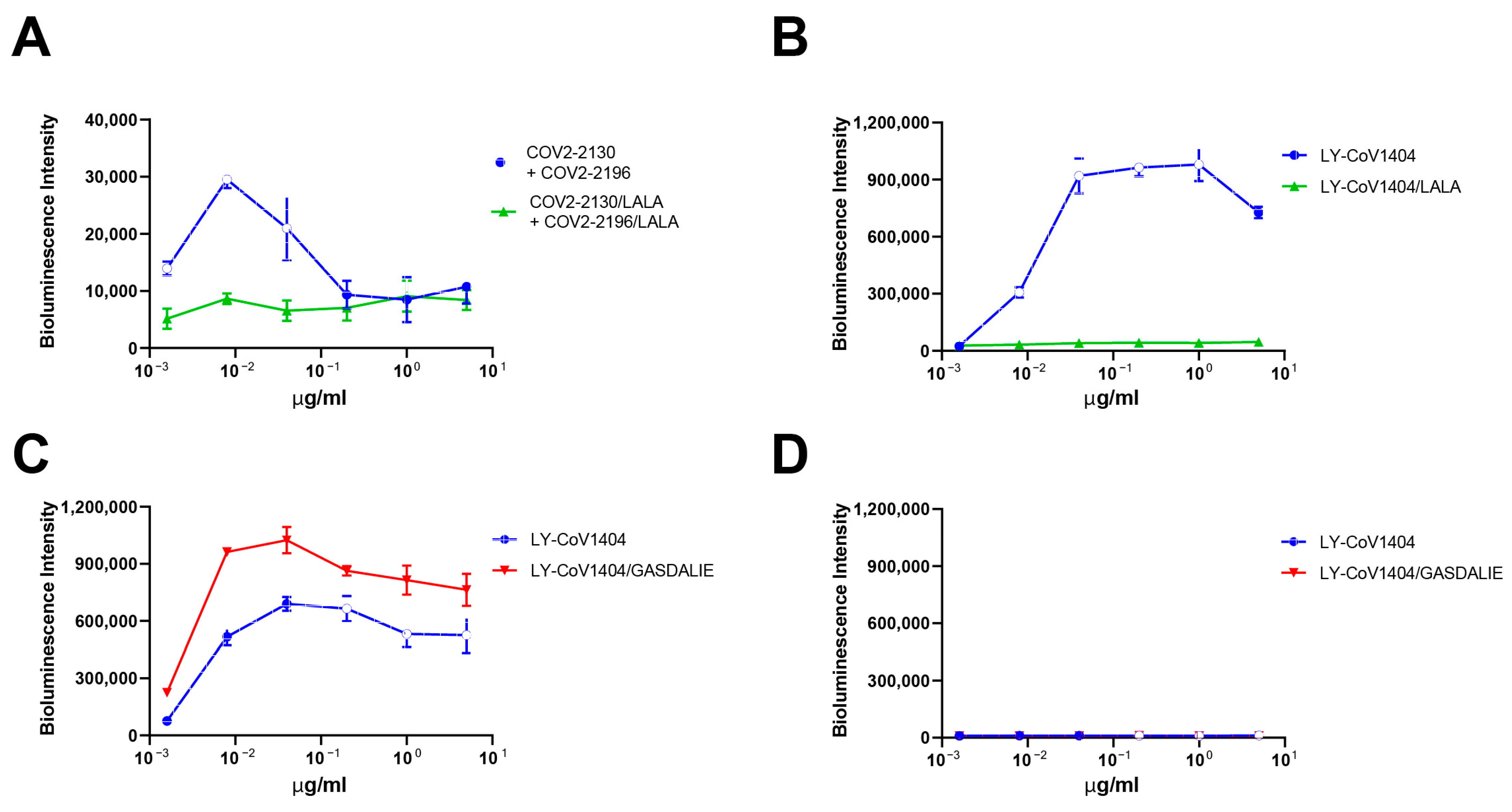
3.3. In Vitro ADE Activities Could Be Eliminated by Adding Human Serum/IgG
3.4. In Vitro ADE Activities Could Be Eliminated by Modification of Antibody Fc Region
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shivalkar, S.; Pingali, M.S.; Verma, A.; Singh, A.; Singh, V.; Paital, B.; Das, D.; Varadwaj, P.K.; Samanta, S.K. Outbreak of COVID-19: A Detailed Overview and Its Consequences. Adv. Exp. Med. Biol. 2021, 1353, 23–45. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Barajas-Nava, L.A. Development of SARS-CoV-2 vaccines. Bol. Med. Hosp. Infant. Mex. 2021, 78, 66–74. [Google Scholar] [CrossRef]
- Okuya, K.; Hattori, T.; Saito, T.; Takadate, Y.; Sasaki, M.; Furuyama, W.; Marzi, A.; Ohiro, Y.; Konno, S.; Hattori, T.; et al. Multiple Routes of Antibody-Dependent Enhancement of SARS-CoV-2 Infection. Microbiol. Spectr. 2022, 10, e0155321. [Google Scholar] [CrossRef]
- Farshadpour, F.; Taherkhani, R. Antibody-Dependent Enhancement and the Critical Pattern of COVID-19: Possibilities and Considerations. Med. Princ. Pract. 2021, 30, 422–429. [Google Scholar] [CrossRef]
- Lee, W.S.; Wheatley, A.K.; Kent, S.J.; DeKosky, B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 2020, 5, 1185–1191. [Google Scholar] [CrossRef]
- Ricke, D.O. Two Different Antibody-Dependent Enhancement (ADE) Risks for SARS-CoV-2 Antibodies. Front. Immunol. 2021, 12, 640093. [Google Scholar] [CrossRef]
- Sánchez-Zuno, G.A.; Matuz-Flores, M.G.; González-Estevez, G.; Nicoletti, F.; Turrubiates-Hernández, F.J.; Mangano, K.; Muñoz-Valle, J.F. A review: Antibody-dependent enhancement in COVID-19: The not so friendly side of antibodies. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211050199. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Barnes, C.O.; Jette, C.A.; Abernathy, M.E.; Dam, K.A.; Esswein, S.R.; Gristick, H.B.; Malyutin, A.G.; Sharaf, N.G.; Huey-Tubman, K.E.; Lee, Y.E.; et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 2020, 588, 682–687. [Google Scholar] [CrossRef]
- Porterfield, J.S. Antibody-dependent enhancement of viral infectivity. Adv. Virus Res. 1986, 31, 335–355. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Toltzis, P. 50 Years Ago in The Journal of Pediatrics: Atypical Exanthem after Exposure to Natural Measles: Eleven Cases in Children Previously Inoculated with Killed Vaccine. J. Pediatr. 2018, 193, 84. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Kuwahara, K.; Li, L.; Liu, Z.; Li, T.; Zhu, H.; Liu, J.; Xu, Y.; Xie, J.; et al. Immunodominant SARS Coronavirus Epitopes in Humans Elicited both Enhancing and Neutralizing Effects on Infection in Non-human Primates. ACS Infect. Dis. 2016, 2, 361–376. [Google Scholar] [CrossRef]
