Surveillance of Daughter Micronodule Formation Is a Key Factor for Vaccine Evaluation Using Experimental Infection Models of Tuberculosis in Macaques
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
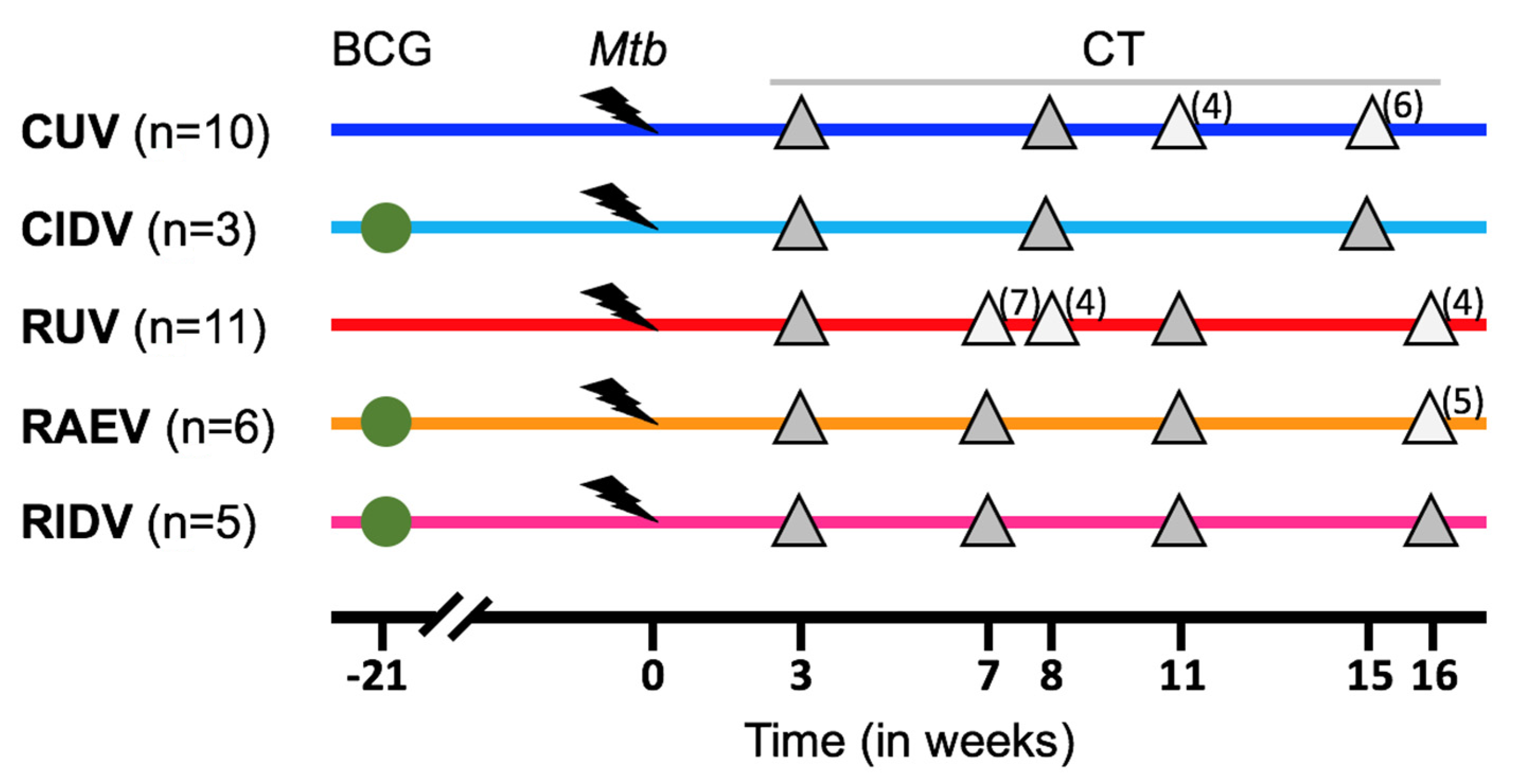
2.2. Experimental Groups
2.3. CT Images
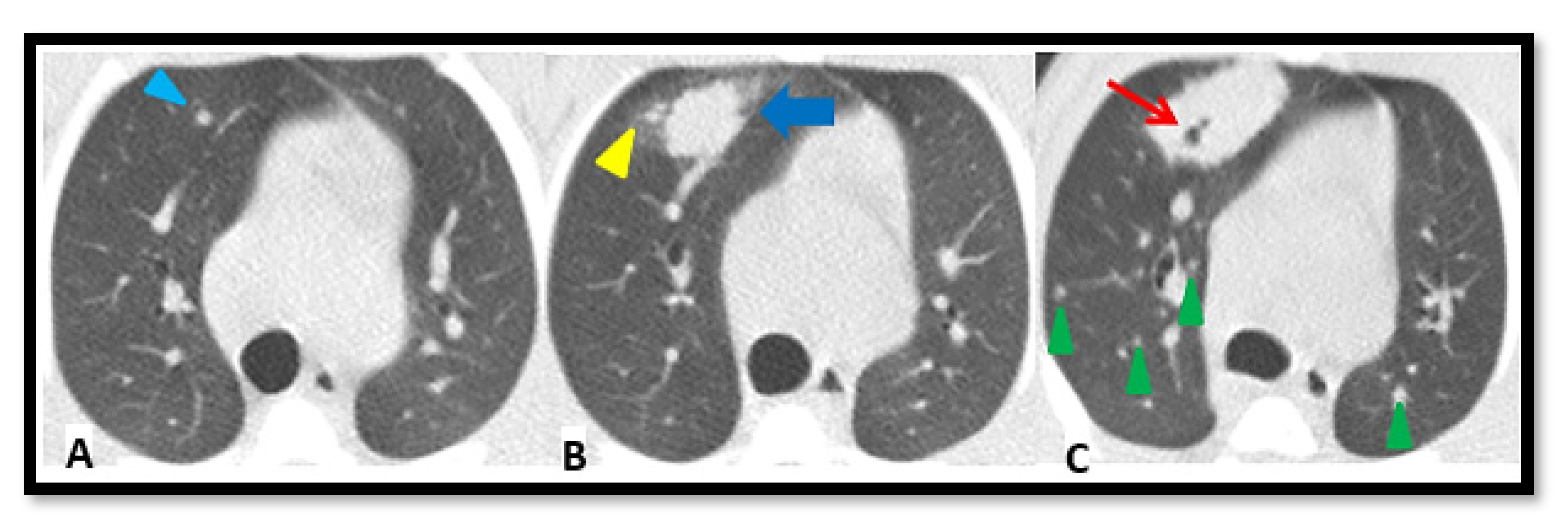
2.4. TB Lung Lesions Analysis from CT Images
- A.
- Micronodule is a small solid lung nodule with smooth margins.
- B.
- Consolidation (also referred to as consolidated lesion) is a pneumonic patch or necrotizing consolidative process that occupies and even destroys alveoli, and it is described in CT images as a soft tissue lesion within lung parenchyma showing irregular margins.
- C.
- D.
- Pleural distance is defined as the distance between the margin of each consolidation to the closest pleura or fissure.
