Taugt17b1 Overexpression in Trichoderma atroviride Enhances Its Ability to Colonize Roots and Induce Systemic Defense of Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Identification, Characterization and Acquisition of Overexpression Strains
2.3. In Vitro Mycoparasite Effect of Trichoderma Mutants on Growth Plant Pathogenic Fungi
2.4. Determining Root Colonization Ability of T. atroviride H18-1-1 WT and H18-1-1-t Strains and Root Activity
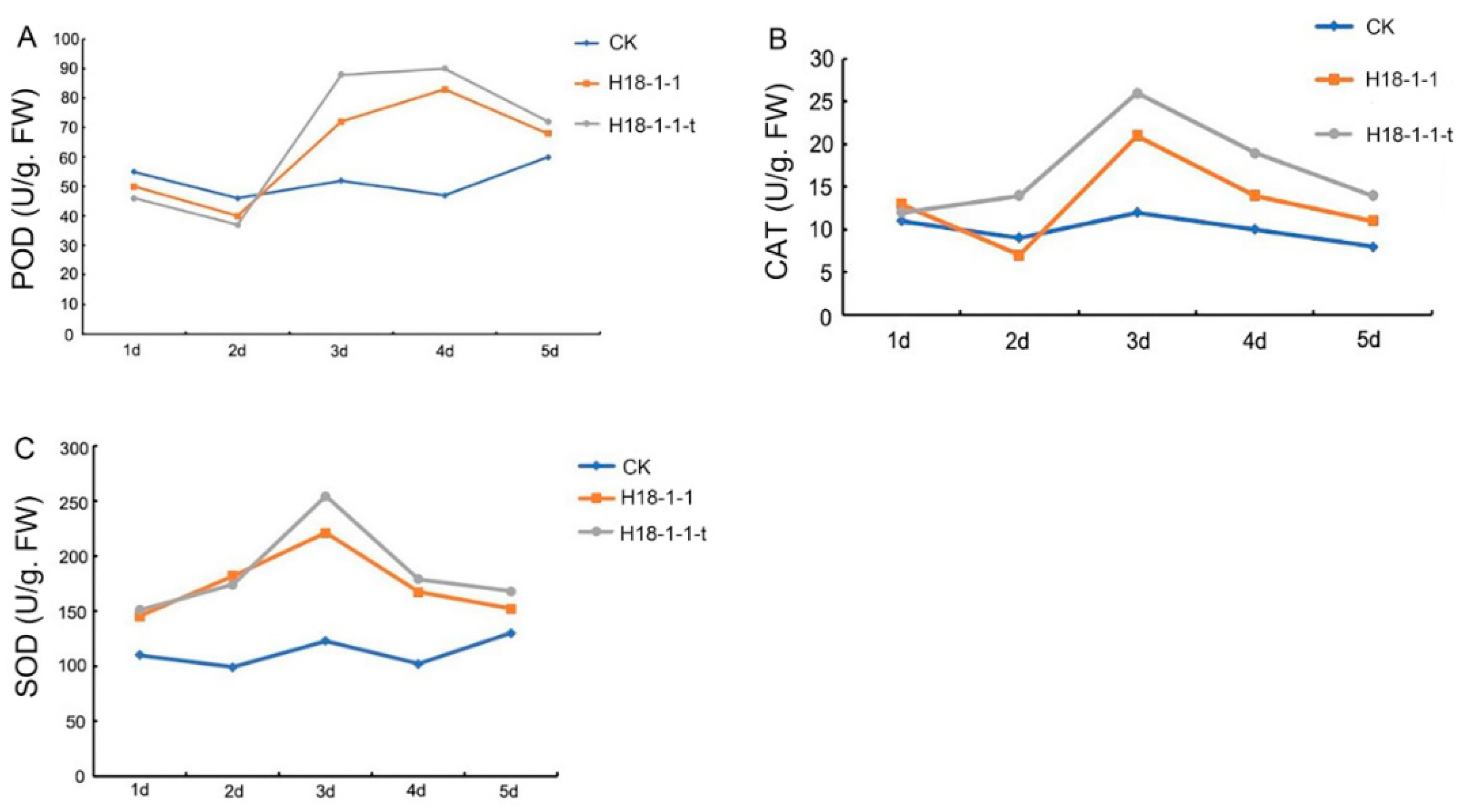
2.5. Effect of T. atroviride H18-1-1-t on Plant Defensive Enzymatic System
2.6. Effect of T. atroviride H18-1-1 WT on Disease Resistance in Wheat
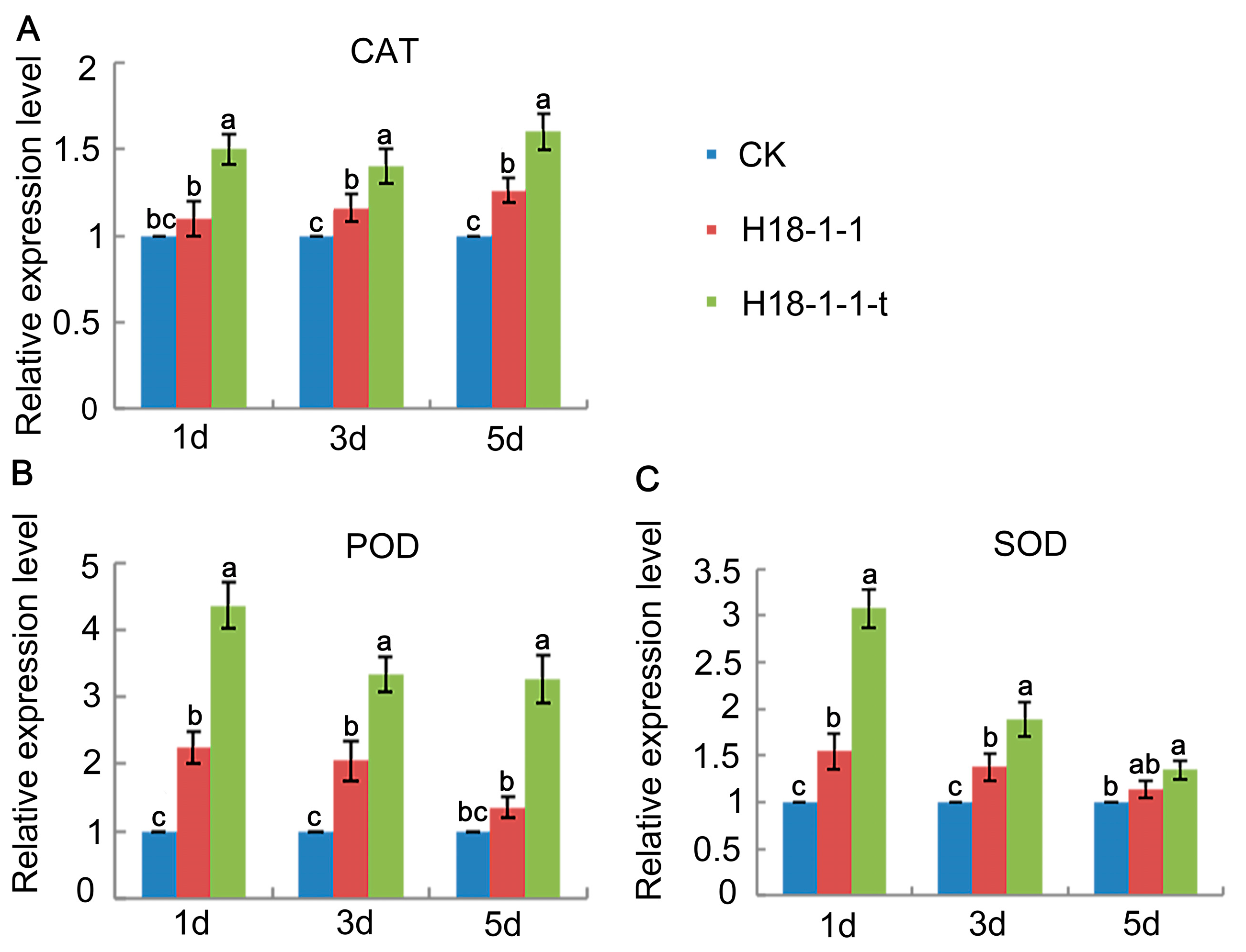
2.7. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Assays
2.8. Statistical Analysis
3. Results
3.1. Results and Analysis
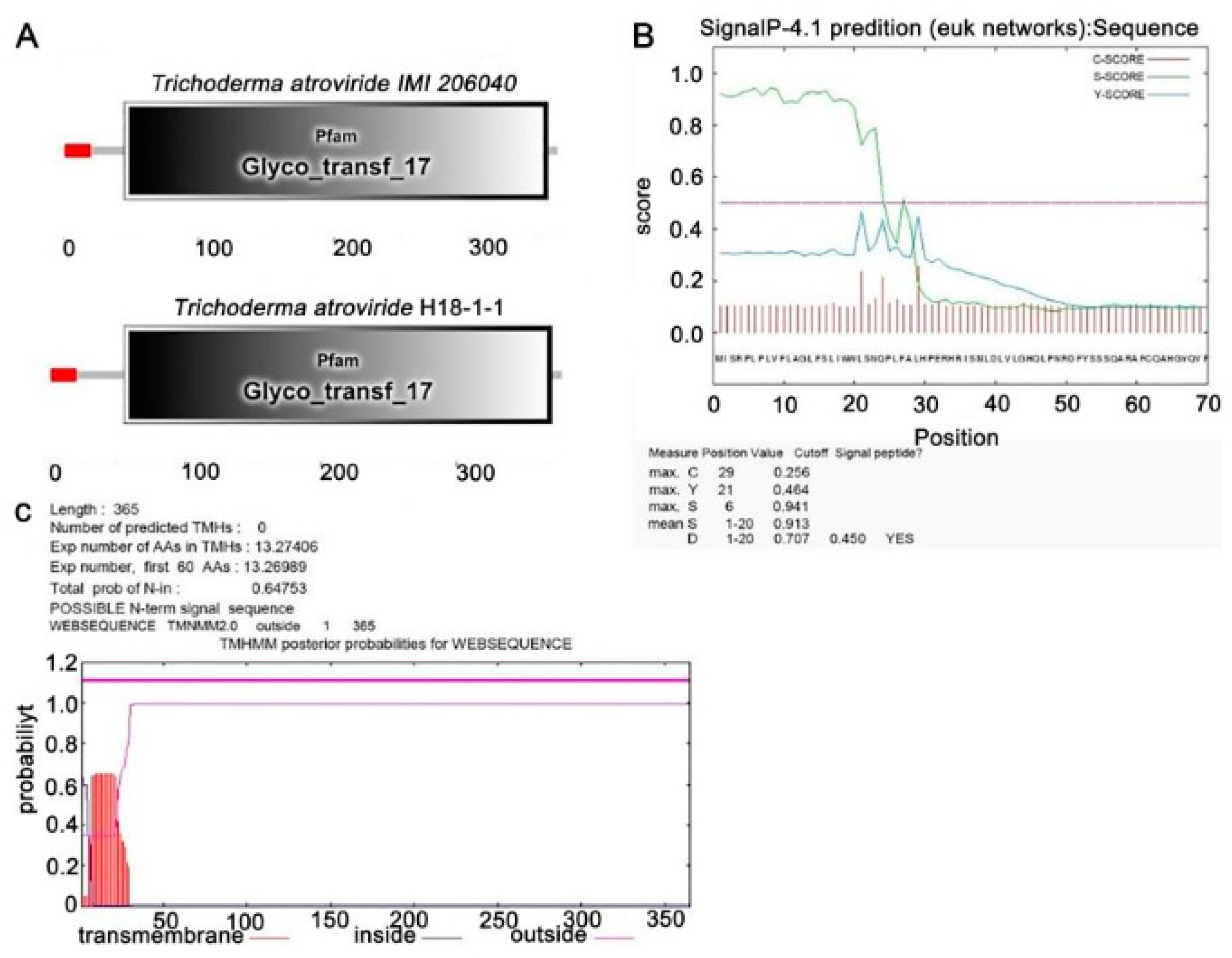
3.1.1. Identification of the Taugt17b1
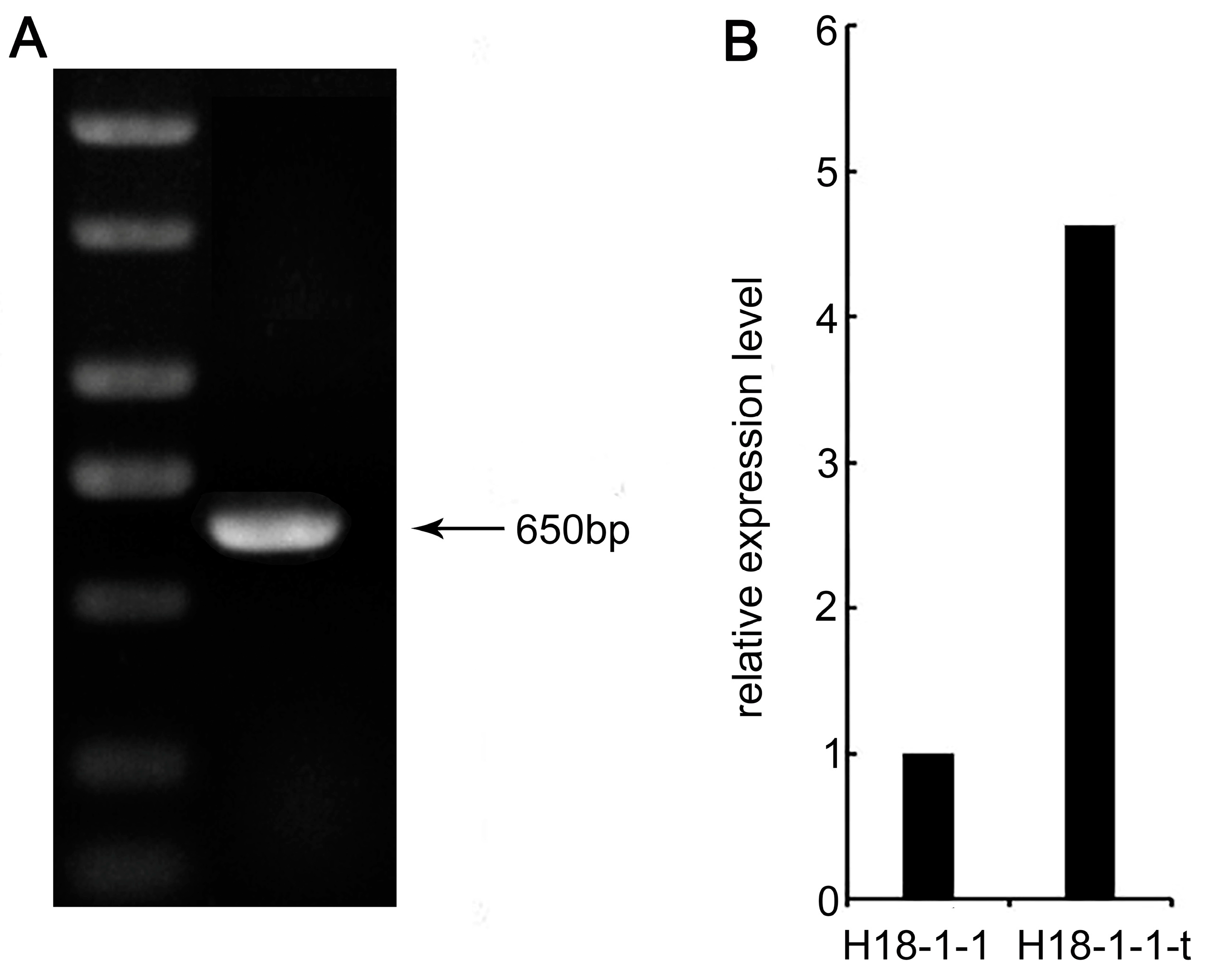
3.1.2. Taugt17b1 Overexpression Obtained Was Stably Inherited
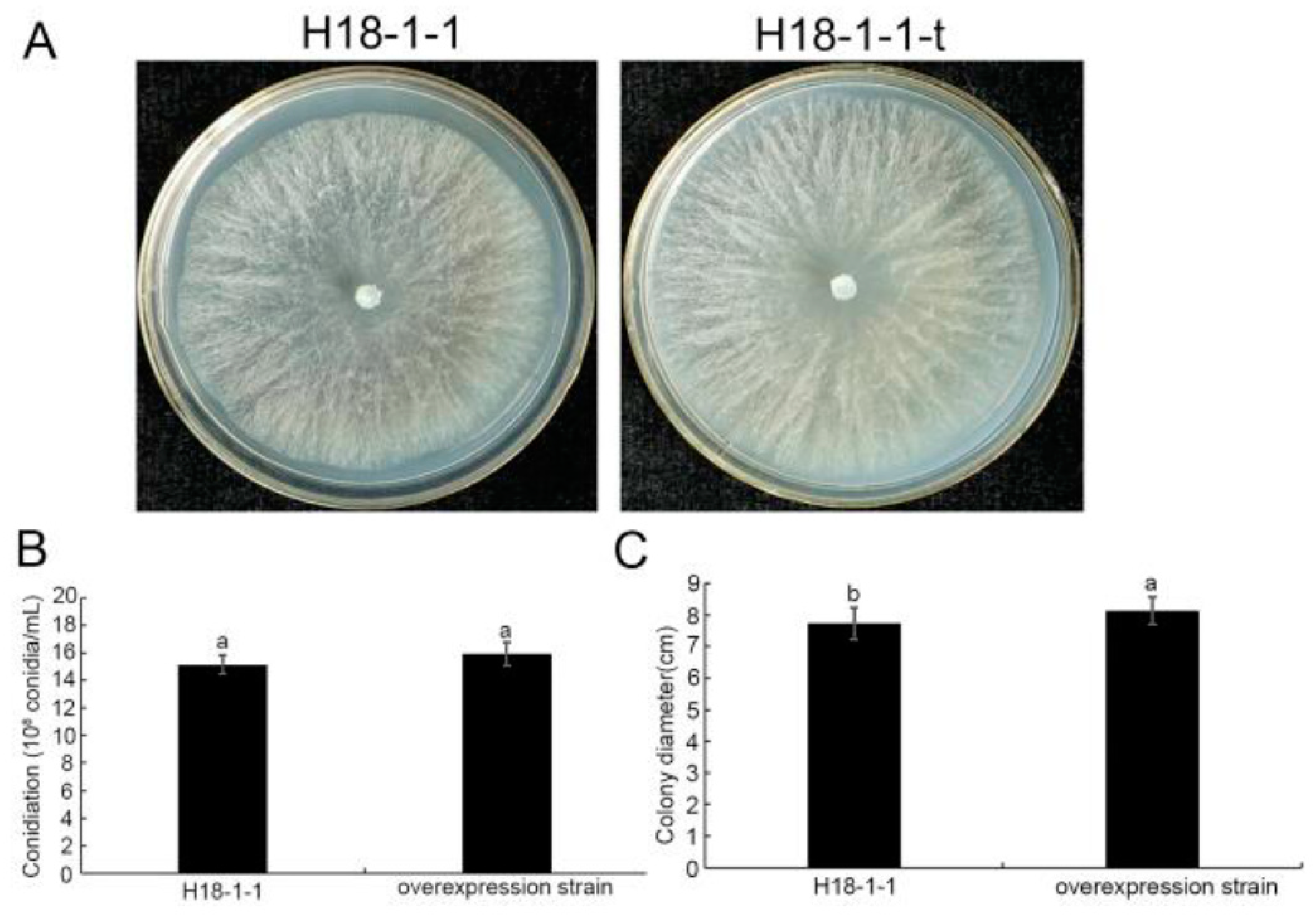
3.1.3. Taugt17b1 Overexpression Has No Effect on the Growth and Conidiation of T. atroviride
3.1.4. Antagonistic Effects of Strains H18-1-1 and H18-1-1-t on Phytopathogenic Fungi
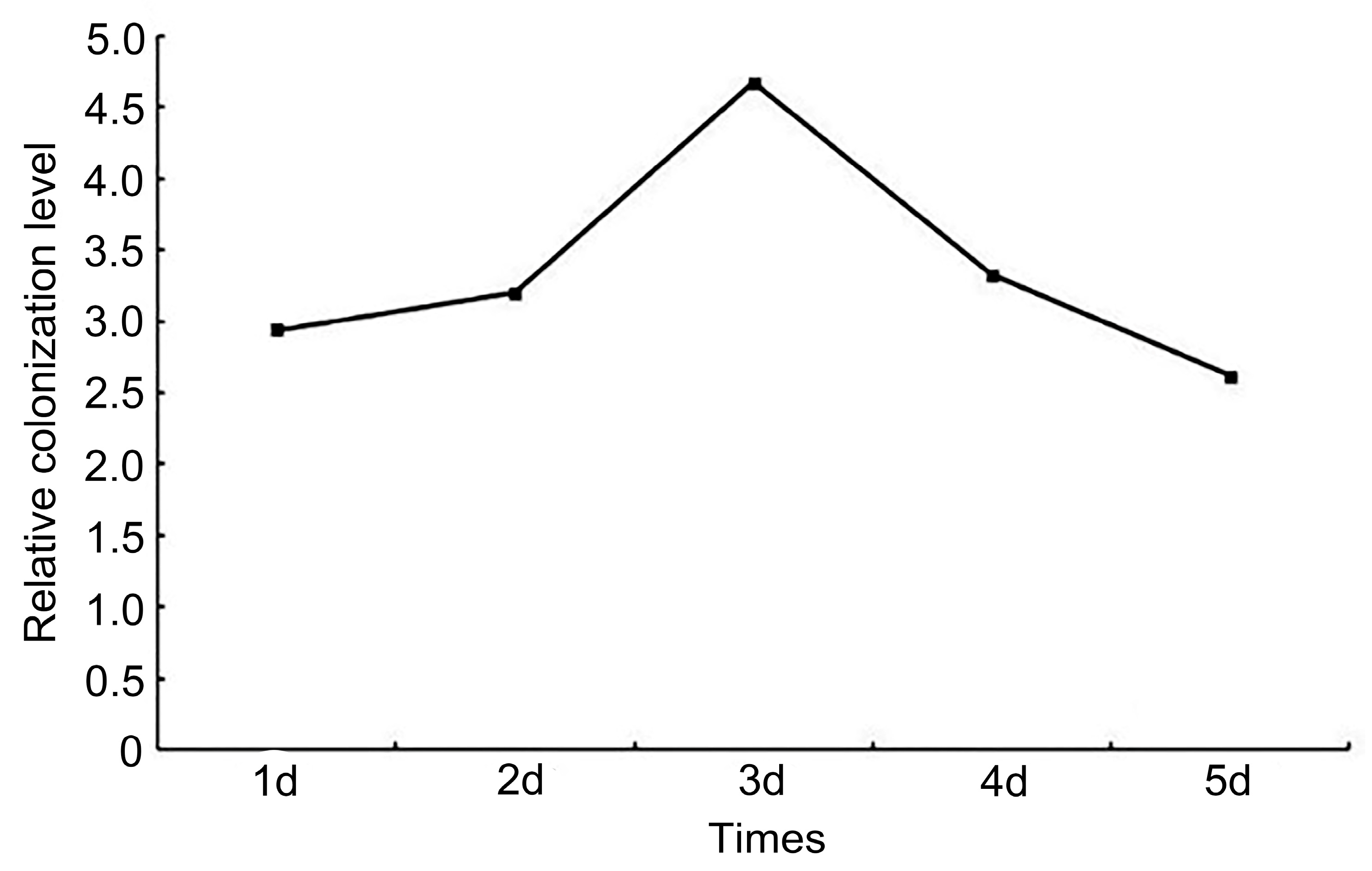
3.1.5. Overexpression of Taugt17b1 Affects Colonization Ability of H18-1-1 and Root Activity
3.1.6. Overexpression of the Taugt17b1 Improved Activity and Expression of Plant Defense Enzymes