- Agrawal, A.S.; Tao, X.; Algaissi, A.; Garron, T.; Narayanan, K.; Peng, B.H.; Couch, R.B.; Tseng, C.T. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum. Vaccin. Immunother. 2016, 12, 2351–2356. [Google Scholar] [CrossRef]
- Ulrich, H.; Pillat, M.M.; Tárnok, A. Dengue Fever, COVID-19 (SARS-CoV-2), and Antibody-Dependent Enhancement (ADE): A Perspective. Cytometry A 2020, 97, 662–667. [Google Scholar] [CrossRef]
- Tetro, J.A. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020, 22, 72–73. [Google Scholar] [CrossRef]
- Wang, S.F.; Tseng, S.P.; Yen, C.H.; Yang, J.Y.; Tsao, C.H.; Shen, C.W.; Chen, K.H.; Liu, F.T.; Liu, W.T.; Chen, Y.M.; et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem. Biophys. Res. Commun. 2014, 451, 208–214. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody Responses to SARS-CoV-2 in Patients with Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef]
- Maemura, T.; Kuroda, M.; Armbrust, T.; Yamayoshi, S.; Halfmann, P.J.; Kawaoka, Y. Antibody-Dependent Enhancement of SARS-CoV-2 Infection Is Mediated by the IgG Receptors FcγRIIA and FcγRIIIA but Does Not Contribute to Aberrant Cytokine Production by Macrophages. mBio 2021, 12, e0198721. [Google Scholar] [CrossRef]
- Wieczorek, L.; Zemil, M.; Merbah, M.; Dussupt, V.; Kavusak, E.; Molnar, S.; Heller, J.; Beckman, B.; Wollen-Roberts, S.; Peachman, K.K.; et al. Evaluation of Antibody-Dependent Fc-Mediated Viral Entry, as Compared with Neutralization, in SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 901217. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.R.; Li, Q.; Li, H.L.; Wang, X.; Wang, Q.; Zheng, X.S.; Geng, R.; Zhang, Y.L.; Li, B.; Jiang, R.D.; et al. Antibody-Dependent Enhancement of SARS-CoV-2 Infection of Human Immune Cells: In Vitro Assessment Provides Insight in COVID-19 Pathogenesis. Viruses 2021, 13, 2483. [Google Scholar] [CrossRef]
- Zhou, W.; Tang, B.; Bai, Y.; Shao, Y.; Xiao, Y.; Tang, S. The resurgence risk of COVID-19 in China in the presence of immunity waning and ADE: A mathematical modelling study. Vaccine 2022, 40, 7141–7150. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Song, J.; Wu, J.; Zhu, Y.; Li, M.; Cui, Y.; Chen, Y.; Yang, L.; Liu, J.; et al. Homologous or heterologous booster of inactivated vaccine reduces SARS-CoV-2 Omicron variant escape from neutralizing antibodies. Emerg. Microbes Infect. 2022, 11, 477–481. [Google Scholar] [CrossRef]
- Ai, J.; Wang, X.; He, X.; Zhao, X.; Zhang, Y.; Jiang, Y.; Li, M.; Cui, Y.; Chen, Y.; Qiao, R.; et al. Antibody evasion of SARS-CoV-2 Omicron BA.1, BA.1.1, BA.2, and BA.3 sub-lineages. Cell Host Microbe 2022, 30, 1077–1083.e1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Cui, Y.; Qiao, R.; Li, M.; Chen, Y.; Yang, L.; Jiang, S.; Wang, P. Neutralization of distinct Omicron sublineages by longitudinal vaccination sera. J. Med. Virol. 2022, 94, 5090–5092. [Google Scholar] [CrossRef]
