Plant-Derived Products with Therapeutic Potential against Gastrointestinal Bacteria
Abstract
1. Introduction
2. Proposed Mechanisms of Action of Plant-Derived Products
3. Preclinical and Clinical Studies of Herbal Products in the Management of GI Infectious Diarrhoeal Disorders
| Disease | Treatment | No. of Patients | Study Design | Dosage/Duration | Outcomes | Reference |
|---|---|---|---|---|---|---|
| Diarrhoea-predominant irritable bowel syndrome |
| 22 | Double-blind, cross-over randomized clinical trial | Twice daily/4 weeks followed by a one week wash out period | No significant improvement in the symptoms of diarrhoea-pre-dominant irritable bowel syndrome compared to placebo. | [128] |
| Diarrhoea-predominant irritable bowel syndrome |
| 119 | Double-blind, randomized clinical trial | Twice daily/8 weeks | No significant difference was observed in symptom and Quality of Life (QoL) scores between two groups. | [129] |
| Diarrhoea |
| 161 | Prospective clinical trial | Daily with a follow-up after 4 to 48 h | The two treatments did not differ significantly in terms of diarrhoea duration and weight gain | [130] |
| Acute infective diarrhoea |
| 55 | Randomized clinical trial | Initial dose 40 mg followed by 20 mg every 4 h for 5 days | Faster recovery using berberine compared to standard antibiotic therapy with a clinical cure 72% | [103] |
| Antibiotic-associated diarrhoea (AAD) |
| 63 | Prospective study | NR | Cure rates 84 and 92% for C. difficile and non-C. difficile AAD, respectively | [112] |
| Acute gastroenteritis and dysentery |
| 129 | Clinical trial | 300 mg/day for 7 days | Combinations of berberine with antibiotics were more effective than berberine and antibiotics alone | [131] |
| Acute nonspecific diarrhoea |
| 94 | An open-label parallel comparison study bismuth subsalicylate | 4900 mg/day for 2 days | Loperamide was significantly effective for diarrhoea treatment than bismuth subsalicylate | [121] |
| Enterotoxigenic E. coli and V. cholerae diarrhoea |
| 165 | Randomized clinical trial | 400 mg or 1200 mg berberine sulphate in a single oral dose | Reduced mean stool volume during three consecutive 8-hr periods after treatment | [107] |
| Infectious gastroenteritis |
| 100 | Randomized clinical trial | 500 mg every 8 h for 3 days | Decreased the duration of abdominal pain with no significant changes in the consistency and frequency of liquid stools compared with the control group. | [101] |
4. OMICS Applications for Studying Biological Effects of Herbal Products against GI Bacteria
4.1. Metabolomics in Antimicrobial Medicinal Plant-Based Drug Discovery
4.2. Transcriptomics and Functional Analysis of Proteins in Medicinal Plants
5. Safety Implications Regarding the Use of Plant-Derived Antimicrobials
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thielman, N.M.; Guerrant, R.L. Acute infectious diarrhea. N. Engl. J. Med. 2004, 350, 38–47. [Google Scholar] [CrossRef]
- Casburn-Jones, A.C.; Farthing, M.J. Management of infectious diarrhoea. Gut 2004, 53, 296–305. [Google Scholar] [CrossRef]
- Zboromyrska, Y.; Vila, J. Advanced PCR-based molecular diagnosis of gastrointestinal infections: Challenges and opportunities. Expert Rev. Mol. Diagn. 2016, 16, 631–640. [Google Scholar] [CrossRef]
- WHO. Diarrhoeal Disease. Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 5 December 2022).
- Fischer Walker, C.L.; Sack, D.; Black, R.E. Etiology of diarrhea in older children, adolescents and adults: A systematic review. PLoS Negl. Trop. Dis. 2010, 4, e768. [Google Scholar] [CrossRef]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Fazly Bazzaz, B.S. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef]
- Spellberg, B.; Powers, J.H.; Brass, E.P.; Miller, L.G.; Edwards, J.E., Jr. Trends in Antimicrobial Drug Development: Implications for the Future. Clin. Infect. Dis. 2004, 38, 1279–1286. [Google Scholar] [CrossRef]
- Pan, S.Y.; Litscher, G.; Gao, S.H.; Zhou, S.F.; Yu, Z.L.; Chen, H.Q.; Zhang, S.F.; Tang, M.K.; Sun, J.N.; Ko, K.M. Historical perspective of traditional indigenous medical practices: The current renaissance and conservation of herbal resources. Evid. Based Complement. Altern. Med. 2014, 2014, 525340. [Google Scholar] [CrossRef]
- Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Indian Traditional Ayurvedic System of Medicine and Nutritional Supplementation. Evid.-Based Complement. Altern. Med. 2013, 2013, 376327. [Google Scholar] [CrossRef]
- Ezekwesili-Ofili Josephine, O.; Okaka Antoinette Nwamaka, C. Herbal Medicine in African Traditional Medicine. In Herbal. Medicine; Philip, F.B., Ed.; IntechOpen: Rijeka, Croatia, 2019; p. 191. [Google Scholar]
- Tillisch, K. Complementary and alternative medicine for functional gastrointestinal disorders. Gut 2006, 55, 593–596. [Google Scholar] [CrossRef]
- Radulović, N.S.; Blagojević, P.D.; Stojanović-Radić, Z.Z.; Stojanović, N.M. Antimicrobial plant metabolites: Structural diversity and mechanism of action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar] [CrossRef]
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M.R. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharm. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Env. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef]
- Lambert, R.J.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
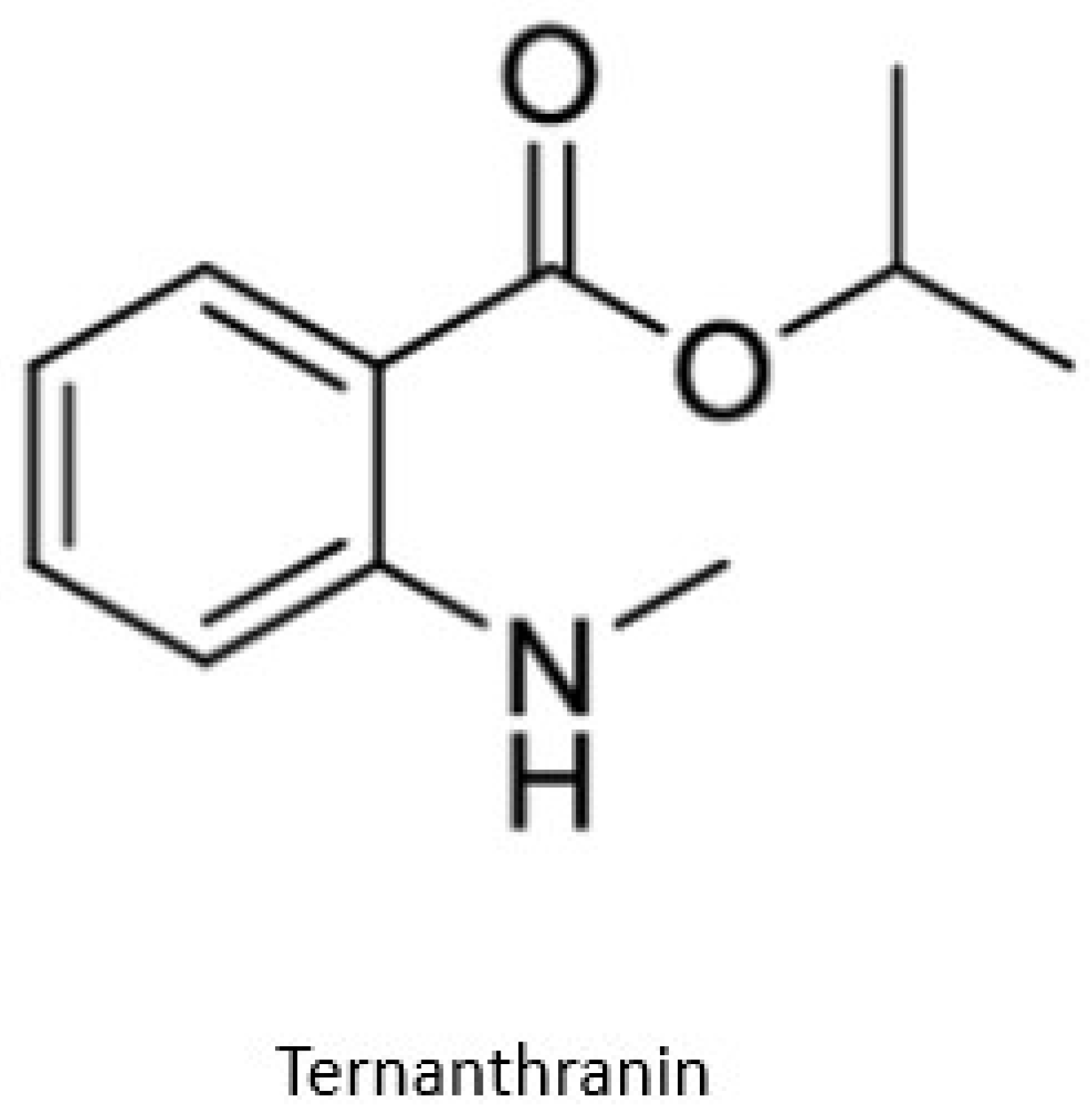