2.5. Statistical Assessment
3. Results
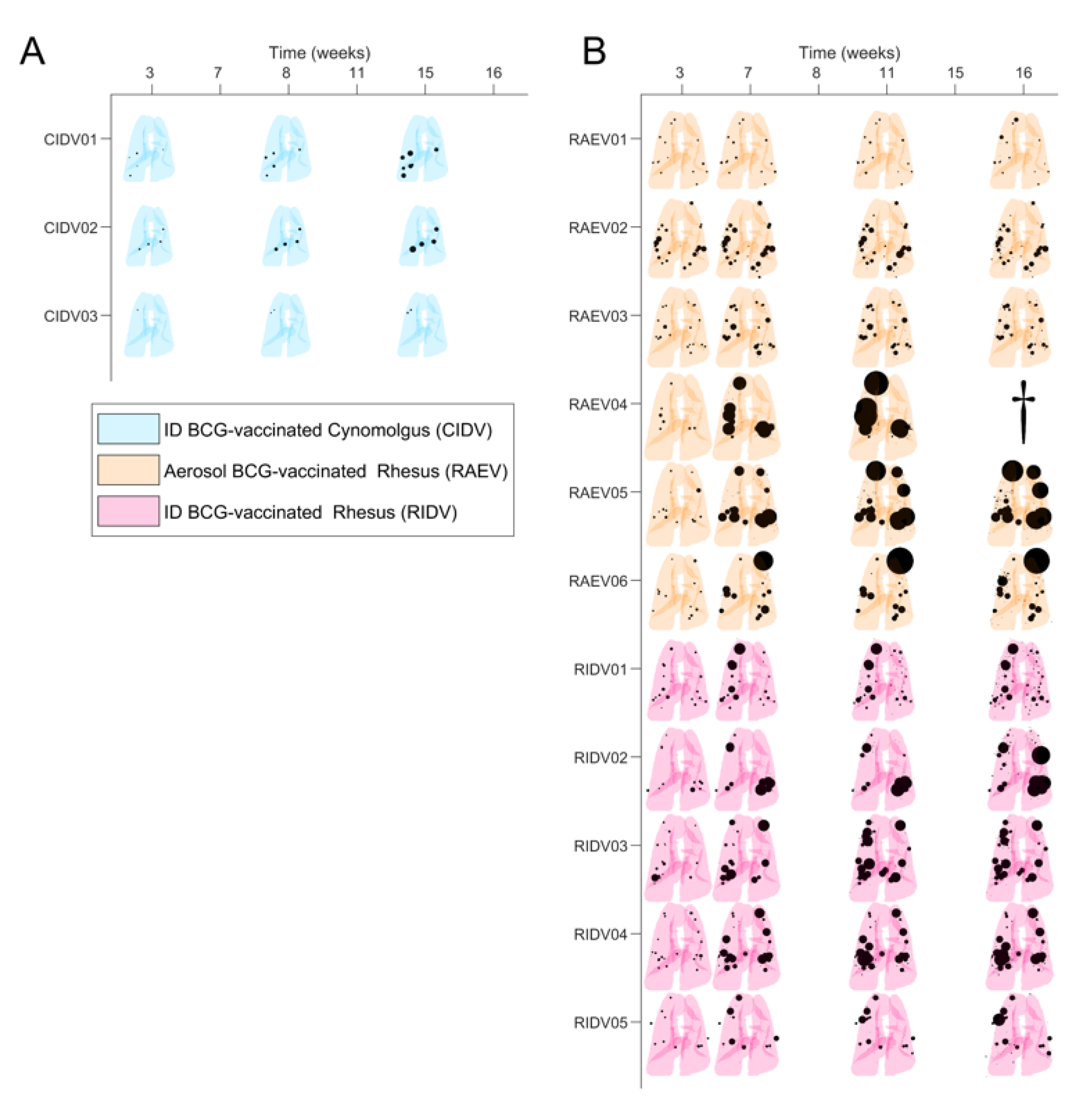
3.1. Preliminary Images’ Assessment and Anatomy
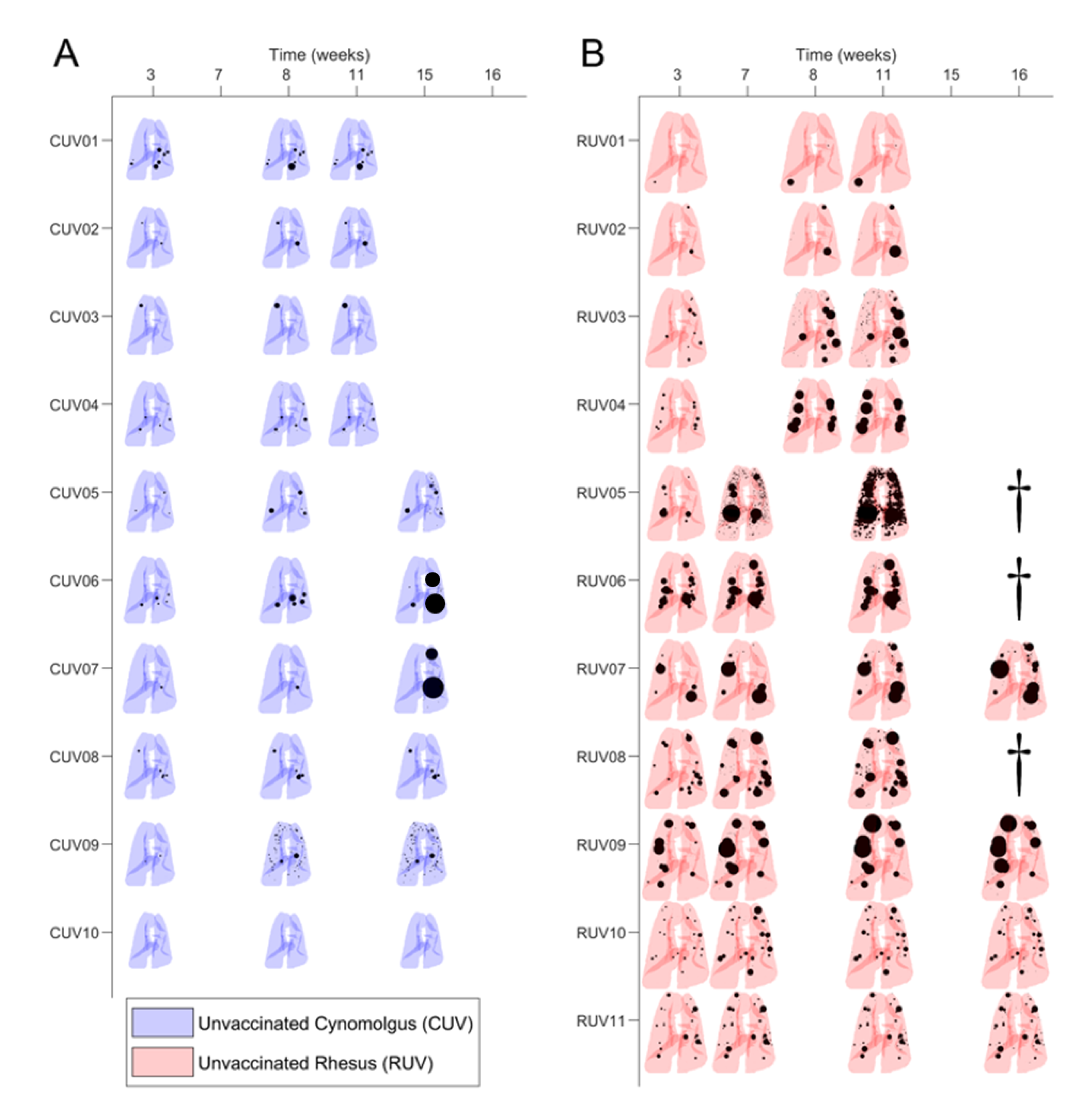
3.2. Mtb-Induced Disease Is Worse in Rhesus Macaques
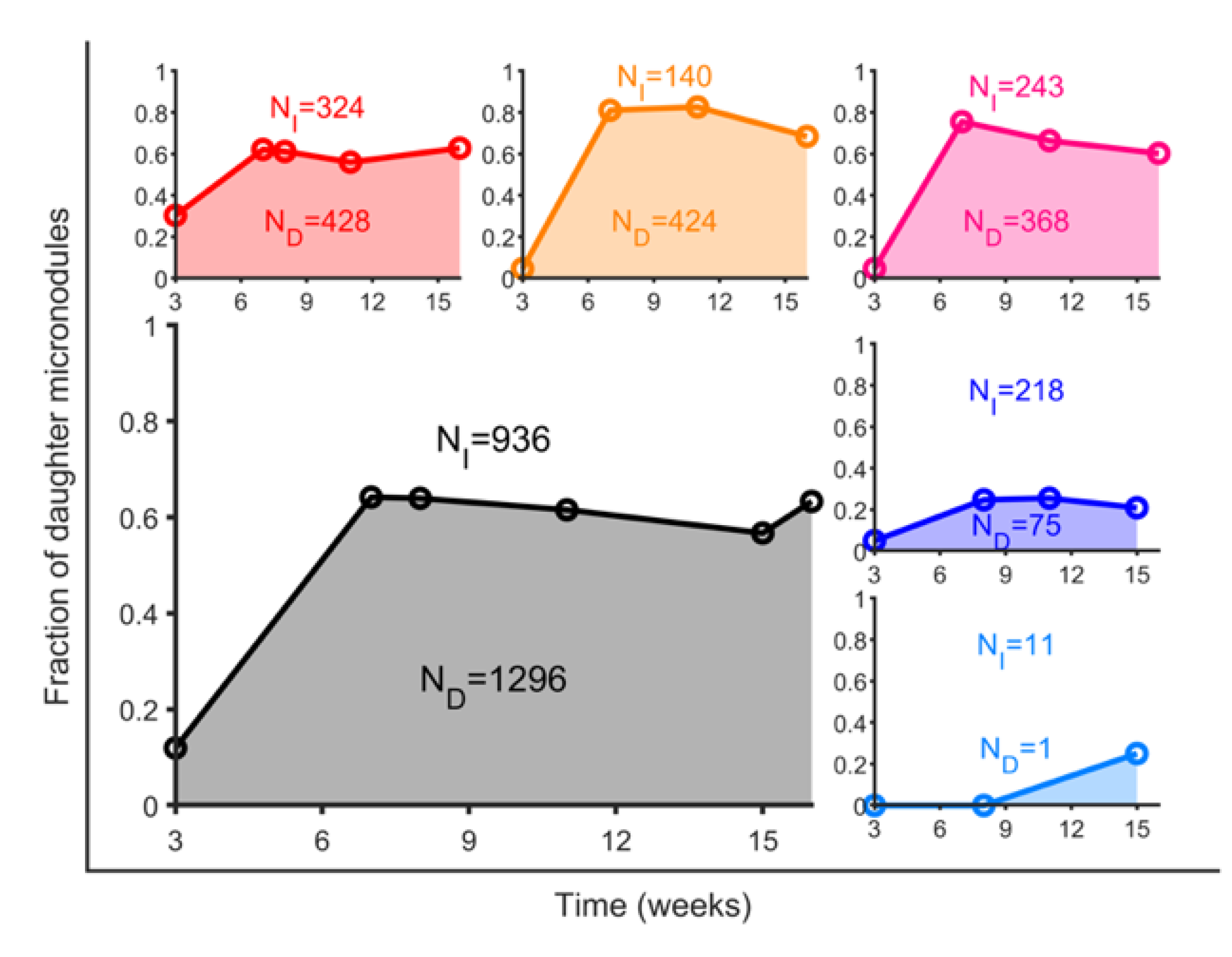
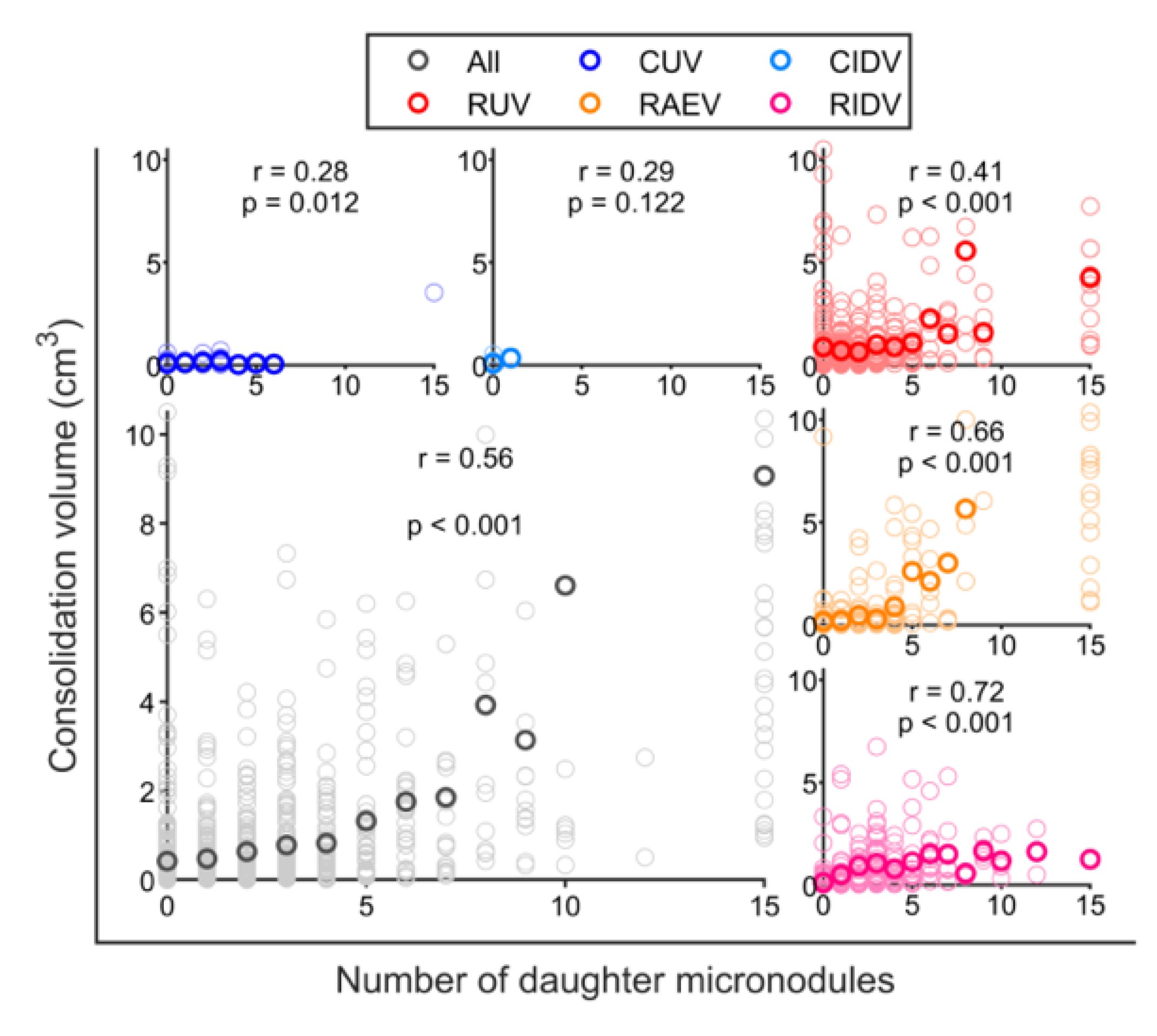
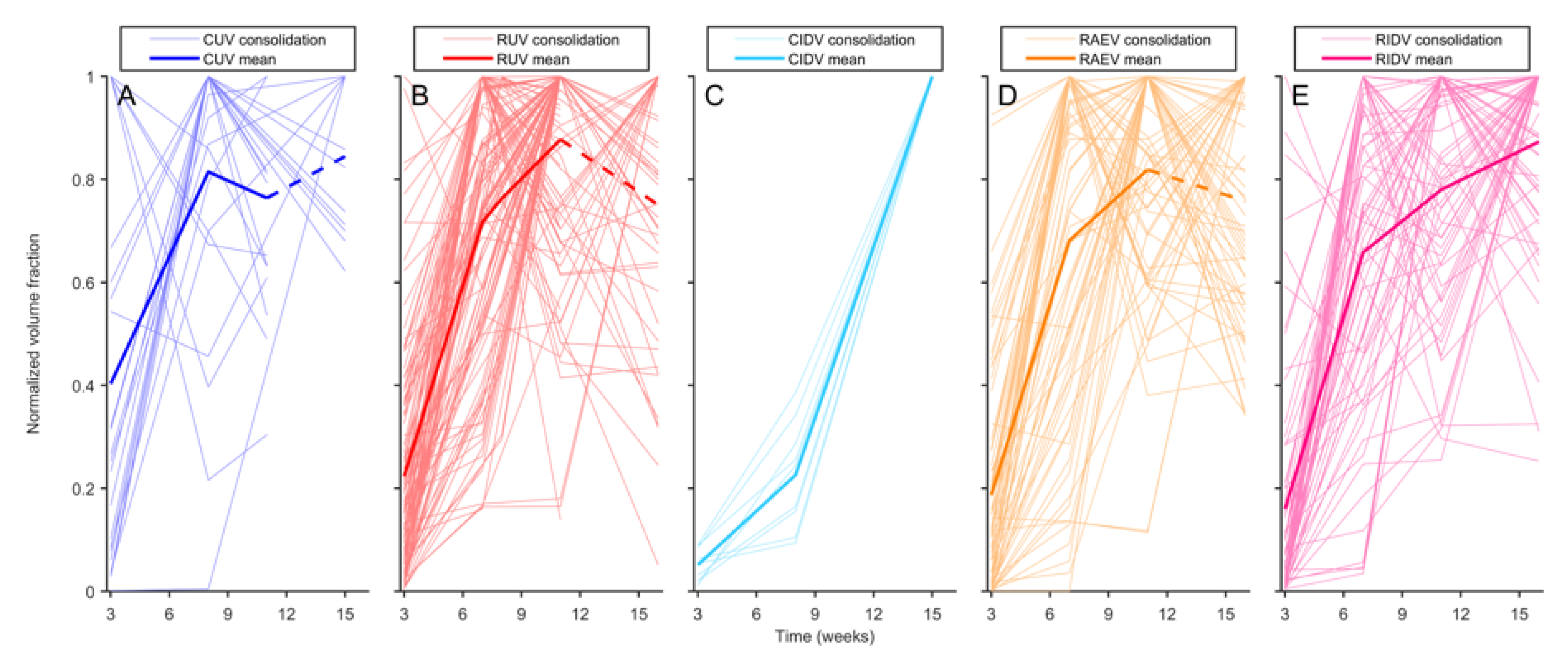
3.3. BCG Vaccination in Rhesus Macaques Reduces the Number of Lesions, Although Intradermal Vaccination Better Controls Daughter Micronodules
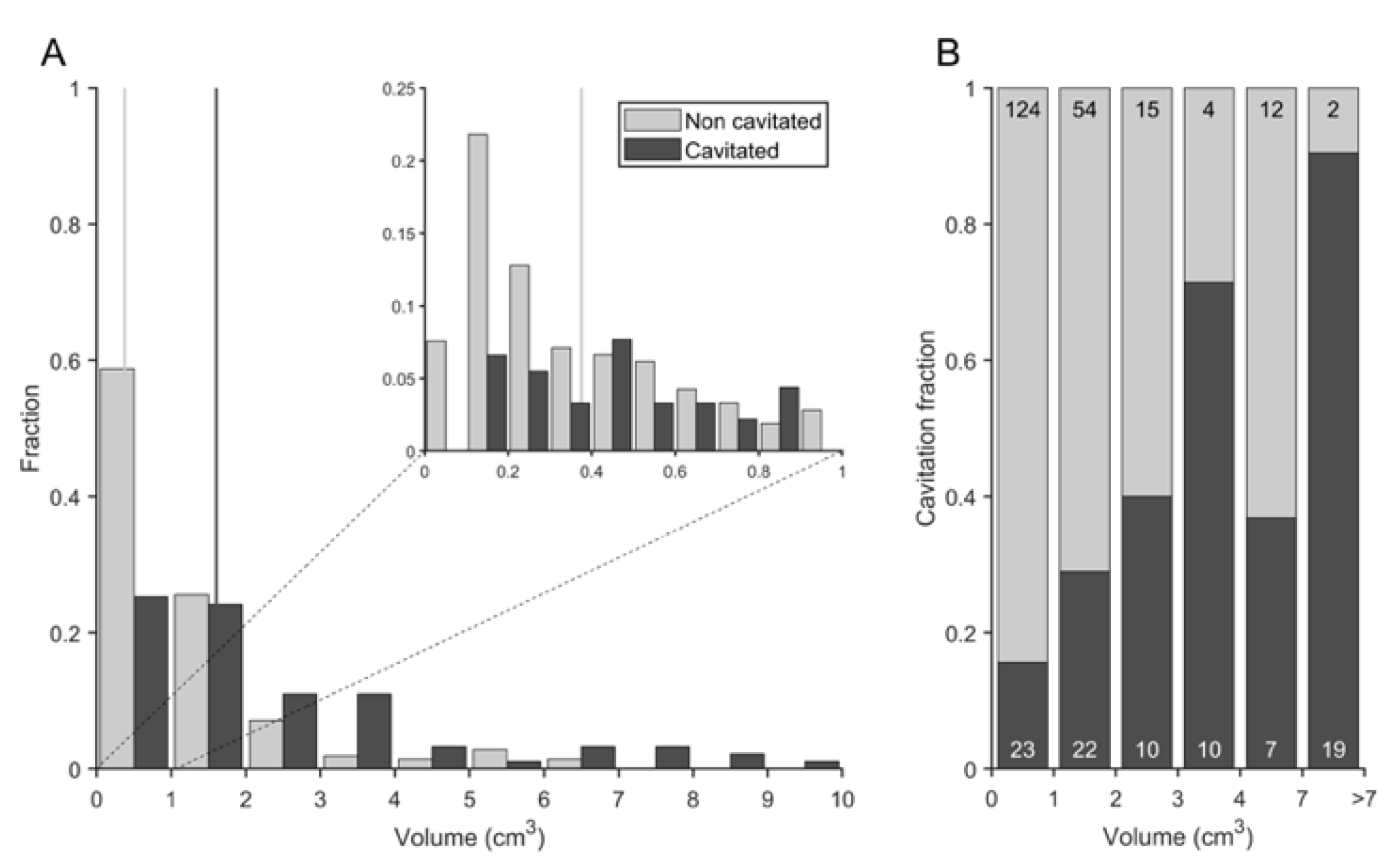
3.4. Cavitation Is More Probable in Larger Lesions and Contact with Pleura Appear to Impact on TB Progression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- World Health Organization. Impact of the COVID-19 Pandemic on TB Detection and Mortality in 2020; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Pai, M.; Kasaeva, T.; Swaninathan, S. COVID-19’s devastating effect on tuberculosis care—A path to recovery. N. Engl. J. Med. 2022, 386, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. End TB Strategy; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Cardona, P.J. What we have learned and what we have missed in tuberculosis pathophysiology for a new vaccine design: Searching for the “Pink Swan”. Front. Immunol. 2017, 8, 556. [Google Scholar] [CrossRef]
- Wallis, R.S.; Maeurer, M.; Mwaba, P.; Chakaya, J.; Rustomjee, R.; Migliori, G.B.; Marais, B.; Schito, M.; Churchyard, G.; Swaminathan, S.; et al. Tuberculosis-advances in development of new drugs, treatment regimens, host-directed therapies, and biomarkers. Lancet Infect. Dis. 2016, 16, e34–e46. [Google Scholar] [CrossRef] [PubMed]
- Urbanowski, M.E.; Ordonez, A.A.; Ruiz-Bedoya, C.A.; Jain, S.K.; Bishai, W.R. Cavitary tuberculosis: The gateway of disease transmission. Lancet Infect. Dis. 2020, 20, e117–e128. [Google Scholar] [CrossRef]
- Ihms, E.A.; Urbanowski, M.E.; Bishai, W.R. Diverse cavity types and evidence that mechanical action on the necrotic granuloma drives tuberculous cavitation. Am. J. Pathol. 2018, 188, 1666–1675. [Google Scholar] [CrossRef]
- Gil, O.; Díaz, I.; Vilaplana, C.; Tapia, G.; Díaz, J.; Fort, M.; Cáceres, N.; Pinto, S.; Caylà, J.; Corner, L.; et al. Granuloma encapsulation is a key factor for containing tuberculosis infection in minipigs. PLoS ONE 2010, 5, e10030. [Google Scholar] [CrossRef]
- Wong, E.A.; Joslyn, L.; Grant, N.L.; Klein, E.; Lin, P.L.; Kirschner, D.E.; Flynn, J.A.L. Low levels of T cell exhaustion in tuberculous lung granulomas. Infect. Immun. 2018, 86, e00426-18. [Google Scholar] [CrossRef]
- Lin, P.L.; Coleman, T.; Carney, J.P.J.; Lopresti, B.J.; Tomko, J.; Fillmore, D.; Dartois, V.; Scanga, C.; Frye, L.J.; Janssen, C.; et al. Radiologic responses in cynomolgus macaques for assessing tuberculosis chemotherapy regimens. Antimicrob. Agents Chemother. 2013, 57, 4237–4244. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 2012, 12, 352–366. [Google Scholar] [CrossRef]
- White, A.D.; Sibley, L.; Gullick, J.; Sarfas, C.; Clark, S.; Fagrouch, Z.; Verschoor, E.; Salguero, F.J.; Dennis, M.; Sharpe, S. TB and SIV coinfection; a model for evaluating vaccine strategies against TB reactivation in asian origin cynomolgus macaques: A pilot study using BCG vaccination. Vaccines 2021, 9, 945. [Google Scholar] [CrossRef]