- Wang, X.; Ai, J.; Li, X.; Zhao, X.; Wu, J.; Zhang, H.; He, X.; Zhao, C.; Qiao, R.; Li, M.; et al. Neutralization of Omicron BA.4/BA.5 and BA.2.75 by booster vaccination or BA.2 breakthrough infection sera. Cell Discov. 2022, 8, 110. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y.; Wang, R.; Jiao, S.; Wang, M.; Huang, W.; Shan, C.; Jiang, W.; Li, Z.; Gu, C.; et al. Characterization of neutralizing antibody with prophylactic and therapeutic efficacy against SARS-CoV-2 in rhesus monkeys. Nat. Commun. 2020, 11, 5752. [Google Scholar] [CrossRef] [PubMed]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Westendorf, K.; Žentelis, S.; Wang, L.; Foster, D.; Vaillancourt, P.; Wiggin, M.; Lovett, E.; van der Lee, R.; Hendle, J.; Pustilnik, A.; et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022, 39, 110812. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schäfer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020, 584, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Baum, A.; Pascal, K.E.; Russo, V.; Giordano, S.; Wloga, E.; Fulton, B.O.; Yan, Y.; Koon, K.; Patel, K.; et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 2020, 369, 1010–1014. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Yu, X.; Jiang, W.; Chen, S.; Wang, R.; Wang, M.; Jiao, S.; Yang, Y.; Wang, W.; et al. Antibody-dependent enhancement (ADE) of SARS-CoV-2 pseudoviral infection requires FcγRIIB and virus-antibody complex with bivalent interaction. Commun. Biol. 2022, 5, 262. [Google Scholar] [CrossRef]
- Wang, Y.T.; Allen, R.D., 3rd; Kim, K.; Shafee, N.; Gonzalez, A.J.; Nguyen, M.N.; Valentine, K.M.; Cao, X.; Lu, L.; Pai, C.I.; et al. SARS-CoV-2 monoclonal antibodies with therapeutic potential: Broad neutralizing activity and No evidence of antibody-dependent enhancement. Antiviral Res. 2021, 195, 105185. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Keremane, S.R.; Vielmetter, J.; Bjorkman, P.J. Structural characterization of GASDALIE Fc bound to the activating Fc receptor FcγRIIIa. J. Struct. Biol. 2016, 194, 78–89. [Google Scholar] [CrossRef]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286.e278. [Google Scholar] [CrossRef]
- Li, D.; Luan, N.; Li, J.; Zhao, H.; Zhang, Y.; Long, R.; Jiang, G.; Fan, S.; Xu, X.; Cao, H.; et al. Waning antibodies from inactivated SARS-CoV-2 vaccination offer protection against infection without antibody-enhanced immunopathology in rhesus macaque pneumonia models. Emerg. Microbes Infect. 2021, 10, 2194–2198. [Google Scholar] [CrossRef]