- Radulović, N.S.; Miltojević, A.B.; McDermott, M.; Waldren, S.; Parnell, J.A.; Pinheiro, M.M.G.; Fernandes, P.D.; de Sousa Menezes, F. Identification of a new antinociceptive alkaloid isopropyl N-methylanthranilate from the essential oil of Choisya ternata Kunth. J. Ethnopharmacol. 2011, 135, 610–619. [Google Scholar] [CrossRef]
- Jahan, H.; Choudhary, M.I.; Atta, A.; Khan, K.M.; Ur-Rahman, A. Anthranilic Acid Derivatives: Novel Inhibitors of Protein Glycation and the Associated Oxidative Stress in the Hepatocytes. Med. Chem. 2018, 14, 516–523. [Google Scholar] [CrossRef]
- Asif, M. Study of Anthranylic Acid Derivatives: Mefenamic Acid and Its Various Analogues. Am. J. Med. Stud. 2014, 2, 24–30. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef]
- Ranfaing, J.; Dunyach-Remy, C.; Lavigne, J.-P.; Sotto, A. Propolis potentiates the effect of cranberry (Vaccinium macrocarpon) in reducing the motility and the biofilm formation of uropathogenic Escherichia coli. PLoS ONE 2018, 13, e0202609. [Google Scholar] [CrossRef]
- Shane, A.L.; Mody, R.K.; Crump, J.A.; Tarr, P.I.; Steiner, T.S.; Kotloff, K.; Langley, J.M.; Wanke, C.; Warren, C.A.; Cheng, A.C.; et al. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin. Infect. Dis. 2017, 65, e45–e80. [Google Scholar] [CrossRef]
- Chen, J.C.; Huang, L.J.; Wu, S.L.; Kuo, S.C.; Ho, T.Y.; Hsiang, C.Y. Ginger and its bioactive component inhibit enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea in mice. J. Agric. Food. Chem. 2007, 55, 8390–8397. [Google Scholar] [CrossRef]
- Sugita-Konishi, Y.; Hara-Kudo, Y.; Amano, F.; Okubo, T.; Aoi, N.; Iwaki, M.; Kumagai, S. Epigallocatechin gallate and gallocatechin gallate in green tea catechins inhibit extracellular release of Vero toxin from enterohemorrhagic Escherichia coli O157:H7. Biochim. Biophys. Acta. 1999, 1472, 42–50. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J.; Sharma, S.; Sahu, D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur. J. Pharm. Sci. 2020, 152, 105424. [Google Scholar] [CrossRef]
- Dadi, P.K.; Ahmad, M.; Ahmad, Z. Inhibition of ATPase activity of Escherichia coli ATP synthase by polyphenols. Int. J. Biol. Macromol. 2009, 45, 72–79. [Google Scholar] [CrossRef]
- Amin, A.H.; Subbaiah, T.V.; Abbasi, K.M. Berberine sulfate: Antimicrobial activity, bioassay, and mode of action. Can. J. Microbiol. 1969, 15, 1067–1076. [Google Scholar] [CrossRef]
- Sack, R.B.; Froehlich, J.L. Berberine inhibits intestinal secretory response of Vibrio cholerae and Escherichia coli enterotoxins. Infect. Immun. 1982, 35, 471–475. [Google Scholar] [CrossRef]
- Baskaran, S.A.; Kollanoor-Johny, A.; Nair, M.S.; Venkitanarayanan, K. Efficacy of Plant-Derived Antimicrobials in Controlling Enterohemorrhagic Escherichia coli Virulence In Vitro. J. Food. Prot. 2016, 79, 1965–1970. [Google Scholar] [CrossRef]
- Mooyottu, S.; Kollanoor-Johny, A.; Flock, G.; Bouillaut, L.; Upadhyay, A.; Sonenshein, A.L.; Venkitanarayanan, K. Carvacrol and trans-cinnamaldehyde reduce Clostridium difficile toxin production and cytotoxicity in vitro. Int. J. Mol. Sci. 2014, 15, 4415–4430. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, R.; Wang, J.; Yu, P.; Liu, Q.; Zeng, D.; Song, H.; Kuang, Z. Protective effects of baicalin on LPS-induced injury in intestinal epithelial cells and intercellular tight junctions. Can. J. Physiol. Pharmacol. 2015, 93, 233–237. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Chen, Y.; Niu, X.; Zhang, Y.; Yang, C.; Wang, Q.; Li, X.; Deng, X. Baicalin inhibits the lethality of Shiga-like toxin 2 in mice. Antimicrob. Agents Chemother. 2015, 59, 7054–7060. [Google Scholar] [CrossRef]
- Liu, I.X.; Durham, D.G.; Richards, R.M. Baicalin synergy with beta-lactam antibiotics against methicillin-resistant Staphylococcus aureus and other beta-lactam-resistant strains of S. aureus. J. Pharm. Pharm. 2000, 52, 361–366. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, Z.; Liu, Y.; He, W.; Yang, C.; Wang, Q.; Dong, J.; Deng, X. Baicalin Protects Mice from Lethal Infection by Enterohemorrhagic Escherichia coli. Front. Microbiol. 2017, 8, 395. [Google Scholar] [CrossRef]
- Thapa, D.; Louis, P.; Losa, R.; Zweifel, B.; Wallace, R.J. Essential oils have different effects on human pathogenic and commensal bacteria in mixed faecal fermentations compared with pure cultures. Microbiology 2015, 161, 441–449. [Google Scholar] [CrossRef]
- Khan, U.A.; Rahman, H.; Qasim, M.; Hussain, A.; Azizllah, A.; Murad, W.; Khan, Z.; Anees, M.; Adnan, M. Alkanna tinctoria leaves extracts: A prospective remedy against multidrug resistant human pathogenic bacteria. BMC. Complement. Altern. Med. 2015, 15, 127. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, Y. Antibacterial activity of matrine and oxymatrine on pathogenic bacteria causing cow endometritis in vitro. Prog. Vet. Med. 2017, 38, 65–68. [Google Scholar] [CrossRef]
- Nowicki, D.; Maciąg-Dorszyńska, M.; Bogucka, K.; Szalewska-Pałasz, A.; Herman-Antosiewicz, A. Various modes of action of dietary phytochemicals, sulforaphane and phenethyl isothiocyanate, on pathogenic bacteria. Sci. Rep. 2019, 9, 13677. [Google Scholar] [CrossRef]
- Gupta, S.; Yadava, J.N.S.; Tandon, J.S. Antisecretory (Antidiarrhoeal) Activity of Indian Medicinal Plants against Escherichia Coli Enterotoxin-Induced Secretion in Rabbit and Guinea Pig Ileal Loop Models. Int. J. Pharmacogn. 1993, 31, 198–204. [Google Scholar] [CrossRef]