3.1.7. The Control Effects of H18-1-1-t in Wheat Sharp Eyespot Was Better Than H18-1-1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Salas-Marina, M.A.; Isordia-Jasso, M.I.; Islas-Osuna, M.A.; Delgado-Sánchez, P.; Jiménez-Bremont, J.F.; Rodríguez-Kessler, M.; Rosales-Saavedra, M.T.; Herrera-Estrella, A.; Ecasas-Flores, S. The Epl1 and Sm1 proteins from Trichoderma atroviride and Trichoderma virens differentially modulate systemic disease resistance against different life style pathogens in Solanum lycopersicum. Front. Plant Sci. 2015, 6, 77. [Google Scholar] [CrossRef]
- Wiest, A.; Grzegorski, D.; Xu, B.W.; Goulard, C.; Rebuffat, S.; Ebbole, D.J.; Bodo, B.; Kenerley, C. Identification of peptaibols from Trichoderma virens and cloning of a peptaibol synthetase. J. Biol. Chem. 2002, 77, 20862–20868. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Baek, J.M.; Uribe, P.; Kenerley, C.M.; Cook, D.R. Cloning and characterization of multiple glycosyl hydrolase genes from Trichoderma virens. Curr. Genet. 2002, 40, 374–384. [Google Scholar] [CrossRef]
- Zhang, J.L.; Tang, W.L.; Huang, Q.R.; Li, Y.Z.; Wei, M.L.; Jiang, L.L.; Liu, C.; Yu, X.; Zhu, H.-W.; Chen, G.-Z.; et al. Trichoderma: A treasure house of structurally diverse secondary metabolites with medicinal importance. Front. Microbiol. 2021, 12, 723828. [Google Scholar] [CrossRef]
- Weindling, R. Trichoderma lignorum as a parasite of other soil fungi. Phytopathology 1932, 22, 837–845. [Google Scholar]