- Sharpe, S.; White, A.; Gleeson, F.; McIntyre, A.; Smyth, D.; Clark, S.; Sarfas, C.; Laddy, D.; Rayner, E.; Hall, G.; et al. Ultra-low dose aerosol challenge with Mycobacterium tuberculosis leads to divergent outcomes in rhesus and cynomolgus macaques. Tuberculosis 2016, 96, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.L.; Rodgers, M.; Smith, L.; Bigbee, M.; Myers, A.; Bigbee, C.; Chiosea, I.; Capuano, S.V.; Fuhrman, C.; Klein, E.; et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect. Immun. 2009, 77, 4631–4642. [Google Scholar] [CrossRef]
- Lin, P.L.; Flynn, J.L. The end of the binary era: Revisiting the spectrum of tuberculosis. J. Immunol. 2018, 201, 2541–2548. [Google Scholar] [CrossRef]
- Zwerling, A.; Behr, M.A.; Verma, A.; Brewer, T.F.; Menzies, D.; Pai, M. The BCG world atlas: A database of global BCG vaccination policies and practices. PLoS Med. 2011, 8, e1001012. [Google Scholar] [CrossRef] [PubMed]
- Lancione, S.; Alvarez, J.V.; Alsdurf, H.; Pai, M.; Zwerling, A.A. Tracking changes in national BCG vaccination policies and practices using the BCG world atlas. BMJ Glob Health 2022, 7, e007462. [Google Scholar] [CrossRef]
- Abubakar, I.; Pimpin, L.; Ariti, C.; Beynon, R.; Mangtani, P.; Sterne, J.; Fine, P.; Smith, P.; Lipman, M.; Elliman, D.; et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guérin vaccination against tuberculosis. Health Technol. Assess. 2013, 17, 1–372. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Diwan, V.K.; Wheeler, J.G. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: A meta-analysis. Int. J. Epidemiol. 1993, 22, 1154–1158. [Google Scholar] [CrossRef]
- Dockrell, H.M.; Smith, S.G. What have we learnt about BCG vaccination in the last 20 years? Front. Immunol. 2017, 13, 1134. [Google Scholar] [CrossRef]
- Trunz, B.B.; Fine, P.; Dye, C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet 2006, 367, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A.; Brewer, T.F.; Berkey, C.S.; Wilson, M.E.; Burdick, E.; Fineberg, H.V.; Mosteller, F. Efficacy of BCG vaccine in the prevention of tuberculosis: Meta-analysis of the published literature. JAMA 1994, 271, 698–702. [Google Scholar] [CrossRef]
- Sutherland, I.; Lindgren, I. The protective effect of BCG vaccination as indicated by autopsy studies. Tubercle. 1979, 60, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E.; Weiner, J.; von Reyn, C.F. Novel approaches to tuberculosis vaccine development. Int. J. Infect. Dis. 2017, 56, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Whitlow, E.; Mustafa, A.S.; Hanif, S.N.M. An overview of the development of new vaccines for tuberculosis. Vaccines 2020, 8, 586. [Google Scholar] [CrossRef]
- Cardona, P.J. The progress of therapeutic vaccination with regard to tuberculosis. Front. Microbiol. 2016, 7, 1536. [Google Scholar] [CrossRef]
- White, A.D.; Sarfas, C.; Sibley, L.S.; Gullick, J.; Clark, S.; Rayner, E.; Gleeson, F.; Català, M.; Nogueira, I.; Cardona, P.J.; et al. Protective efficacy of inhaled BCG vaccination against ultra-low dose aerosol M. tuberculosis challenge in rhesus macaques. Pharmaceutics 2020, 12, 394. [Google Scholar] [CrossRef] [PubMed]
- Sibley, L.; White, A.D.; Gooch, K.E.; Stevens, L.M.; Tanner, R.; Jacobs, A.; Daykin-Pont, O.; Gleeson, F.; McIntyre, A.; Basaraba, R.; et al. High-dose Mycobacterium tuberculosis aerosol challenge cannot overcome BCG-induced protection in chinese origin cynomolgus macaques; implications of natural resistance for vaccine evaluation. Sci. Rep. 2021, 11, 12274. [Google Scholar] [CrossRef]