- Pereira da Cruz, R.; Sampaio de Freitas, T.; Socorro Costa, M.D.; Lucas Dos Santos, A.T.; Ferreira Campina, F.; Pereira, R.L.S.; Bezerra, J.W.A.; Quintans-Júnior, L.J.; De Souza Araújo, A.A.; Júnior, J.P.S.; et al. Effect of α-Bisabolol and Its β-Cyclodextrin Complex as TetK and NorA Efflux Pump Inhibitors in Staphylococcus aureus Strains. Antibiot 2020, 9, 28. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Rangel, J.M.; Sparling, P.H.; Crowe, C.; Griffin, P.M.; Swerdlow, D.L. Epidemiology of Escherichia coli O157: H7 outbreaks, united states, 1982–2002. Emerg. Infect. Dis. 2005, 11, 603. [Google Scholar] [CrossRef]
- Chen, J.-C.; Ho, T.-Y.; Chang, Y.-S.; Wu, S.-L.; Hsiang, C.-Y. Anti-diarrheal effect of Galla Chinensis on the Escherichia coli heat-labile enterotoxin and ganglioside interaction. J. Ethnopharmacol. 2006, 103, 385–391. [Google Scholar] [CrossRef]
- Tian, F.; Li, B.; Ji, B.; Yang, J.; Zhang, G.; Chen, Y.; Luo, Y. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinensis: The polarity affects the bioactivities. Food. Chem. 2009, 113, 173–179. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Lee, C.O.; Kweon, J.H.; Ahn, J.W.; Park, J.H. Growth-inhibitory effects of Galla Rhois-derived tannins on intestinal bacteria. J. Appl. Microbiol. 1998, 84, 439–443. [Google Scholar] [CrossRef]
- Song, X.; Yang, Y.; Li, J.; He, M.; Zou, Y.; Jia, R.; Li, L.; Hang, J.; Cui, M.; Bai, L.; et al. Tannins extract from Galla Chinensis can protect mice from infection by Enterotoxigenic Escherichia coli O101. BMC. Complement. Med. Ther. 2021, 21, 84. [Google Scholar] [CrossRef]
- Caturla, N.; Vera-Samper, E.; Villalaín, J.; Mateo, C.R.; Micol, V. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free. Radic. Biol. Med. 2003, 34, 648–662. [Google Scholar] [CrossRef]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food. Res. 2007, 51, 116–134. [Google Scholar] [CrossRef]
- Zeller, W.E. Activity, purification, and analysis of condensed tannins: Current state of affairs and future endeavors. Crop. Sci. 2019, 59, 886–904. [Google Scholar] [CrossRef]
- Doughari, J.H.; Ndakidemi, P.A.; Human, I.S.; Benade, S. Antioxidant, antimicrobial and antiverotoxic potentials of extracts of Curtisia dentata. J. Ethnopharmacol. 2012, 141, 1041–1050. [Google Scholar] [CrossRef]
- Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A Promising Weapon in the Arsenal against Antibiotic-Resistant Bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef]
- Di Martino, P.; Agniel, R.; David, K.; Templer, C.; Gaillard, J.L.; Denys, P.; Botto, H. Reduction of Escherichia coli adherence to uroepithelial bladder cells after consumption of cranberry juice: A double-blind randomized placebo-controlled cross-over trial. World J. Urolog. 2006, 24, 21–27. [Google Scholar] [CrossRef]
- Enioutina, E.Y.; Teng, L.; Fateeva, T.V.; Brown, J.C.S.; Job, K.M.; Bortnikova, V.V.; Krepkova, L.V.; Gubarev, M.I.; Sherwin, C.M.T. Phytotherapy as an alternative to conventional antimicrobials: Combating microbial resistance. Expert. Rev. Clin. Pharmacol. 2017, 10, 1203–1214. [Google Scholar] [CrossRef]
- Petronio Petronio, G.; Cutuli, M.A.; Magnifico, I.; Venditti, N.; Pietrangelo, L.; Vergalito, F.; Pane, A.; Scapagnini, G.; Di Marco, R. In Vitro and In Vivo Biological Activity of Berberine Chloride against Uropathogenic, E. coli Strains Using Galleria mellonella as a Host Model. Molecules 2020, 25, 5010. [Google Scholar] [CrossRef]
- Xu, C.; Wang, F.; Huang, F.; Yang, M.; He, D.; Deng, L. Targeting effect of berberine on type I fimbriae of Salmonella Typhimurium and its effective inhibition of biofilm. Appl. Microbiol. Biotechnol. 2021, 105, 1563–1573. [Google Scholar] [CrossRef]
- Domadia, P.N.; Bhunia, A.; Sivaraman, J.; Swarup, S.; Dasgupta, D. Berberine targets assembly of Escherichia coli cell division protein FtsZ. Biochemistry 2008, 47, 3225–3234. [Google Scholar] [CrossRef]
- Boberek, J.M.; Stach, J.; Good, L. Genetic evidence for inhibition of bacterial division protein FtsZ by berberine. PLoS ONE 2010, 5, e13745. [Google Scholar] [CrossRef]
- Jin, J.L.; Hua, G.Q.; Meng, Z.; Gao, P.J. J. Antibacterial Mechanisms of Berberine and Reasons for Little Resistance of Bacteria. Chin. Herb. Med. 2010, 3, 27–35. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Lorenz, P.; Tawara, J.N.; Zenewicz, L.A.; Lewis, K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 2000, 97, 1433–1437. [Google Scholar] [CrossRef]
- Su, F.; Wang, J. Berberine inhibits the MexXY-OprM efflux pump to reverse imipenem resistance in a clinical carbapenem-resistant Pseudomonas aeruginosa isolate in a planktonic state. Exp. Med. 2018, 15, 467–472. [Google Scholar] [CrossRef]
- Sun, T.; Xiaodong, L.; Hong, J.; Liu, C.; Zhang, X.-L.; Zheng, J.-P.; Xu, Y.-J.; Ou, Z.-Y.; Zheng, J.-L.; Yu, D.-J. Inhibitory Effect of Two Traditional Chinese Medicine Monomers, Berberine and Matrine, on the Quorum Sensing System of Antimicrobial-Resistant Escherichia coli. Front. Microbiol. 2019, 10, 2584. [Google Scholar] [CrossRef]
- Shilei, Z.; Ze, J.; Xianghe, Z.; Chunguang, W.; Tie, Z. The Effect of Berberine on the Transcriptome and Proteome of E. coli. bioRxiv 2018, 318733. [Google Scholar] [CrossRef]
- Lorenz, C.; Dougherty, T.J.; Lory, S. Transcriptional Responses of Escherichia coli to a Small-Molecule Inhibitor of LolCDE, an Essential Component of the Lipoprotein Transport Pathway. J. Bacteriol. 2016, 198, 3162–3175. [Google Scholar] [CrossRef]