- Singh, A.; Shukla, N.; Kabadwal, B.C.; Tewari, A.K.; Kumar, J. Review on Plant-Trichoderma-Pathogen Interaction. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 2382–2397. [Google Scholar] [CrossRef]
- Liu, H.; Li, T.; Li, Y.; Wang, X.; Chen, J. Effects of Trichoderma atroviride SG3403 and Bacillus subtilis 22 on the biocontrol of wheat head blight. J. Fungi 2022, 8, 1250. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N.; Kapulnik, Y.; Chet, I. Induction and accumulation of PR proteins activity during early stages of root colonization by the mycoparasite Trichoderma harzianum strain T-203. Plant Physiol. Biochem. 2000, 38, 863–873. [Google Scholar] [CrossRef]
- Fan, L.L.; Fu, K.H.; Yu, C.J.; Li, Y.Y.; Li, Y.Q.; Chen, J. Thc6 protein, isolated from Trichoderma harzianum, can induce maize defense response against Curvularia lunata. J. Basic Microbiol. 2015, 55, 591–600. [Google Scholar] [CrossRef]
- Levy, N.O.; Harel, Y.M.; Haile, Z.M.; Elad, Y.; Rav-David, E.; Jurkevitch, E.; Jurkevitch, E.; Katan, J. Induced resistance to foliar diseases by soil solarization and Trichoderma harzianum. Plant Pathol. 2015, 64, 365–374. [Google Scholar] [CrossRef]
- Kuc, J. Concepts and direction of induced systemic resistance in plants and its application. Eur. J. Plant Pathol. 2001, 107, 7–12. [Google Scholar] [CrossRef]
- Kogel, K.H.; Langen, G. Induced disease resistance and gene expression in cereals. Cell. Microbiol. 2005, 7, 1555–1564. [Google Scholar] [CrossRef]