- Sibley, L.; Dennis, M.; Sarfas, C.; White, A.; Clark, S.; Gleeson, F.; McIntyre, A.; Rayner, E.; Pearson, G.; Williams, A.; et al. Route of delivery to the airway influences the distribution of pulmonary disease but not the outcome of Mycobacterium tuberculosis infection in rhesus macaques. Tuberculosis 2016, 96, 141–149. [Google Scholar] [CrossRef]
- White, A.D.; Sarfas, C.; West, K.; Sibley, L.S.; Wareham, A.S.; Clark, S.; Dennis, M.J.; Williams, A.; Marsh, P.D.; Sharpe, S.A. Evaluation of the immunogenicity of mycobacterium bovis BCG delivered by aerosol to the lungs of macaques. Clin. Vaccine Immunol. 2015, 22, 992–1003. [Google Scholar] [CrossRef]
- Sharpe, S.A.; White, A.D.; Sibley, L.; Gleeson, F.; Hall, G.A.; Basaraba, R.J.; McIntyre, A.; Clark, S.O.; Gooch, K.; Marsh, P.D.; et al. An aerosol challenge model of tuberculosis in mauritian cynomolgus macaques. PLoS ONE 2017, 12, e0171906. [Google Scholar] [CrossRef]
- Bannister, S.; Sudbury, E.; Villanueva, P.; Perrett, K.; Curtis, N. The safety of BCG revaccination: A systematic review. Vaccine 2021, 39, 2736–2745. [Google Scholar] [CrossRef]
- Mahasha, P.W.; Ndwandwe, D.E.; Mavundza, E.J.; Shey, M.; Wiysonge, C.S. Systematic review protocol on bacillus Calmette-Guérin (BCG)) revaccination and protection against tuberculosis. BMJ Open 2019, 9, e027033. [Google Scholar] [CrossRef]
- Lowenstine, L.J.; Osborn, K.G. Respiratory system diseases of nonhuman primates. In Nonhuman Primates in Biomedical Research; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 413–481. [Google Scholar]
- Peña, J.C.; Ho, W.-Z. Non-human primate models of tuberculosis. Microbiol. Spectr. 2016, 4, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Foreman, T.W.; Mehra, S.; Lackner, A.A.; Kaushal, D. Translational research in the nonhuman primate model of tuberculosis. ILAR J. 2017, 58, 151–159. [Google Scholar] [CrossRef]
- Scanga, C.A.; Flynn, J.L. Modeling tuberculosis in nonhuman primates. Cold Spring Harb. Perspect. Med. 2014, 4, a018564. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.L.; Pawar, S.; Myers, A.; Pegu, A.; Fuhrman, C.; Reinhart, T.A.; Capuano, S.V.; Klein, E.; Flynn, J.A.L. Early events in Mycobacterium tuberculosis infection in cynomolgus macaques. Infect. Immun. 2006, 74, 3790–3803. [Google Scholar] [CrossRef]
- Langermans, J.A.; Andersen, P.; van Soolingen, D.; Vervenne, R.A.; Frost, P.A.; van der Laan, T.; van Pinxteren, L.A.; van den Hombergh, J.; Kroon, S.; Peekel, I.; et al. Divergent effect of bacillus Calmette-Guérin (BCG) vaccination on Mycobacterium tuberculosis infection in highly related macaque species: Implications for primate models in tuberculosis vaccine research. Proc. Natl. Acad. Sci. USA. 2001, 98, 11497–11502. [Google Scholar] [CrossRef] [PubMed]
- Verreck, F.A.W.; Tchilian, E.Z.; Vervenne, R.A.W.; Sombroek, C.C.; Kondova, I.; Eissen, O.A.; Sommandas, V.; van der Werff, N.M.; Verschoor, E.; Braskamp, G.; et al. Variable BCG Efficacy in rhesus populations: Pulmonary BCG provides protection where standard intra-dermal vaccination fails. Tuberculosis 2017, 104, 46–57. [Google Scholar] [CrossRef]
- Rayner, E.L.; Pearson, G.R.; Hall, G.A.; Basaraba, R.J.; Gleeson, F.; McIntyre, A.; Clark, S.; Williams, A.; Dennis, M.J.; Sharpe, S.A. Early lesions following aerosol infection of rhesus macaques (Macaca mulatta) with Mycobacterium tuberculosis strain H37RV. J. Comp. Pathol. 2013, 149, 475–485. [Google Scholar] [CrossRef]