- Thong, S.; Ercan, B.; Torta, F.; Fong, Z.Y.; Wong, H.Y.A.; Wenk, M.R.; Chng, S.-S. Defining key roles for auxiliary proteins in an ABC transporter that maintains bacterial outer membrane lipid asymmetry. eLife 2016, 5, e19042. [Google Scholar] [CrossRef]
- Majdalani, N.; Heck, M.; Stout, V.; Gottesman, S. Role of RcsF in signaling to the Rcs phosphorelay pathway in Escherichia coli. J. Bacteriol. 2005, 187, 6770–6778. [Google Scholar] [CrossRef]
- Ghachi, M.E.; Derbise, A.; Bouhss, A.; Mengin-Lecreulx, D. Identification of Multiple Genes Encoding Membrane Proteins with Undecaprenyl Pyrophosphate Phosphatase (UppP) Activity in Escherichia coli*. J. Biol. Chem. 2005, 280, 18689–18695. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Env. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef]
- Dineen, S.S.; McBride, S.M.; Sonenshein, A.L. Integration of metabolism and virulence by Clostridium difficile CodY. J. Bacteriol. 2010, 192, 5350–5362. [Google Scholar] [CrossRef]
- Mooyottu, S.; Flock, G.; Upadhyay, A.; Upadhyaya, I.; Maas, K.; Venkitanarayanan, K. Protective Effect of Carvacrol against Gut Dysbiosis and Clostridium difficile Associated Disease in a Mouse Model. Front. Microbiol. 2017, 8, 625. [Google Scholar] [CrossRef]
- Roshan, N.; Riley, T.V.; Hammer, K.A. Antimicrobial activity of natural products against Clostridium difficile in vitro. J. Appl. Microbiol. 2017, 123, 92–103. [Google Scholar] [CrossRef]
- Roshan, N.; Riley, T.V.; Knight, D.R.; Hammer, K.A. Effect of natural products on the production and activity of Clostridium difficile toxins in vitro. Sci. Rep. 2018, 8, 15735. [Google Scholar] [CrossRef]
- Lv, Z.; Peng, G.; Liu, W.; Xu, H.; Su, J. Berberine blocks the relapse of Clostridium difficile infection in C57BL/6 mice after standard vancomycin treatment. Antimicrob. Agents. Chemother. 2015, 59, 3726–3735. [Google Scholar] [CrossRef]
- Pal, R.; Seleem, M.N. Discovery of a novel natural product inhibitor of Clostridioides difficile with potent activity in vitro and in vivo. PLoS ONE 2022, 17, e0267859. [Google Scholar] [CrossRef]
- Pal, R.; Dai, M.; Seleem, M.N. High-throughput screening identifies a novel natural product-inspired scaffold capable of inhibiting Clostridioides difficile in vitro. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Shilling, M.; Matt, L.; Rubin, E.; Visitacion, M.P.; Haller, N.A.; Grey, S.F.; Woolverton, C.J. Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile. J. Med. Food. 2013, 16, 1079–1085. [Google Scholar] [CrossRef]
- Fraternale, D.; Flamini, G.; Ricci, D. Essential oil composition and antimicrobial activity of Angelica archangelica L.(Apiaceae) roots. J. Med. Food. 2014, 17, 1043–1047. [Google Scholar] [CrossRef]
- Finegold, S.M.; Summanen, P.H.; Corbett, K.; Downes, J.; Henning, S.M.; Li, Z. Pomegranate extract exhibits in vitro activity against Clostridium difficile. Nutrition 2014, 30, 1210–1212. [Google Scholar] [CrossRef]
- Nadeau, É.; Messier, S.; Quessy, S. Comparison of Campylobacter isolates from poultry and humans: Association between in vitro virulence properties, biotypes, and pulsed-field gel electrophoresis clusters. Appl. Environ. Microbiol. 2003, 69, 6316–6320. [Google Scholar] [CrossRef]
- Mahajan, S.; Rodgers, F.G. Isolation, characterization, and host-cell-binding properties of a cytotoxin from Campylobacter jejuni. J. Clin. Microbiol. 1990, 28, 1314–1320. [Google Scholar] [CrossRef]
- Valtierra-Rodríguez, D.; Heredia, N.L.; García, S.; Sánchez, E. Reduction of Campylobacter jejuni and Campylobacter coli in poultry skin by fruit extracts. J. Food. Prot. 2010, 73, 477–482. [Google Scholar] [CrossRef]
- Castillo, S.L.; Heredia, N.; Contreras, J.F.; García, S. Extracts of Edible and Medicinal Plants in Inhibition of Growth, Adherence, and Cytotoxin Production of Campylobacter jejuni and Campylobacter coli. J. Food. Sci. 2011, 76, M421–M426. [Google Scholar] [CrossRef]
- Toda, M.; Okubo, S.; Ohnishi, R.; Shimamura, T. Antibacterial and bactericidal activities of Japanese green tea. Nihon. Saikingaku. Zasshi. Jpn. J. Bacteriol. 1989, 44, 669–672. [Google Scholar] [CrossRef]
- Zhu, J.; Hua, X.; Hou, J.; Zhao, W. The virulence determinants of Campylobacter jejuni and its ability to colonize hosts. Rev. Med. Microbiol. 2008, 19, 13–18. [Google Scholar] [CrossRef]
- Lengsfeld, C.; Faller, G.; Hensel, A. Okra polysaccharides inhibit adhesion of Campylobacter jejuni to mucosa isolated from poultry in vitro but not in vivo. Anim. Feed. Sci. Technol. 2007, 135, 113–125. [Google Scholar] [CrossRef]
- Samy, R.; Gopalakrishnakone, P. Review: Therapeutic potential of plants asanti-microbials for drug discovery. J. Evid. -Based Compl. Altern. Med. 2008, 10, 1093. [Google Scholar] [CrossRef]
- Stickel, F.; Schuppan, D. Herbal medicine in the treatment of liver diseases. Dig. Liver. Dis. 2007, 39, 293–304. [Google Scholar] [CrossRef]
- CDC. Diarrhea: Common Illness, Global Killer. Available online: https://stacks.cdc.gov/view/cdc/13557 (accessed on 11 December 2022).
- Groombridge, B.; Jenkins, M. World Atlas of Biodiversity: Earth’s Living Resources in the 21st Century; University of California Press: Berkeley, CA, USA, 2002; p. 340. ISBN 0-520-23668-8. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines on Safety Monitoring of Herbal Medicines in Pharmacovigilance Systems; World Health Organization: Geneva, Switzerland, 2004.
