Molecular Detection and Phylogenetic Analysis of Riemerella anatipestifer in Poultry and Wild Geese in Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and DNA Isolation
2.2. Detection of Riemerella anatipestifer DNA with PCR
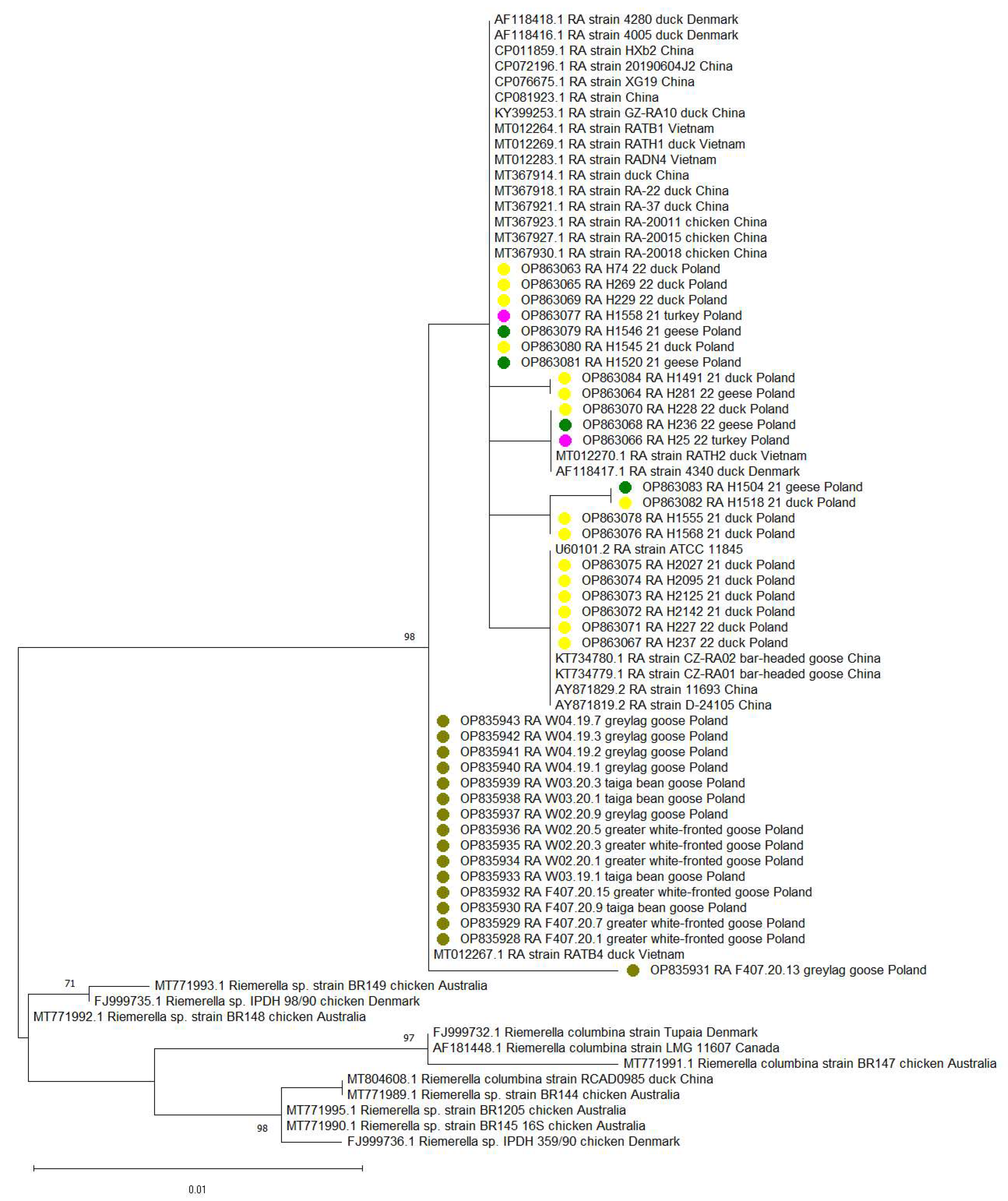
2.3. Sequencing and Phylogenetic Analysis
2.4. Statistical Analysis
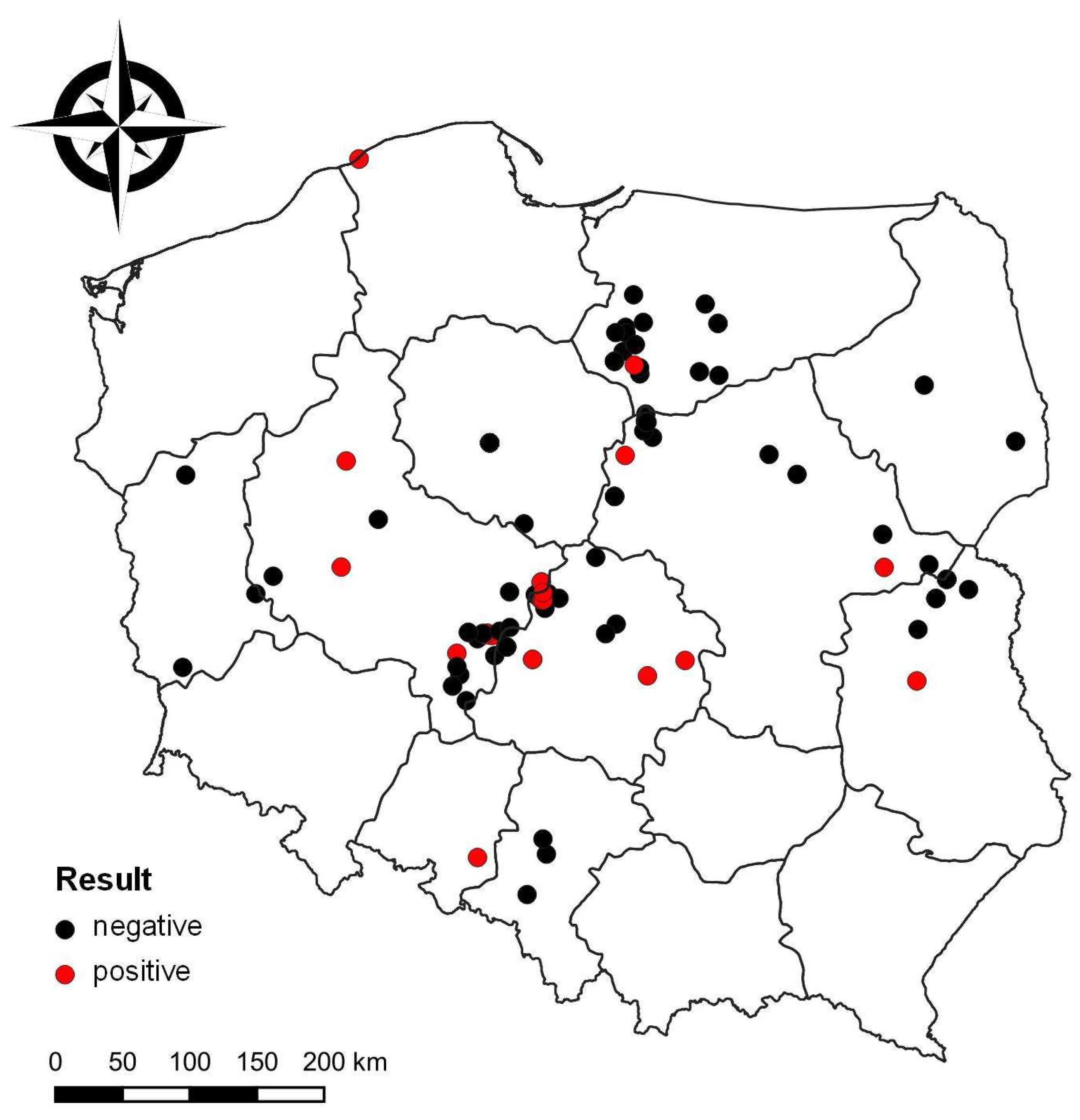
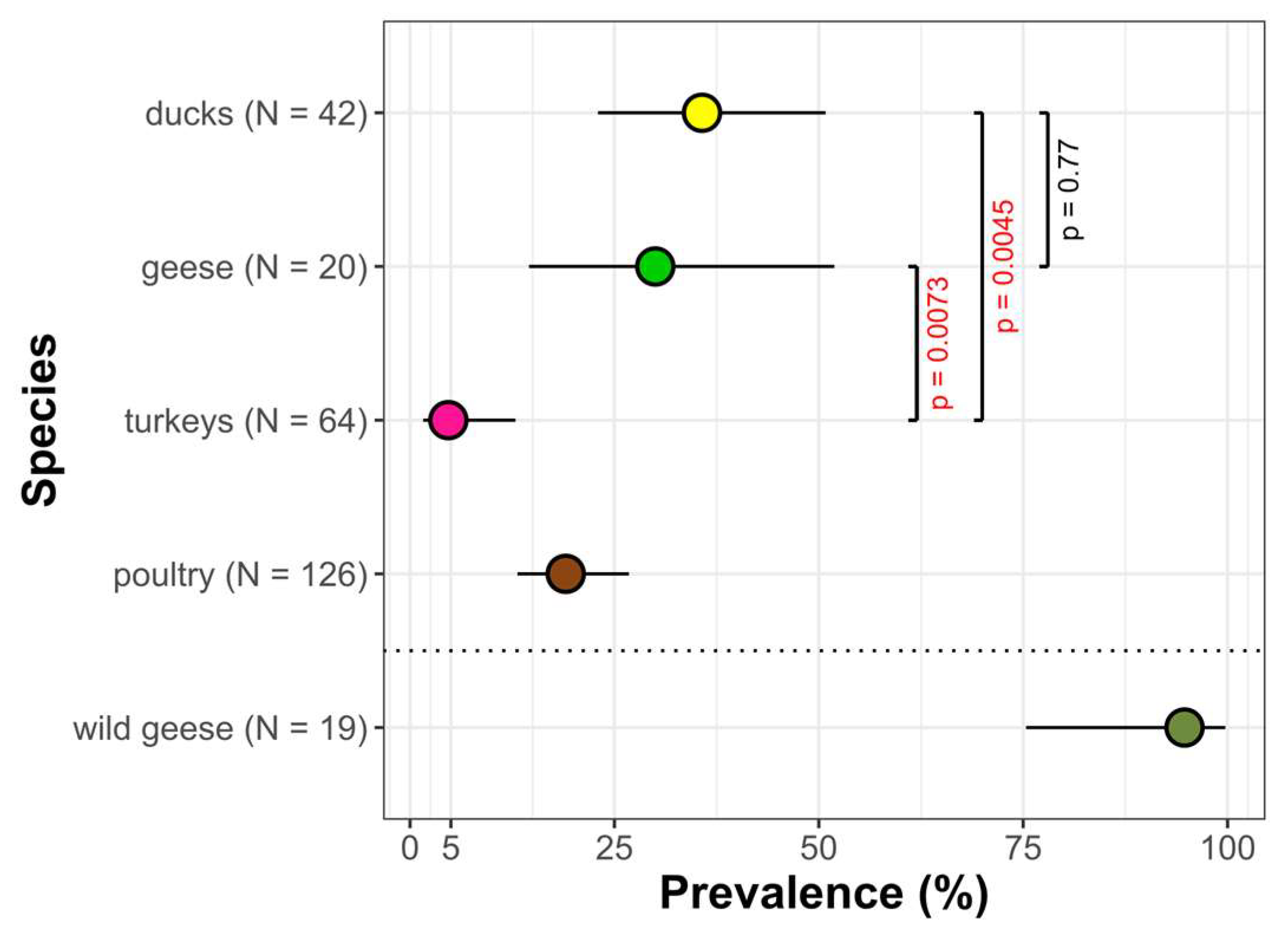
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segers, P.; Mannheim, W.; Vancanneyt, M.; De Brandt, K.; Hinz, K.-H.; Kersters, K.; Vandamme, P. Riemerella anatipestifer gen. nov., comb. nov., the causative agent of septicemia anserum exsudativa, and its