- Brotman, Y.; Lisec, J.; Méret, M.; Chet, I.; Willmitzer, L.; Viterbo, A. Transcript and metabolite analysis of the Trichoderma induced systemic resistance response to Pseudomonas syringae in Arabidopsis thaliana. Microbiology 2012, 158, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Cottier, F.; Mühlschlegel, F.A. Communication in fungi. Int. J. Microbiol. 2012, 2012, 351832. [Google Scholar] [CrossRef]
- Morán-Diez, E.; Hermosa, R.; Ambrosino, P.; Cardoza, R.E.; Gutiérrez, S.; Lorito, M.; Monte, E. The ThPG1 endopolygalacturonase is required for the Trichoderma harzianum plant beneficial interaction. Mol. Plant Microbe Interact. 2009, 22, 1021–1031. [Google Scholar] [CrossRef]
- Samolski, I.; Rincón, A.M.; Pinzón, L.M.; Viterbo, A.; Monte, E. The qid74 gene from Trichoderma harzianum has a role in root architecture and plant biofertilization. Microbiology 2012, 158, 129–138. [Google Scholar] [CrossRef]
- Lamdan, N.L.; Shalaby, S.; Ziv, T.; Kenerley, C.M.; Horwitz, B.A. Secretome of Trichoderma interacting with maize roots: Role in induced systemic resistance. Mol. Cell. Proteom. 2015, 14, 1054–1063. [Google Scholar] [CrossRef]
- Schmoll, M.; Dattenbock, C.; Carreras-Villasenor, N.; Mendoza-Mendoza, A.; Tisch, D.; Aleman, M.I.; Baker, S.E.; Brown, C.; Cervantes-Badillo, M.G.; Cetz-Chel, J.; et al. The genomes of three uneven siblings: Footprints of the lifestyles of three Trichoderma species. Microbiol. Mol. Biol. Rev. 2016, 80, 205–327. [Google Scholar] [CrossRef]