- Dijkman, K.; Vervenne, R.A.W.; Sombroek, C.C.; Boot, C.; Hofman, S.O.; van Meijgaarden, K.E.; Ottenhoff, T.H.M.; Kocken, C.H.M.; Haanstra, K.G.; Vierboom, M.P.M.; et al. Disparate tuberculosis disease development in macaque species is associated with innate immunity. Front. Immunol. 2019, 10, 2479. [Google Scholar] [CrossRef]
- Capuano, S.V.; Croix, D.A.; Pawar, S.; Zinovik, A.; Myers, A.; Lin, P.L.; Bissel, S.; Fuhrman, C.; Klein, E.; Flynn, J.A.L. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect. Immun. 2003, 71, 5831–5844. [Google Scholar] [CrossRef]
- Maiello, P.; DiFazio, R.M.; Cadena, A.M.; Rodgers, M.A.; Lin, P.L.; Scanga, C.A.; Flynn, J.A.L. Rhesus macaques are more susceptible to progressive tuberculosis than cynomolgus macaques: A quantitative comparison. Infect. Immun. 2018, 86, e00505-17. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, S.A.; Eschelbach, E.; Basaraba, R.J.; Gleeson, F.; Hall, G.A.; McIntyre, A.; Williams, A.; Kraft, S.L.; Clark, S.; Gooch, K.; et al. Determination of lesion volume by mri and stereology in a macaque model of tuberculosis. Tuberculosis 2009, 89, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, S.A.; McShane, H.; Dennis, M.J.; Basaraba, R.J.; Gleeson, F.; Hall, G.; McIntyre, A.; Gooch, K.; Clark, S.; Beveridge, N.E.R.; et al. Establishment of an aerosol challenge model of tuberculosis in rhesus macaques and an evaluation of endpoints for vaccine testing. Clin. Vaccine Immunol. 2010, 17, 1170–1182. [Google Scholar] [CrossRef]
- White, A.G.; Maiello, P.; Coleman, M.T.; Tomko, J.A.; Frye, L.J.; Scanga, C.A.; Lin, P.L.; Flynn, J.A.L. Analysis of 18FDG PET/CT imaging as a tool for studying Mycobacterium tuberculosis infection and treatment in non-human primates. J. Vis. Exp. 2017, 127, e56375. [Google Scholar]
- Prats, C.; Vilaplana, C.; Valls, J.; Marzo, E.; Cardona, P.J.; López, D. Local inflammation, dissemination and coalescence of lesions are key for the progression toward active tuberculosis: The Bubble Model. Front. Microbiol. 2016, 7, 33. [Google Scholar] [CrossRef]
- Català, M.; Bechini, J.; Tenesa, M.; Pérez, R.; Moya, M.; Vilaplana, C.; Valls, J.; Alonso, S.; López, D.; Cardona, P.J.; et al. Modelling the dynamics of tuberculosis lesions in a virtual lung: Role of the bronchial tree in endogenous reinfection. PLoS Comput. Biol. 2020, 16, e1007772. [Google Scholar] [CrossRef]
- Cardona, P.J. The key role of exudative lesions and their encapsulation: Lessons learned from the pathology of human pulmonary tuberculosis. Front. Microbiol. 2015, 6, 612. [Google Scholar] [CrossRef] [PubMed]
- Cardona, P.J. A dynamic reinfection hypothesis of latent tuberculosis infection. Infection 2009, 37, 80–86. [Google Scholar] [CrossRef]
- Cardona, P.J. Revisiting the natural history of tuberculosis: The inclusion of constant reinfection, host tolerance, and damage-response frameworks leads to a better understanding of latent infection and its evolution towards active disease. Arch. Immunol. Ther. Exp. 2010, 58, 7–14. [Google Scholar] [CrossRef]
- Marzo, E.; Vilaplana, C.; Tapia, G.; Diaz, J.; Garcia, V.; Cardona, P.J. Damaging role of neutrophilic infiltration in a mouse model of progressive tuberculosis. Tuberculosis 2014, 94, 55–64. [Google Scholar] [CrossRef]
- Lin, P.L.; Maiello, P.; Gideon, H.P.; Coleman, M.T.; Cadena, A.M.; Rodgers, M.A.; Gregg, R.; O’Malley, M.; Tomko, J.; Fillmore, D.; et al. PET-CT identifies reactivation risk in cynomolgus macaques with latent M. tuberculosis. PLoS Pathog. 2016, 12, e1005739. [Google Scholar] [CrossRef]