phylogenetic affiliation within the Flavobacterium-Cytophaga rRNA homology group. Int. J. Syst. Bacteriol. 1993, 43, 768–776. [Google Scholar] [CrossRef]
- Ruiz, J.A.; Sandhu, T.S. Riemerella anatipestifer Infection. In Diseases of Poultry; Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., de Wit, S., Grimes, T., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 846–853. [Google Scholar]
- Glünder, G.; Hinz, K.-H. Isolation of Moraxella anatipestifer from embryonated goose eggs. Avian Pathol. 1989, 18, 351–355. [Google Scholar] [CrossRef]
- Hess, C.; Enichlmayr, H.; Jandreski-Cvetkovic, D.; Liebhart, D.; Bilic, I.; Hess, M. Riemerella anatipestifer outbreaks in commercial goose flocks and identification of isolates by MALDI-TOF mass spectrometry. Avian Pathol. 2013, 42, 151–156. [Google Scholar] [CrossRef]
- Cooper, G.L. Pasteurella anatipestifer infections in California turkey flocks: Circumstantial evidence of a mosquito vector. Avian Dis. 1989, 33, 809–815. [Google Scholar] [CrossRef]
- Chang, F.-F.; Chen, C.-C.; Wang, S.-H.; Chen, C.-L. Epidemiology and antibiogram of Riemerella anatipestifer isolated from waterfowl slaughterhouses in Taiwan. J. Vet. Res. 2019, 63, 79–86. [Google Scholar] [CrossRef]
- El-Rawy, E.M.; Khader, A.A.; Mahmoud, M.S.; Zaki, E.S.A.; Salma, S.S.; El-moneim, W.S.A.; Mwafy, A. Preparation and evaluation of combined oil adjuvant vaccine against duck pasteurellosis and Riemerella anatipestifer infection in ducks. Int. J. Vet. Sci. Anim. Husb. 2020, 5, 18–21. [Google Scholar]
- Molnar, S. Production and Trade of Duck Products in Global View. Ann. Polish Assoc. Agric. Agribus. Econ. 2017, XIX, 199–205. [Google Scholar] [CrossRef]
- Utnik-Banaś, K.; Żmija, J. The Geese market in Poland. Ann. Polish Assoc. Agric. Agribus. Econ. 2018, XX, 157–163. [Google Scholar] [CrossRef]
- Kuliś, M.; Przypaśniak, J.; Dach-Oleszek, R.W.I.; Tylkowska-Siek, A. Statistics Poland, Agriculture Department, Farm animals in 2021. 2022. Available online: https://stat.gov.pl/ (accessed on 2 December 2022).