- Silva, M.; Bittner, M.; Mueller, M.S.; Mechler, E. Medicinal Plants in Tropical Countries, Traditional Use—Experience—Facts; Georg Thieme Verlag: Stuttgart, Germany, 2005; ISBN 1-58890-253-6. [Google Scholar] [CrossRef]
- Palombo, E.A. Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: Modes of action and effects on intestinal function. Phytother. Res. 2006, 20, 717–724. [Google Scholar] [CrossRef]
- Hirudkar, J.R.; Parmar, K.M.; Prasad, R.S.; Sinha, S.K.; Lomte, A.D.; Itankar, P.R.; Prasad, S.K. The antidiarrhoeal evaluation of Psidium guajava L. against enteropathogenic Escherichia coli induced infectious diarrhoea. J. Ethnopharmacol. 2020, 251, 112561. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.P.; Mitchell, S.; Solis, R.V. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 1–27. [Google Scholar] [CrossRef]
- Galvez, J.; Zarzuelo, A.; Crespo, M.E.; Lorente, M.D.; Ocete, M.A.; Jiménez, J. Antidiarrhoeic activity of Euphorbia hirta extract and isolation of an active flavonoid constituent. Planta. Med. 1993, 59, 333–336. [Google Scholar] [CrossRef]
- Gupta, P.; Birdi, T. Psidium guajava leaf extract prevents intestinal colonization of Citrobacter rodentium in the mouse model. J. Ayurveda Integr. Med. 2015, 6, 50. [Google Scholar] [CrossRef]
- Shittu, O.B.; Ajayi, O.L.; Bankole, S.O.; Popoola, T.O. Intestinal ameliorative effects of traditional Ogi-tutu, Vernonia amygdalina and Psidium guajava in mice infected with Vibrio cholera. Afr. Health. Sci. 2016, 16, 620–628. [Google Scholar] [CrossRef]
- Lozoya, X.; Reyes-Morales, H.; Chávez-Soto, M.A.; del Carmen Martínez-García, M.a.; Soto-González, Y.; Doubova, S.V. Intestinal anti-spasmodic effect of a phytodrug of Psidium guajava folia in the treatment of acute diarrheic disease. J. Ethnopharmacol. 2002, 83, 19–24. [Google Scholar] [CrossRef]
- Manekeng, H.T.; Mbaveng, A.T.; Ntyam Mendo, S.A.; Agokeng, A.D.; Kuete, V. Evaluation of Acute and Subacute Toxicities of Psidium guajava Methanolic Bark Extract: A Botanical with In Vitro Antiproliferative Potential. Evid. Based Complement. Altern. Med. 2019, 2019, 8306986. [Google Scholar] [CrossRef]
- Chauhan, R.K.; Jain, A.M.; Dube, M.K.; Bhandari, B. A combination of sulfadimidine, neomycin and berberine in the treatment of infectious diarrhoea. Indian. J. Pediatr. 1969, 36, 242–244. [Google Scholar] [CrossRef]
- Chauhan, R.K.S.; Jain, A.M.; Bhandari, B. Berberine in the treatment of childhood diarrhoea. Indian. J. Pediatr. 1970, 37, 577–579. [Google Scholar] [CrossRef]
- Sharda, D.C. Berberine in the treatment of diarrhoea of infancy and childhood. J. Indian. Med. Assoc. 1970, 54, 22–24. [Google Scholar] [CrossRef]
- Sharma, R.; Joshi, C.K.; Goyal, R.K. Berberine tannate in acute diarrhoea. Indian. Pediatr. 1970, 7, 496–501. [Google Scholar]
- Rabbani, G.H.; Butler, T.; Knight, J.; Sanyal, S.C.; Alam, K. Randomized controlled trial of berberine sulfate therapy for diarrhea due to enterotoxigenic Escherichia coli and Vibrio cholerae. J. Infect. Dis. 1987, 155, 979–984. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takase, H.; Abe, K.; Saito, Y.; Suzuki, A. [Pharmacological studies on antidiarrheal effects of a preparation containing berberine and geranii herba]. Nihon Yakurigaku Zasshi 1993, 101, 169–175. [Google Scholar] [CrossRef]
- Tsai, C.S.; Ochillo, R.F. Pharmacological effects of berberine on the longitudinal muscle of the guinea-pig isolated ileum. Arch. Int. Pharm. 1991, 310, 116–131. [Google Scholar]
- Zhang, Y.; Wang, X.; Sha, S.; Liang, S.; Zhao, L.; Liu, L.; Chai, N.; Wang, H.; Wu, K. Berberine increases the expression of NHE3 and AQP4 in sennosideA-induced diarrhoea model. Fitoterapia 2012, 83, 1014–1022. [Google Scholar] [CrossRef]
- Cernáková, M.; Kostálová, D. Antimicrobial activity of berberine--A constituent of Mahonia aquifolium. Folia. Microbiol. 2002, 47, 375–378. [Google Scholar] [CrossRef]
- Zhou, F.F.; Wu, S.; Klena, J.D.; Huang, H.H. Clinical characteristics of Clostridium difficile infection in hospitalized patients with antibiotic-associated diarrhea in a university hospital in China. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1773–1779. [Google Scholar] [CrossRef][Green Version]
- He, C.; Gao, P. Meta-analysis of antibiotic-associated diarrhea treated by traditional Chinese medicine. Chin. J. TCM. WM. Crit. Care 2010, 17, 69–72. [Google Scholar] [CrossRef]
- Shu, Q.; Peng, S.; Feng, Y.; Zhang, Z.; Xie, Y.; Zhou, S. The influences of Chinese herbal compounds on in vitro expression of toxic genes tcd A/tcd B of Clostridium difficile. Chin. J. Microecol. 2013, 25, 373–380. [Google Scholar]
- Singh, N.; Sharma, B. Toxicological Effects of Berberine and Sanguinarine. Front. Mol. Biosci. 2018, 5, 21. [Google Scholar] [CrossRef]
- Rad, S.Z.K.; Rameshrad, M.; Hosseinzadeh, H. Toxicology effects of Berberis vulgaris (barberry) and its active constituent, berberine: A review. Iran. J. Basic. Med. Sci. 2017, 20, 516–529. [Google Scholar] [CrossRef]
- Ai, X.; Yu, P.; Peng, L.; Luo, L.; Liu, J.; Li, S.; Lai, X.; Luan, F.; Meng, X. Berberine: A Review of its Pharmacokinetics Properties and Therapeutic Potentials in Diverse Vascular Diseases. Front. Pharmacol. 2021, 12, 3104. [Google Scholar] [CrossRef]
- Baska, A.; Leis, K.; Gałązka, P. Berberine in the Treatment of Diabetes Mellitus: A Review. Endocr. Metab. Immune. Disord. Drug Targets. 2021, 21, 1379–1386. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Steffen, R.; Mathewson, J.J.; Ericsson, C.D.; DuPont, H.L.; Helminger, A.; Balm, T.K.; Wolff, K.; Witassek, F. Travelers’ diarrhea in West Africa and Mexico: Fecal transport systems and liquid bismuth subsalicylate for self-therapy. J. Infect. Dis. 1988, 157, 1008–1013. [Google Scholar] [CrossRef][Green Version]
- DuPont, H.L.; Flores Sanchez, J.; Ericsson, C.D.; Mendiola Gomez, J.; DuPont, M.W.; Cruz Luna, A.; Mathewson, J.J. Comparative efficacy of loperamide hydrochloride and bismuth subsalicylate in the management of acute diarrhea. Am. J. Med. 1990, 88, 15s–19s. [Google Scholar] [CrossRef]
- Figueroa-Quintanilla, D.; Salazar-Lindo, E.; Sack, R.B.; León-Barúa, R.; Sarabia-Arce, S.; Campos-Sánchez, M.; Eyzaguirre-Maccan, E. A controlled trial of bismuth subsalicylate in infants with acute watery diarrheal disease. N. Engl. J. Med. 1993, 328, 1653–1658. [Google Scholar] [CrossRef]
- Pitz, A.M.; Park, G.W.; Lee, D.; Boissy, Y.L.; Vinjé, J. Antimicrobial activity of bismuth subsalicylate on Clostridium difficile, Escherichia coli O157:H7, norovirus, and other common enteric pathogens. Gut Microbes. 2015, 6, 93–100. [Google Scholar] [CrossRef]