- Hansell, D.M.; Armstrong, P.; Lynch, D.A.; McAdams, H.P. Imaging of Diseases of the Chest, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2005; ISBN 0-323-03660-0-2005. [Google Scholar]
- Webb, W.R.; Müller, N.L.; Naidich, D.P. High-Resolution CT of the Lung, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003; ISBN 0-0817-2278-0-2001. [Google Scholar]
- Leung, A.N. State-of-the-art: Pulmonary Tuberculosis. Radiology 1996, 1992, 307–322. [Google Scholar]
- Andreu, J.; Cáceres, J.; Pallisa, E.; Martinez-Rodriguez, M. Radiological manifestations of pulmonary tuberculosis. Eur. J. Radiol. 2004, 51, 139–149. [Google Scholar] [CrossRef]
- Nachiappan, A.C.; Rahbar, K.; Shi, X.; Guy, E.S.; Mortani Barbosa, E.J.; Shroff, G.S.; Ocazionez, D.; Schlesinger, A.E.; Katz, S.I.; Hammer, M.M. Pulmonary tuberculosis: Role of radiology in diagnosis and management. Radiographics 2017, 37, 52–72. [Google Scholar] [CrossRef]
- Rozenshtein, A.; Hao, F.; Starc, M.T.; Pearson, G.D.N. Radiographic appearance of pulmonary tuberculosis: Dogma disproved. Am. J. Roentgenol. 2015, 204, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Liu, Z.; Shen, G.; Zhang, Y.; Li, L.; Wu, Z.; Luo, D.; Gu, Q.; Mao, H.; Wang, L. MRI evaluation of pulmonary lesions and lung tissue changes induced by tuberculosis. Int. J. Infect. Dis. 2019, 82, 138–146. [Google Scholar] [CrossRef]
- Sharpe, S.A.; Smyth, D.; McIntyre, A.; Gleeson, F.; Dennis, M.J. Refinement and reduction through application of a quantitative score system for estimation of TB-induced disease burden using computed tomography. Lab. Anim. 2018, 52, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Hislop, A.; Howard, S.; Fairweather, D.V.I. Morphometric studies on the structural development of the lung in Macaca fascicularis during fetal and postnatal life. J. Anat. 1984, 138, 95–112. [Google Scholar]
- Chase, R.E. Lung Lobation in rhesus monkey, compared with man. Amer Jour Phys Anthropol. 1942, 29, 267–286. [Google Scholar] [CrossRef]
- Tyler, N.K.; Hyde, D.M.; Hendrickx, A.G.; Plopper, C.G. Morphogenesis of the Respiratory Bronchiole in Rhesus Monkey Lungs. Am J Anat. 1988, 182, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Asgharian, B.; Price, O.; McClellan, G.; Corley, R.; Einstein, D.R.; Jacob, R.E.; Harkema, J.; Carey, S.A.; Schelegle, E.; Hyde, D.; et al. Development of a rhesus monkey lung geometry model and application to particle deposition in comparison to humans. Inhal Toxicol. 2012, 24, 869–899. [Google Scholar] [CrossRef] [PubMed]
- Mercer, R.R.; Crapo, J.D. Lower respiratory tract structure of laboratory animals and humans: Dosimetry implications. Aerosol Sci. Technol. 1993, 18, 257–271. [Google Scholar]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef]
- Rossi, S.E.; Franquet, T.; Volpacchio, M.; Giménez, A.; Aguilar, G. Tree-in-bud pattern at thin-section CT of the lungs: Radiologic-pathologic overview. Radiographics 2005, 25, 789–801. [Google Scholar] [CrossRef]
- Hunter, R.L. Tuberculosis as a three-act play: A new paradigm for the pathogenesis of pulmonary tuberculosis. Tuberculosis 2016, 97, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Darrah, P.A.; Zeppa, J.J.; Maiello, P.; Hackney, J.A.; Wadsworth, M.H.; Hughes, T.K.; Pokkali, S.; Swanson, P.A.; Grant, N.L.; Rodgers, M.A.; et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 2020, 577, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Laddy, D.J.; Bonavia, A.; Hanekom, W.A.; Kaushal, D.; Williams, A.; Roederer, M.; Seder, R.A.; Sharpe, S.A.; Verreck, F.A.W.; Darrah, P.A. Toward tuberculosis vaccine development: Recommendations for nonhuman primate study design. Infect. Immun. 2018, 86, e00776-17. [Google Scholar] [CrossRef]