- Śmietanka, K.; Świętoń, E.; Wyrostek, K.; Kozak, E.; Tarasiuk, K.; Styś-Fijoł, N.; Dziadek, K.; Niemczuk, K. Highly pathogenic avian influenza H5Nx in Poland in 2020/2021: A descriptive epidemiological study of a large-scale epidemic. J. Vet. Res. 2022, 66, 1–7. [Google Scholar] [CrossRef]
- Śmietanka, K.; Swiȩtoń, E.; Kozak, E.; Wyrostek, K.; Tarasiuk, K.; Tomczyk, G.; Konopka, B.; Welz, M.; Domańska-Blicharz, K.; Niemczuk, K. Highly pathogenic avian influenza H5N8 in Poland in 2019-2020. J. Vet. Res. 2020, 64, 469–476. [Google Scholar] [CrossRef]
- Rubbenstroth, D.; Ryll, M.; Behr, K.; Rautenschlein, S. Pathogenesis of Riemerella anatipestifer in turkeys after experimental mono-infection via respiratory routes or dual infection together with the avian metapneumovirus. Avian Pathol. 2009, 38, 497–507. [Google Scholar] [CrossRef]
- Banda, A.; Galloway-Haskins, R.I.; Sandhu, T.S.; Schat, K.A. Genetic analysis of a duck circovirus detected in commercial Pekin ducks in New York. Avian Dis. 2007, 51, 90–95. [Google Scholar] [CrossRef]
- Shawky, S.; Sandhu, T.; Shivaprasad, H.L. Pathogenicity of a low-virulence duck virus enteritis isolate with apparent immunosuppressive ability. Avian Dis. 2000, 44, 590–599. [Google Scholar] [CrossRef]
- Soike, D.; Köhler, B.; Albrecht, K. A circovirus-like infection in geese related to a runting syndrome. Avian Pathol. 1999, 28, 199–202. [Google Scholar] [CrossRef]
- Tsai, H.; Liu, Y.; Tseng, C.; Pan, M. Genetic variation of the ompA and 16S rRNA genes of Riemerella anatipestifer. Avian Pathol. 2005, 34, 55–64. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Found. Stat. Comput. Vienna, Austria. 2022. Available online: https://www.r-project.org/ (accessed on 30 November 2022).
- Hervé, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R Package Version 0.9-81-2. Available online: https://cran.r-project.org/web/packages/RVAideMemoire/index.html (accessed on 7 December 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–260. [Google Scholar]
- QGIS Development Team QGIS Geographic Information System. Open Source Geospatial Foundation Project. Version 3.28.0. Available online: https://qgis.org/ (accessed on 25 November 2022).
- Chikuba, T.; Uehara, H.; Fumikura, S.; Takahashi, K.; Suzuki, Y.; Hoshinoo, K.; Yamamoto, Y. Riemerella anatipestifer infection in domestic ducks in Japan, 2014. J. Vet. Med. Sci. 2016, 78, 1635–1638. [Google Scholar] [CrossRef]
- Doley, M.K.; Das, S.; Sharma, R.K.; Borah, P.; Sarma, D.K.; Buragohain, L.; Hazarika, R.; Baruah, N. Isolation and molecular characterization of Riemerella anatipestifer from domesticated ducks of Assam, India. Indian J. Anim. Res. 2021, I, 1–7. [Google Scholar] [CrossRef]
- Phonvisay, M.; Liou, J.-J.; Cheng, L.-T.; Chen, Y.-P.; Wu, H.-C.; Liu, C.-H.; Lee, J.-W.; Chu, C.-Y. Survey of a Riemerella anatipestifer outbreak in Southern Taiwan duck farms. Taiwan Vet. J. 2017, 43, 165–170. [Google Scholar] [CrossRef]
- Sarker, R.R.; Rahman, M.S.; Haque, M.E.; Rima, U.K.; Hossain, M.Z.; Barman, B.C.; Mahna, K. Identification of ribonuclease Z gene from an outbreak of Riemerella anatipestifer infection in ducks of Bangladesh. Poult. Fish. Wildl. Sci. 2017, 5, 185. [Google Scholar]
- Gyuris, É.; Nemes, C.; Magyar, T. Data on the epidemiology and pathology of anatipestifer disease in Hungary (2010–2014). Acta Vet. Hung. 2018, 66, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Tzora, A.; Skoufos, S.; Bonos, E.; Fotou, K.; Karamoutsios, A.; Nelli, A.; Giannenas, I.; Tsinas, A.; Skoufos, I. Identification by MALDI-TOF MS and antibiotic resistance of Riemerella anatipestifer, isolated from a clinical case in commercial broiler chickens. Vet. Sci. 2021, 8, 29. [Google Scholar] [CrossRef]
- Lozica, L.; Mazić, M.; Gottstein, Ž. A case study of a Riemerella anatipestifer infection on a commercial turkey farm in Croatia. Europ. Poult. Sci. 2021, 85, 1–7. [Google Scholar] [CrossRef]
- Hasan, A.; Bose, P.; Aktar, M.T.; Haque, Z.F.; Islam, M.R.; Hossain, M.T.; Siddique, M.P. Molecular Detection and Antibiogram Profile of Riemerella anatipestifer in Duck Farming Areas of Bangladesh (PREPRINT Version 1). Available online: https://europepmc.org/article/ppr/ppr509478 (accessed on 11 December 2022).