- Chowdhury, H.R.; Yunus, M.; Zaman, K.; Rahman, A.; Faruque, S.M.; Lescano, A.G.; Sack, R.B. The efficacy of bismuth subsalicylate in the treatment of acute diarrhoea and the prevention of persistent diarrhoea. Acta. Paediatr. 2001, 90, 605–610. [Google Scholar] [CrossRef]
- Halani, S.; Wu, P.E. Salicylate toxicity from chronic bismuth subsalicylate use. BMJ. Case Rep. 2020, 13, e236929. [Google Scholar] [CrossRef]
- Thakkar, S.; Anklam, E.; Xu, A.; Ulberth, F.; Li, J.; Li, B.; Hugas, M.; Sarma, N.; Crerar, S.; Swift, S.; et al. Regulatory landscape of dietary supplements and herbal medicines from a global perspective. Regul. Toxicol. Pharmacol. 2020, 114, 104647. [Google Scholar] [CrossRef]
- Alibi, S.; Crespo, D.; Navas, J. Plant-Derivatives Small Molecules with Antibacterial Activity. Antibiotics 2021, 10, 231. [Google Scholar] [CrossRef]
- Lauche, R.; Kumar, S.; Hallmann, J.; Lüdtke, R.; Rampp, T.; Dobos, G.; Langhorst, J. Efficacy and safety of Ayurvedic herbs in diarrhoea-predominant irritable bowel syndrome: A randomised controlled crossover trial. Complement. Ther. Med. 2016, 26, 171–177. [Google Scholar] [CrossRef]
- Leung, W.K.; Wu, J.C.Y.; Liang, S.M.; Chan, L.S.; Chan, F.K.L.; Xie, H.; Fung, S.S.L.; Hui, A.J.; Wong, V.W.S.; Che, C.-T.; et al. Treatment of Diarrhea-Predominant Irritable Bowel Syndrome with Traditional Chinese Herbal Medicine: A Randomized Placebo-Controlled Trial. Off. J. Am. Coll. Gastroenterol. ACG. 2006, 101, 1574–1580. [Google Scholar] [CrossRef]
- Tal-Dia, A.; Toure, K.; Sarr, O.; Sarr, M.; Cisse, M.F.; Garnier, P.; Wone, I. A baobab solution for the prevention and treatment of acute dehydration in infantile diarrhea. Dakar. Med. 1997, 42, 68–73. [Google Scholar]
- Khin Maung, U.; Myo, K.; Nyunt Nyunt, W.; Aye, K.; Tin, U. Clinical trial of berberine in acute watery diarrhoea. Br. Med. J. Clin. Res. Ed. 1985, 291, 1601–1605. [Google Scholar] [CrossRef]
- Chakraborty, P. Herbal genomics as tools for dissecting new metabolic pathways of unexplored medicinal plants and drug discovery. Biochim. Open. 2018, 6, 9–16. [Google Scholar] [CrossRef]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.J.; Ewald, J.C..; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods. 2021, 18, 747–756. [Google Scholar] [CrossRef]
- Wang, M.; Lamers, R.J.A.; Korthout, H.A.; van Nesselrooij, J.H.; Witkamp, R.F.; van der Heijden, R.; Voshol, P.J.; Havekes, L.M.; Verpoorte, R.; van der Greef, J. Metabolomics in the context of systems biology: Bridging traditional Chinese medicine and molecular pharmacology. Phytother. Res. Int. J. Devoted. Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2005, 19, 173–182. [Google Scholar] [CrossRef]
- Medema, M.H.; Osbourn, A. Computational genomic identification and functional reconstitution of plant natural product biosynthetic pathways. Nat. Prod. Rep. 2016, 33, 951–962. [Google Scholar] [CrossRef]
- Goodacre, R.; Vaidyanathan, S.; Dunn, W.B.; Harrigan, G.G.; Kell, D.B. Metabolomics by numbers: Acquiring and understanding global metabolite data. TRENDS Biotechnol. 2004, 22, 245–252. [Google Scholar] [CrossRef]
- Zhou, Z.; Luo, M.; Zhang, H.; Yin, Y.; Cai, Y.; Zhu, Z.-J. Metabolite annotation from knowns to unknowns through knowledge-guided multi-layer metabolic networking. Nat. Commun. 2022, 13, 6656. [Google Scholar] [CrossRef]
- Yi, Z.-B.; Yu, Y.; Liang, Y.-Z.; Zeng, B. Evaluation of the antimicrobial mode of berberine by LC/ESI-MS combined with principal component analysis. J. Pharm. Biomed. Anal. 2007, 44, 301–304. [Google Scholar] [CrossRef]
- Budeyri Gokgoz, N.; Avci, F.G.; Yoneten, K.K.; Alaybeyoglu, B.; Ozkirimli, E.; Sayar, N.A.; Kazan, D.; Sariyar Akbulut, B. Response of Escherichia coli to prolonged berberine exposure. Microb. Drug. Resist. 2017, 23, 531–544. [Google Scholar] [CrossRef]
- Karaosmanoglu, K.; Sayar, N.A.; Kurnaz, I.A.; Akbulut, B.S. Assessment of berberine as a multi-target antimicrobial: A multi-omics study for drug discovery and repositioning. Omics. A. J. Integr. Biol. 2014, 18, 42–53. [Google Scholar] [CrossRef]
- Ozbalci, Ç.; Unsal, Ç.; Kazan, D.; Sariyar-Akbulut, B. Proteomic response of Escherichia coli to the alkaloid extract of Papaver polychaetum. Ann. Microbiol. 2010, 60, 709–717. [Google Scholar] [CrossRef]
- Yu, Y.; Yi, Z.B.; Liang, Y.Z. Validate antibacterial mode and find main bioactive components of traditional Chinese medicine Aquilegia oxysepala. Bioorg. Med. Chem. Lett. 2007, 17, 1855–1859. [Google Scholar] [CrossRef]
- Pandita, D.; Pandita, A.; Wani, S.H.; Abdelmohsen, S.A.M.; Alyousef, H.A.; Abdelbacki, A.M.M.; Al-Yafrasi, M.A.; Al-Mana, F.A.; Elansary, H.O. Crosstalk of Multi-Omics Platforms with Plants of Therapeutic Importance. Cells 2021, 10, 1296. [Google Scholar] [CrossRef]
- Dinda, B.; Chowdhury, D.R.; Mohanta, B.C. Naturally Occurring Iridoids, Secoiridoids and Their Bioactivity. An Updated Review, Part 3. Chem. Pharm. Bull. 2009, 57, 765–796. [Google Scholar] [CrossRef]
- Geu-Flores, F.; Sherden, N.H.; Courdavault, V.; Burlat, V.; Glenn, W.S.; Wu, C.; Nims, E.; Cui, Y.; O’Connor, S.E. An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis. Nature 2012, 492, 138–142. [Google Scholar] [CrossRef]
- Giddings, L.-A.; Liscombe, D.K.; Hamilton, J.P.; Childs, K.L.; DellaPenna, D.; Buell, C.R.; O’Connor, S.E. A stereoselective hydroxylation step of alkaloid biosynthesis by a unique cytochrome P450 in Catharanthus roseus. J. Biol. Chem. 2011, 286, 16751–16757. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Statti, G.A.; Menichini, F. Biological and pharmacological activities of iridoids: Recent developments. Mini. Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef]
- Lao, Y.; Wang, X.; Xu, N.; Zhang, H.; Xu, H. Application of proteomics to determine the mechanism of action of traditional Chinese medicine remedies. J. Ethnopharmacol. 2014, 155, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, R.-Y.; He, Q.-Y. Proteomic analysis of anticancer TCMs targeted at mitochondria. Evid.-Based Complement. Altern. Med. 2015, 2015, 539260. [Google Scholar] [CrossRef]
- Wang, L.; Tian, R. Proteomic profiling of salmonella under berberine stress. IOP. Conf. Ser. Mater. Sci. Eng. 2020, 768, 052054. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; MacDonald, I.C.; Martell, J.D.; Takahashi, N.; Chan, C.T.; Lobritz, M.A.; Braff, D.; Schwarz, E.G.; et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 2014, 111, E2100–E2109. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Camacho, D.M.; Kohanski, M.A.; Callura, J.M.; Collins, J.J. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Mol. Cell. 2012, 46, 561–572. [Google Scholar] [CrossRef]