- Taniguchi, N.; Honke, K.; Fukuda, M. Handbook of Glycosyltransferases and Related Genes; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Breton, C.; Snajdrova, L.; Jeanneau, C.; Koca, J.; Imberty, A. Structures and mechanisms of glycosyltransferases. Glycobiology 2006, 16, 29R–37R. [Google Scholar] [CrossRef]
- Artemio, M.M.; Rinat, Z.; Robert, L.; Rosa, H.; Enrique, M.; Benjamin, A.; Mukherjee, P.K. Molecular dialogues between Trichoderma and roots: Role of the fungal secretome. Fungal Biol. 2018, 2, 62–85. [Google Scholar]
- Longa, C.M.; Savazzini, F.; Tosi, S.; Elad, Y.; Pertot, I. Evaluating the survival and environmental fate of the biocontrol agent Trichoderma atroviride SC1 in vineyards in northern Italy. J. Appl. Microb. 2009, 106, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yu, P.B.; Wang, Y.; Hou, W.X.; Yang, X.; Fan, J.L.; Wu, X.-H.; Lv, X.-L.; Zhang, N.; Zhao, L.; et al. Development of a rapid approach for detecting sharp eyespot resistance in seedling-stage wheat and its application in Chinese wheat cultivars. Plant Dis. 2020, 104, 1662–1667. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Herrera-Estrella, A.; Seidl-Seiboth, V.; Martinez, D.A.; Druzhinina, I.S.; Thon, M.; Zeilinger, S.; Casas-Flores, S.; Horwitz, B.A.; Mukherjee, P.K.; et al. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011, 12, R40. [Google Scholar] [CrossRef]
- Catlett, N.L.; Lee, B.N.; Yoder, O.C.; Turgeon, B.G. Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet. Rep. 2003, 50, 9–11. [Google Scholar] [CrossRef]