- Donahue, J.M.; Olson, L.D. Survey of wild ducks and geese for Pasteurella spp. Bull. Wildl. Dis. Assoc. 1969, 5, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Karstad, L.; Lusis, P.; Long, J.R. Pasteurella anatipestifer as a cause of mortality in captive wild waterfowl. J. Wildl. Dis. 1970, 6, 408–413. [Google Scholar] [CrossRef]
- Munday, B.L.; Corbould, A.; Heddleston, K.L.; Harry, E.G. Isolation of Pasteurella anatipestifer from black swan (Cygnus atratus). Aust. Vet. J. 1970, 46, 322–325. [Google Scholar] [CrossRef]
- Hinz, K.H.; Ryll, M.; Köhler, B.; Glünder, G. Phenotypic characteristics of Riemerella anatipestifer and similar micro-organisms from various hosts. Avian Pathol. 1998, 27, 33–42. [Google Scholar] [CrossRef]
- Cha, S.; Seo, H.; Wei, B.; Kang, M.; Roh, J.; Yoon, R.; Kim, J.; Jang, H. Surveillance and characterization of Riemerella anatipestifer from wild birds in South Korea. J. Wildl. Dis. 2015, 51, 341–347. [Google Scholar] [CrossRef]
- Prüter, H.; Czirják, G.Á.; Twietmeyer, S.; Harder, T.; Grund, C.; Mühldorfer, K.; Lüschow, D. Sane and sound: A serologic and molecular survey for selected infectious agents in neozootic Egyptian geese (Alopochen aegyptiacus) in Germany. Eur. J. Wildl. Res. 2018, 64, 71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawicka-Durkalec, A.; Tomczyk, G.; Gerilovych, I.; Kursa, O. Molecular Detection and Phylogenetic Analysis of Riemerella anatipestifer in Poultry and Wild Geese in Poland. Pathogens 2023, 12, 256. https://doi.org/10.3390/pathogens12020256
Sawicka-Durkalec A, Tomczyk G, Gerilovych I, Kursa O. Molecular Detection and Phylogenetic Analysis of Riemerella anatipestifer in Poultry and Wild Geese in Poland. Pathogens. 2023; 12(2):256. https://doi.org/10.3390/pathogens12020256
Chicago/Turabian StyleSawicka-Durkalec, Anna, Grzegorz Tomczyk, Iryna Gerilovych, and Olimpia Kursa. 2023. "Molecular Detection and Phylogenetic Analysis of Riemerella anatipestifer in Poultry and Wild Geese in Poland" Pathogens 12, no. 2: 256. https://doi.org/10.3390/pathogens12020256
APA StyleSawicka-Durkalec, A., Tomczyk, G., Gerilovych, I., & Kursa, O. (2023). Molecular Detection and Phylogenetic Analysis of Riemerella anatipestifer in Poultry and Wild Geese in Poland. Pathogens, 12(2), 256. https://doi.org/10.3390/pathogens12020256