- Cohen, N.R.; Lobritz, M.A.; Collins, J.J. Microbial persistence and the road to drug resistance. Cell. Host. Microbe. 2013, 13, 632–642. [Google Scholar] [CrossRef]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends. Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef]
- Bent, S. Herbal medicine in the United States: Review of efficacy, safety, and regulation: Grand rounds at University of California, San Francisco Medical Center. J. Gen. Intern. Med. 2008, 23, 854–859. [Google Scholar] [CrossRef]
- Tangjitman, K.; Wongsawad, C.; Kamwong, K.; Sukkho, T.; Trisonthi, C. Ethnomedicinal plants used for digestive system disorders by the Karen of northern Thailand. J. Ethnobiol. Ethnomed. 2015, 11, 27. [Google Scholar] [CrossRef]
- Yakubu, M.T.; Nurudeen, Q.O.; Salimon, S.S.; Yakubu, M.O.; Jimoh, R.O.; Nafiu, M.O.; Akanji, M.A.; Oladiji, A.T.; Williams, F.E. Antidiarrhoeal Activity of Musa paradisiaca Sap in Wistar Rats. Evid. Based. Complement. Altern. Med. 2015, 2015, 683726. [Google Scholar] [CrossRef]
- Ugbogu, E.A.; Ude, V.C.; Elekwa, I.; Arunsi, U.O.; Uche-Ikonne, C.; Nwakanma, C. Toxicological profile of the aqueous-fermented extract of Musa paradisiaca in rats. Avicenna. J. Phytomed. 2018, 8, 478–487. [Google Scholar]
- Hossain, F.; Mostofa, M.G.; Alam, A.K. Traditional uses and pharmacological activities of the genus leea and its phytochemicals: A review. Heliyon 2021, 7, e06222. [Google Scholar] [CrossRef]
- Mishra, G.; Khosa, R.; Singh, P.; Jha, K. Hepatoprotective activity of ethanolic extract of Leea indica (Burm. f.) Merr.(Leeaceae) stem bark against paracetamol induced liver toxicity in rats. Niger. J. Exp. Clin. Biosci. 2014, 2, 59. [Google Scholar] [CrossRef]
- Dandiya, P.; Cullumbine, H. Studies on Acorus calamus (III): Some pharmacological actions of the volatile oil. J. Pharmacol. Exp. Ther. 1959, 125, 353–359. [Google Scholar]
- Sharma, V.; Sharma, R.; Gautam, D.S.; Kuca, K.; Nepovimova, E.; Martins, N. Role of Vacha (Acorus calamus Linn.) in Neurological and Metabolic Disorders: Evidence from Ethnopharmacology, Phytochemistry, Pharmacology and Clinical Study. J. Clin. Med. 2020, 9, 1176. [Google Scholar] [CrossRef]
- Oli, A.N.; Obaji, M.; Enweani, I.B. Combinations of Alchornea cordifolia, Cassytha filiformis and Pterocarpus santalinoides in diarrhoegenic bacterial infections. BMC. Res. Notes 2019, 12, 649. [Google Scholar] [CrossRef]
- Libman, A.; Bouamanivong, S.; Southavong, B.; Sydara, K.; Soejarto, D. Medicinal plants: An important asset to health care in a region of Central Laos. J. Ethnopharmacol. 2006, 106, 303–311. [Google Scholar] [CrossRef]
- Nikkhah Bodagh, M.; Maleki, I.; Hekmatdoost, A. Ginger in gastrointestinal disorders: A systematic review of clinical trials. Food. Sci. Nutr. 2019, 7, 96–108. [Google Scholar] [CrossRef]
- Weidner, M.S.; Sigwart, K. Investigation of the teratogenic potential of a Zingiber officinale extract in the rat. Reprod. Toxicol. 2000, 15, 75–80. [Google Scholar] [CrossRef]
- Chivapat, S.; Chavalittumrong, P.; Attawish, A.; Bansiddhi, J.; Padungpat, S. Chronic toxicity of Thunbergia laurifolia Lindl. extract. J. Tradit. Thai. Altern. Med. 2009, 7, 17–25. [Google Scholar]
- Alshehri, M.M.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Tutuncu, S.; Aydar, E.F.; Topkaya, C.; Mertdinc, Z.; Ozcelik, B.; Aital, M.; et al. A Review of Recent Studies on the Antioxidant and Anti-Infectious Properties of Senna Plants. Oxid. Med. Cell. Longev. 2022, 2022, 6025900. [Google Scholar] [CrossRef]
- Nadal, S.R.; Calore, E.E.; Manzione, C.R.; Puga, F.R.; Perez, N.M. Effects of long-term administration of Senna occidentalis seeds in the large bowel of rats. Pathol. -Res. Pract. 2003, 199, 733–737. [Google Scholar] [CrossRef]
- Oladeji, O.S.; Adelowo, F.E.; Oluyori, A.P.; Bankole, D.T. Ethnobotanical Description and Biological Activities of Senna alata. Evid. Based Complement. Altern. Med. 2020, 2020, 2580259. [Google Scholar] [CrossRef]
- Hennebelle, T.; Weniger, B.; Joseph, H.; Sahpaz, S.; Bailleul, F. Senna alata. Fitoterapia 2009, 80, 385–393. [Google Scholar] [CrossRef]
- Ali, M.Z.; Mehmood, M.H.; Saleem, M.; Gilani, A.H. The use of Euphorbia hirta L. (Euphorbiaceae) in diarrhea and constipation involves calcium antagonism and cholinergic mechanisms. BMC. Complement. Med. 2020, 20, 14. [Google Scholar] [CrossRef]
- Adedapo, A.A.; Abatan, M.O.; Olorunsogo, O.O. Toxic effects of some plants in the genus Euphorbia on haematological and biochemical parameters of rats. Vet. Arh. 2004, 74, 53–62. [Google Scholar]
- Rujjanawate, C.; Kanjanapothi, D.; Amornlerdpison, D.; Pojanagaroon, S. Anti-gastric ulcer effect of Kaempferia parviflora. J. Ethnopharmacol. 2005, 102, 120–122. [Google Scholar] [CrossRef]
- Sudwan, P.; Saenphet, K.; Saenphet, S.; Suwansirikul, S. Effect of Kaempferia parviflora Wall. ex. Baker on sexual activity of male rats and its toxicity. Southeast. Asian. J. Trop. Med. Public. Health. 2006, 37 Suppl. 3, 210–215. [Google Scholar]
- Syiem, D.; Khup, P. Evaluation of Flemingia macrophylla L., a traditionally used plant of the north eastern region of India for hypoglycemic and anti-hyperglycemic effect on mice. Pharmacologyonline 2007, 2, 355–366. [Google Scholar]
- Palle, S.; Kanakalatha, A.; Kavitha, C.N. Gastroprotective and Antiulcer Effects of Celastrus paniculatus Seed Oil Against Several Gastric Ulcer Models in Rats. J. Diet. Suppl. 2018, 15, 373–385. [Google Scholar] [CrossRef]
- Borrelli, F.; Borbone, N.; Capasso, R.; Montesano, D.; De Marino, S.; Aviello, G.; Aprea, G.; Masone, S.; Izzo, A.A. Potent relaxant effect of a Celastrus paniculatus extract in the rat and human ileum. J. Ethnopharmacol. 2009, 122, 434–438. [Google Scholar] [CrossRef]
- Nalini, K.; Karanth, K.; Rao, A.; Aroor, A. Effects of Celastrus paniculatus on passive avoidance performance and biogenic amine turnover in albino rats. J. Ethnopharmacol. 1995, 47, 101–108. [Google Scholar] [CrossRef]
- FDA. Complementary and Alternative Medicine Products and their Regulation by the Food and Drug Administration. Available online: https://www.fda.gov/regulatory-information (accessed on 5 December 2022).
- EMA. The Committee on Herbal Medicinal Products (HMPC). Available online: https://www.ema.europa.eu/en/committees/committee-herbal-medicinal-products-hmpc (accessed on 10 December 2022).