- Carrero-Carrón, I.; Trapero-Casas, J.L.; Olivares-García, C.; Monte, E.; Hermosa, R.; Jiménez-Díaz, R.M. Trichoderma asperellum is effective for biocontrol of verticillium wilt in olive caused by the defoliating pathotype of Verticillium dahliae. Crop Prot. 2016, 88, 45–52. [Google Scholar] [CrossRef]
- Hermosa, R.; Rubio, M.B.; Cardoza, R.E. The contribution of Trichoderma to balancing the costs of plant growth and defense. Int. Microbiol. 2013, 16, 69–80. [Google Scholar]
- Atanasova, L.; LeCrom, S.; Gruber, S. Comparative transcriptomics reveal different strategies of Trichoderma mycoparasitism. BMC Genom. 2013, 14, 121. [Google Scholar] [CrossRef]
- Meng, X.; Miao, Y.; Liu, Q.; Ma, L.; Guo, K.; Liu, D.; Ran, W.; Shen, Q. TgSWO from Trichoderma guizhouense NJAU4742 promotes growth in cucumber plants by modifying the root morphology and the cell wall architecture. Microb. Cell Factories 2019, 18, 148. [Google Scholar] [CrossRef]
- Montero-Barrientos, M.; Hermosa, R.; Cardoza, R.E.; Gutiérrez, S.; Nicolás, C.; Monte, E. Transgenic expression of the Trichoderma harzianum hsp70 gene increases Arabidopsis resistance to heat and other abiotic stresses. J. Plant Physiol. 2010, 167, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Hermosa, R.; Monte, E.; Nicolás, C. The Trichoderma harzianum Kelch protein ThKEL1 plays a key role in root colonization and the induction of systemic defense in Brassicaceae plants. Front. Plant Sci. 2019, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guzmán, P.; Alemán-Duarte, M.I.; Delaye, L.; Herrera-Estrella, A.; Olmedo-Monfil, V. Identification of effector-like proteins in Trichoderma spp. and role of a hydrophobin in the plant fungus interaction and mycoparasitism. BMC Genet. 2017, 18, 16. [Google Scholar] [CrossRef]