- Li, D.; Edwards, R.J.; Manne, K.; Martinez, D.R.; Schäfer, A.; Alam, S.M.; Wiehe, K.; Lu, X.; Parks, R.; Sutherland, L.L.; et al. In vitro and in vivo functions of SARS-CoV-2 infection-enhancing and neutralizing antibodies. Cell 2021, 184, 4203–4219.e4232. [Google Scholar] [CrossRef]
- Mu, S.; Song, S.; Hao, Y.; Luo, F.; Wu, R.; Wang, Y.; Han, X.; Li, T.; Hu, C.; Li, S.; et al. Neutralizing antibodies from the rare convalescent donors elicited antibody-dependent enhancement of SARS-CoV-2 variants infection. Front. Med. 2022, 9, 952697. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Z.; Li, S.; Xu, W.; Zhang, Q.; Silva, I.T.; Li, C.; Wu, Y.; Jiang, Q.; Liu, Z.; et al. Enhancement versus neutralization by SARS-CoV-2 antibodies from a convalescent donor associates with distinct epitopes on the RBD. Cell Rep. 2021, 34, 108699. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721.e719. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Castells, M.C.; Phillips, E.J. Maintaining Safety with SARS-CoV-2 Vaccines. N. Engl. J. Med. 2021, 384, 643–649. [Google Scholar] [CrossRef]

| Primer a | Sequence (5′–3′) b |
|---|---|
| L234A/L235A-F | TTGTCCCGCCCCTGAGTTTGAGGGCGGACCTTCCGT |
| L234A/L235A-R | ACGGAAGGTCCGCCCTCAAACTCAGGGGCGGGACAA |
| G117A/S120D-F | CTGAGCTTCTGGCCGGACCTGATGTGTTCCTGTTCCC |
| G117A/S120D-R | GGGAACAGGAACACATCAGGTCCGGCCAGAAGCTCAG |
| A211L/I213E-F | CAACAAGGCCCTGCCCCTACCCGAGGAGAAAACCATCAG |
| A211L/I213E-R | CTGATGGTTTTCTCCTCGGGTAGGGGCAGGGCCTTGTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, M.; Lu, P.; Li, C.; Zhao, C.; Zhao, X.; Qiao, R.; Cui, Y.; Chen, Y.; Li, J.; et al. In Vitro Antibody-Dependent Enhancement of SARS-CoV-2 Infection Could Be Abolished by Adding Human IgG. Pathogens 2023, 12, 1108. https://doi.org/10.3390/pathogens12091108
Wang X, Li M, Lu P, Li C, Zhao C, Zhao X, Qiao R, Cui Y, Chen Y, Li J, et al. In Vitro Antibody-Dependent Enhancement of SARS-CoV-2 Infection Could Be Abolished by Adding Human IgG. Pathogens. 2023; 12(9):1108. https://doi.org/10.3390/pathogens12091108
Chicago/Turabian StyleWang, Xun, Minghui Li, Panpan Lu, Chen Li, Chaoyue Zhao, Xiaoyu Zhao, Rui Qiao, Yuchen Cui, Yanjia Chen, Jiayan Li, and et al. 2023. "In Vitro Antibody-Dependent Enhancement of SARS-CoV-2 Infection Could Be Abolished by Adding Human IgG" Pathogens 12, no. 9: 1108. https://doi.org/10.3390/pathogens12091108
APA StyleWang, X., Li, M., Lu, P., Li, C., Zhao, C., Zhao, X., Qiao, R., Cui, Y., Chen, Y., Li, J., Cai, G., & Wang, P. (2023). In Vitro Antibody-Dependent Enhancement of SARS-CoV-2 Infection Could Be Abolished by Adding Human IgG. Pathogens, 12(9), 1108. https://doi.org/10.3390/pathogens12091108