- Plumlee, C.R.; Duffy, F.J.; Gern, B.H.; Delahaye, J.L.; Cohen, S.B.; Stoltzfus, C.R.; Rustad, T.R.; Hansen, S.G.; Axthelm, M.K.; Picker, L.J.; et al. Ultra-low dose aerosol infection of mice with Mycobacterium tuberculosis more closely models human tuberculosis. Cell Host Microbe 2021, 29, 68–82. [Google Scholar] [CrossRef]
- Spertini, F.; Audran, R.; Chakour, R.; Karoui, O.; Steiner-Monard, V.; Thierry, A.-C.; Mayor, C.E.; Rettby, N.; Jaton, K.; Vallotton, L.; et al. Safety of human immunisation with a live-attenuated Mycobacterium tuberculosis vaccine: A randomised, double-blind, controlled phase I trial. Lancet. Respir. Med. 2015, 3, 953–962. [Google Scholar] [CrossRef]
- Tameris, M.; Mearns, H.; Penn-Nicholson, A.; Gregg, Y.; Bilek, N.; Mabwe, S.; Geldenhuys, H.; Shenje, J.; Luabeya, A.K.K.; Murillo, I.; et al. Live-attenuated Mycobacterium tuberculosis vaccine MTBVAC versus BCG in adults and neonates: A randomised controlled, double-blind dose-escalation trial. Lancet. Respir. Med. 2019, 7, 757–770. [Google Scholar] [CrossRef]
- Aguilo, N.; Gonzalo-Asensio, J.; Alvarez-Arguedas, S.; Marinova, D.; Gomez, A.B.; Uranga, S.; Spallek, R.; Singh, M.; Audran, R.; Spertini, F.; et al. Reactogenicity to major tuberculosis antigens absent in BCG is linked to improved protection against Mycobacterium tuberculosis. Nat. Commun. 2017, 8, 16085. [Google Scholar] [CrossRef]
- Vilaplana, C.; Marzo, E.; Tapia, G.; Diaz, J.; Garcia, V.; Cardona, P.J. Ibuprofen therapy resulted in significantly decreased tissue bacillary loads and increased survival in a new murine experimental model of active tuberculosis. J. Infect. Dis. 2013, 208, 199–202. [Google Scholar] [CrossRef]
- Cardona, P.J. Reactivation or reinfection in adult tuberculosis: Is that the question? Int. J. Mycobacteriol. 2016, 5, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.G.; Zak, D.E.; Xu, G.; Ford, J.C.; Marshall, E.E.; Malouli, D.; Gilbride, R.M.; Hughes, C.M.; Ventura, A.B.; Ainslie, E.; et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat. Med. 2018, 24, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, S.; White, A.; Sarfas, C.; Sibley, L.; Gleeson, F.; McIntyre, A.; Basaraba, R.; Clark, S.; Hall, G.; Rayner, E.; et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: Protection associated with mycobacterial antigen-specific CD4 effector memory T-Cell populations. Tuberculosis 2016, 101, 174–190. [Google Scholar] [CrossRef] [PubMed]
- DiFazio, R.M.; Maiello, P.; Tomko, J.; Frye, L.; Bigbee, C.; Chedrick, C.; Updike, C.; Lin, P.; Agger, E.M.; Andersen, P.; et al. Predicting vaccine efficacy against tuberculosis using immunologic and radiologic measures in the non-human primate model (VAC7P.974). J. Immunol. 2014, 192, 141–149. [Google Scholar] [CrossRef]
- Kraft, S.L.; Dailey, D.; Kovach, M.; Stasiak, K.L.; Bennett, J.; McFarland, C.T.; McMurray, D.N.; Izzo, A.A.; Orme, I.M.; Basaraba, R.J. Magnetic resonance imaging of pulmonary lesions in guinea pigs infected with Mycobacterium tuberculosis. Infect. Immun. 2004, 72, 5963–5971. [Google Scholar] [CrossRef] [PubMed][Green Version]





| EG | Consolidations | Daughter Micronodules | Isolated Micronodules | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Range | % | Median | Range | % | Median | Range | % | |
| CUV (n = 320) | 2 | [0 6] | 9 | 5.5 | [0 30] | 23 | 5.5 | [0 110] | 68 |