- Zhang, F.L.; Chen, C.; Zhang, F.; Gao, L.; Liu, J.D.; Chen, L.; Fan, X.; Liu, C.; Zhang, K.; He, Y.; et al. Trichoderma harzianum containing 1-aminocyclopropane-1-carboxylate deaminase and chitinase improved growth and diminished adverse effect caused by Fusarium oxysporum in soybean. J. Plant Physiol. 2017, 210, 84–94. [Google Scholar] [CrossRef]
- Dubey, S.C.; Suresh, M.; Singh, B. Evaluation of Trichoderma species against Fusarium oxysporum f. sp. ciceris for integrated management of chickpea wilt. Biol. Control 2007, 40, 118–127. [Google Scholar]
- Freeman, S.; Minz, D.; Kolesnik, I.; Barbul, O.; Zreibil, A.; Maymon, M.; Nitzani, Y.; Kirshner, B.; Rav-David, D.; Bilu, A.; et al. Trichoderma biocontrol of Colletotrichum acutatum and Botrytis cinerea, and survival in strawberry. Eur. J. Plant Pathol. 2004, 110, 361–370. [Google Scholar] [CrossRef]
- Vieira, P.M.; Santos, M.P.; Andrade, C.M.; Souza-Neto, O.A.; Ulhoa, C.J.; Aragão, F.J.L. Overexpression of an aquaglyceroporin gene from Trichoderma harzianum improves water-use efficiency and drought tolerance in Nicotiana tabacum. Plant Physiol. Biochem. 2017, 121, 38–47. [Google Scholar] [CrossRef]
- Xia, H.; Li, Y.Y.; Liu, Z.C.; Li, Y.Q.; Chen, J. Transgenic Expression of chit42 gene from Metarhizium anisopliae in Trichoderma harzianum enhances antagonistic activity against Botrytis cinerea. Mol. Biol. 2018, 52, 668–675. [Google Scholar] [CrossRef]