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef]
- Netopilova, M.; Houdkova, M.; Rondevaldova, J.; Kmet, V.; Kokoska, L. Evaluation of in vitro growth-inhibitory effect of carvacrol and thymol combination against Staphylococcus aureus in liquid and vapour phase using new broth volatilization chequerboard method. Fitoterapia 2018, 129, 185–190. [Google Scholar] [CrossRef]
- Abreu, A.C.; McBain, A.J.; Simões, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef]
- Hadacek, F. Secondary Metabolites as Plant Traits: Current Assessment and Future Perspectives. Crit. Rev. Plant. Sci. 2002, 21, 273–322. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar]

| Phytochemicals | Chemical Structure | Plant | Mode of Action | Microorganism | (s) |
|---|---|---|---|---|---|
| Proanthocyanidin | 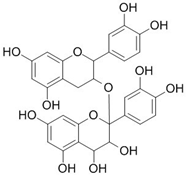 | Vaccinium macrocarpon L. | Modifies biofilm formation | Enterococcus faecalis, E. coli | [25,26] |
| Zingerone | 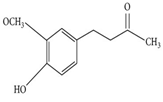 | Zingiber officinale Rosc. | Reduce heat-labile enterotoxin (LT)-induced diarrhoea in ETEC through blocking the binding to GM1 ganglioside receptors | ETEC | [27] |
| Epigallocatechin |  | Camellia sinensis L. | Inhibit extracellular release of Vero toxin from enterohemorrhagic Escherichia coli O157:H7 | EHEC | [28] |
| Quercetin | 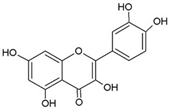 | Allium cepa L. | Inhibition of ATPase activity, elevates extracellular phosphatase and galactosidase. | E. coli | [29,30] |
| Berberine | 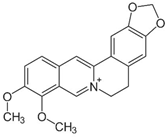 | Berberis vulgaris L. | Targeting proteins responsible for upholding the structure of cells and for cell division | E. coli, Salmonella spp., V. cholerae | [31,32] |
| Eugenol |  | Syzygium aromaticum L. | Inhibition of toxin production | EHEC | [33] |
| Cinnamaldehyde | 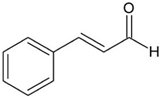 | Cinnamomum verum J. Presl | Inhibition of toxin genes and host receptor expression, reducing toxin-mediated pathology | EHEC, C. difficile | [33,34] |
| Baicalin (5,6,7-trihydroxyflavone) |  | Scutellaria baicalensis Georgi. | Inhibition of toxin production | H. pylori | [35,36,37,38] |
| Thymol | 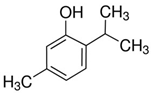 | Thymus vulgaris L. | Disturbance of the cell membrane and cytoplasm | C. difficile | [39] |
| Geraniol | 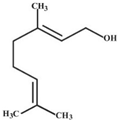 | Monarda fistulosa L. | Disturbance of the cell membrane and disruption of the cytoplasm. | C. difficile | [39] |
| Pyrrolizidine |  | Alkanna tinctoria L. | Disturbance of the cell membrane and cytoplasm | E. coli | [40] |
| Martine | 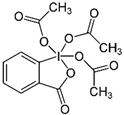 | Sophora flavescens Ait. | Inhibiting the synthesis of proteins | E. coli | [41] |
| Isothiocyanates |  | Brassica oleracea var. botrytis | Acting on cell membranes and leakage of cellular metabolites | Pathogenic E. coli strains | [42] |
| Andrographolide | 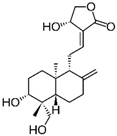 | Andrographis paniculata (Burm.f.) Nees | Anti-secretory activity against enterotoxins (Heat Labile (LT) and Heat Stable (ST) forms) | Pathogenic E. coli | [43] |
| α-bisabolol | 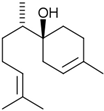 | Matricaria chamomilla L. | Inhibition of efflux pump | E. coli | [44] |
| Plant Species | Common Name | Family | GI Disorder(s) | Toxicological Effects |
|---|---|---|---|---|
| Psidium guajava L. | Guava | Myrtaceae | Acute diarrhoea [96,156] | Minor liver inflammation in rats, LD50 = 1000 mg/kg [102] |
| Musa × paradisiaca L. | Plantain | Musaceae | Infectious diarrhoea [157] | Significant changes in white blood cells, eosinophils, platelets, neutrophils, and monocytes count [158]. |
| Leea indica (Burm.f.) Merr. | Bandicoot berry | Vitaceae | Diarrhoea, dysentery [159] | Liver toxicity in rats [160] |
| Acorus calamus L. | Sweet flag | Acoraceae | GI infections, diarrhoea, dysentery [161,162] | Acute liver, spleen, and kidney toxicity and genotoxic effects in rats, LD50 = 221 g/kg [161] |
| Cassytha filiformis L. | Love-vine | Lauraceae | Diarrhoeagenic bacterial infections [163] | Acute haematological and biochemical toxicity (significant increase in alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total and direct bilirubin in rats, LD50 = 625.8 g/kg [164]) |
| Zingiber officinale Roscoe | Ginger | Zingiberaceae | Gastric ulceration, flatulence, diarrhoea [27,165] | Embryo toxic to pregnant rats [166] |
| Thunbergia laurifolia Lindl. | Laurel clock vine | Acanthaceae | Gastric ulcer, diarrhoea [156] | Decrease red blood cells in male rat [167] |
| Senna occidentalis (L.) Link | Coffee senna | Fabaceae | Constipation, GI infections [168] | Intestinal disturbance in long term use [169] |
| Senna alata (L.) Roxb. | Candle Bush | Fabaceae | Constipation, abdominal pain [168,170] | Decrease haemoglobin and erythrocyte (RBC) count values in rats [171] |
| Euphorbia hartal L. | Hairy Spurge | Euphorbiaceae | Diarrhoea, dysentery, constipation, intestinal parasites [172] | Leucocytosis, dullness, anorexia, stairy haircoat and 20% mortality in rats [173] |
| Euphorbia heterophylla L. | Milkweed | Euphorbiaceae | Intestinal bacterial infections, diarrhoea [172,173] | Leucopaenia in rats [173] |
| Kaempferia parviflora L. | Thai ginseng | Zingiberaceae | Flatulence, gastric ulcer [156,174] | Hepatotoxic to rats [175] |
| Flemingia macrophylla (Willd.) Kuntze ex Merr. | Apa apa | Fabaceae | Flatulence, indigestive [156] | Severe hypoglycaemia followed by death within 24 h after administration to normoglycemic mice [176] |
| Celastrus paniculatus Willd. | Black oil plant | Celastraceae | Diarrhoea, gastric ulcer, bowel spasms [177,178] | Hyperactivity and loss of behavioural responsiveness (loss of righting reflex in rat) [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qassadi, F.I.; Zhu, Z.; Monaghan, T.M. Plant-Derived Products with Therapeutic Potential against Gastrointestinal Bacteria. Pathogens 2023, 12, 333. https://doi.org/10.3390/pathogens12020333
Qassadi FI, Zhu Z, Monaghan TM. Plant-Derived Products with Therapeutic Potential against Gastrointestinal Bacteria. Pathogens. 2023; 12(2):333. https://doi.org/10.3390/pathogens12020333
Chicago/Turabian StyleQassadi, Fatimah I., Zheying Zhu, and Tanya M. Monaghan. 2023. "Plant-Derived Products with Therapeutic Potential against Gastrointestinal Bacteria" Pathogens 12, no. 2: 333. https://doi.org/10.3390/pathogens12020333
APA StyleQassadi, F. I., Zhu, Z., & Monaghan, T. M. (2023). Plant-Derived Products with Therapeutic Potential against Gastrointestinal Bacteria. Pathogens, 12(2), 333. https://doi.org/10.3390/pathogens12020333