| CIDV (n = 22) | 4 | [0 6] | 45 | 0 | [0 1] | 5 | 4 | [2 5] | 50 |
| C (n = 342) | 2 | [0 6] | 11 | 3 | [0 30] | 22 | 4 | [0 110] | 67 |
| RUV (n = 871) | 12.5 | [1 25] | 14 | 36 | [2 96] | 49 | 30 | [1 83] | 37 |
| RAEV (n = 643) | 14.5 | [0 19] | 12 | 54 | [0 151] | 66 | 18.5 | [14 37] | 22 |
| RIDV (n = 697) | 15 | [12 24] | 12 | 55 | [45 138] | 53 | 29 | [21 117] | 35 |
| R (n = 2211) | 14 | [0 25] | 13 | 51 | [0 151] | 55 | 29 | [1 117] | 32 |
| TOTAL (n = 2553) | 10.5 | [0 25] | 13 | 27.5 | [0 151] | 51 | 20 | [0 117] | 37 |
| EG | Number (n) | Fraction DM/IM | ||
|---|---|---|---|---|
| Total | DM | IM | ||
| CUV | 293 | 75 | 218 | 0.34 |
| CIDV | 12 | 1 | 11 | 0.09 |
| C | 304 | 75 | 229 | 0.33 |
| RUV | 752 | 428 | 324 | 1.32 |
| RAEV | 564 | 424 | 140 | 3.03 |
| RIDV | 611 | 368 | 243 | 1.51 |
| R | 1924 | 1216 | 708 | 1.72 |
| EG | Consolidations with DM | DM per Consolidation | ||||
|---|---|---|---|---|---|---|
| n | % | Median | Range | Median | Range | |
| CUV (n = 29) | 19 | 66 | 2 | [0 4] | 3 | [1 15] |
| CIDV (n = 10) | 1 | 10 | 0 | [0 1] | 1 | [1 1] |
| C (n = 39) | 20 | 51 | 1 | [0 4] | 3 | [1 15] |
| RUV (n = 121) | 111 | 92 | 11.5 | [1 24] | 3 | [1 15] |
| RAEV (n = 80) | 79 | 99 | 14.5 | [0 19] | 3.5 | [1 15] |
| RIDV (n = 88) | 84 | 95 | 14 | [11 24] | 3 | [1 15] |
| R (n = 289) | 274 | 95 | 14 | [0 24] | 3 | [1 15] |
| Total (n = 328) | 294 | 90 | 9 | [0 24] | 3 | [1 15] |
| EG | CUV | CIDV | C | RUV | RAEV | RIDV | R |
|---|---|---|---|---|---|---|---|
| CUV | ND | ||||||
| CIDV | 0.3057 | ND | |||||
| C | ND | ND | ND | ||||
| RUV | ND | ND | ND | ND | |||
| RAEV | ND | ND | ND | <0.0001 | ND | ||
| RIDV | ND | ND | ND | 0.32431 | <0.0001 | ND | |
| R | ND | ND | <0.0001 | ND | ND | ND | ND |
| EG | CUV | CIDV | C | RUV | RAEV | RIDV | R |
|---|---|---|---|---|---|---|---|
| CUV | ND | ||||||
| CIDV | 0.0014 | ND | |||||
| C | ND | ND | ND | ||||
| RUV | ND | ND | ND | ND | |||
| RAEV | ND | ND | ND | 0.0585 | ND | ||
| RIDV | ND | ND | ND | 0.0355 | >0.999 | ND | |
| R | ND | ND | <0.0001 | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, I.; Català, M.; White, A.D.; Sharpe, S.A.; Bechini, J.; Prats, C.; Vilaplana, C.; Cardona, P.-J. Surveillance of Daughter Micronodule Formation Is a Key Factor for Vaccine Evaluation Using Experimental Infection Models of Tuberculosis in Macaques. Pathogens 2023, 12, 236. https://doi.org/10.3390/pathogens12020236
Nogueira I, Català M, White AD, Sharpe SA, Bechini J, Prats C, Vilaplana C, Cardona P-J. Surveillance of Daughter Micronodule Formation Is a Key Factor for Vaccine Evaluation Using Experimental Infection Models of Tuberculosis in Macaques. Pathogens. 2023; 12(2):236. https://doi.org/10.3390/pathogens12020236
Chicago/Turabian StyleNogueira, Isabel, Martí Català, Andrew D. White, Sally A Sharpe, Jordi Bechini, Clara Prats, Cristina Vilaplana, and Pere-Joan Cardona. 2023. "Surveillance of Daughter Micronodule Formation Is a Key Factor for Vaccine Evaluation Using Experimental Infection Models of Tuberculosis in Macaques" Pathogens 12, no. 2: 236. https://doi.org/10.3390/pathogens12020236
APA StyleNogueira, I., Català, M., White, A. D., Sharpe, S. A., Bechini, J., Prats, C., Vilaplana, C., & Cardona, P.-J. (2023). Surveillance of Daughter Micronodule Formation Is a Key Factor for Vaccine Evaluation Using Experimental Infection Models of Tuberculosis in Macaques. Pathogens, 12(2), 236. https://doi.org/10.3390/pathogens12020236