| G418 (μg/mL) | 0 | 10 | 15 | 20 | 25 | 30 |
|---|---|---|---|---|---|---|
| H18-1-1 | + | + | + | + | - | - |
| Treatment | Disease Index | Disease Prevention | Root Vigor (μg/g/h) |
|---|---|---|---|
| CK | 38.33a | 0 | 176.45b |
| H18-1-1 | 16.47b | 57.03 | 335.64a |
| Overexpression strain | 13.33b | 65.22 | 389.87a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, S.; Xue, X.; Zhang, R.; Zhang, L.; Yu, J. Taugt17b1 Overexpression in Trichoderma atroviride Enhances Its Ability to Colonize Roots and Induce Systemic Defense of Plants. Pathogens 2023, 12, 264. https://doi.org/10.3390/pathogens12020264
Chi S, Xue X, Zhang R, Zhang L, Yu J. Taugt17b1 Overexpression in Trichoderma atroviride Enhances Its Ability to Colonize Roots and Induce Systemic Defense of Plants. Pathogens. 2023; 12(2):264. https://doi.org/10.3390/pathogens12020264
Chicago/Turabian StyleChi, Shengqi, Xiaoyu Xue, Ronghuan Zhang, Li Zhang, and Jinfeng Yu. 2023. "Taugt17b1 Overexpression in Trichoderma atroviride Enhances Its Ability to Colonize Roots and Induce Systemic Defense of Plants" Pathogens 12, no. 2: 264. https://doi.org/10.3390/pathogens12020264
APA StyleChi, S., Xue, X., Zhang, R., Zhang, L., & Yu, J. (2023). Taugt17b1 Overexpression in Trichoderma atroviride Enhances Its Ability to Colonize Roots and Induce Systemic Defense of Plants. Pathogens, 12(2), 264. https://doi.org/10.3390/pathogens12020264





